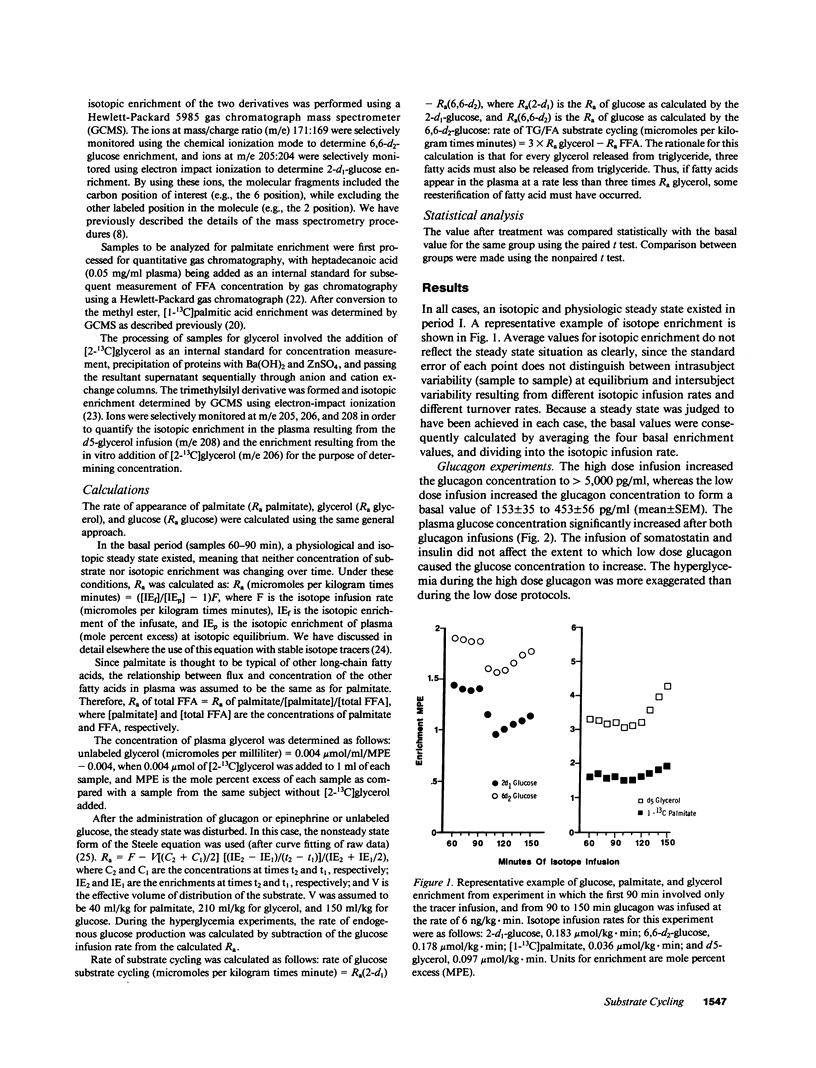
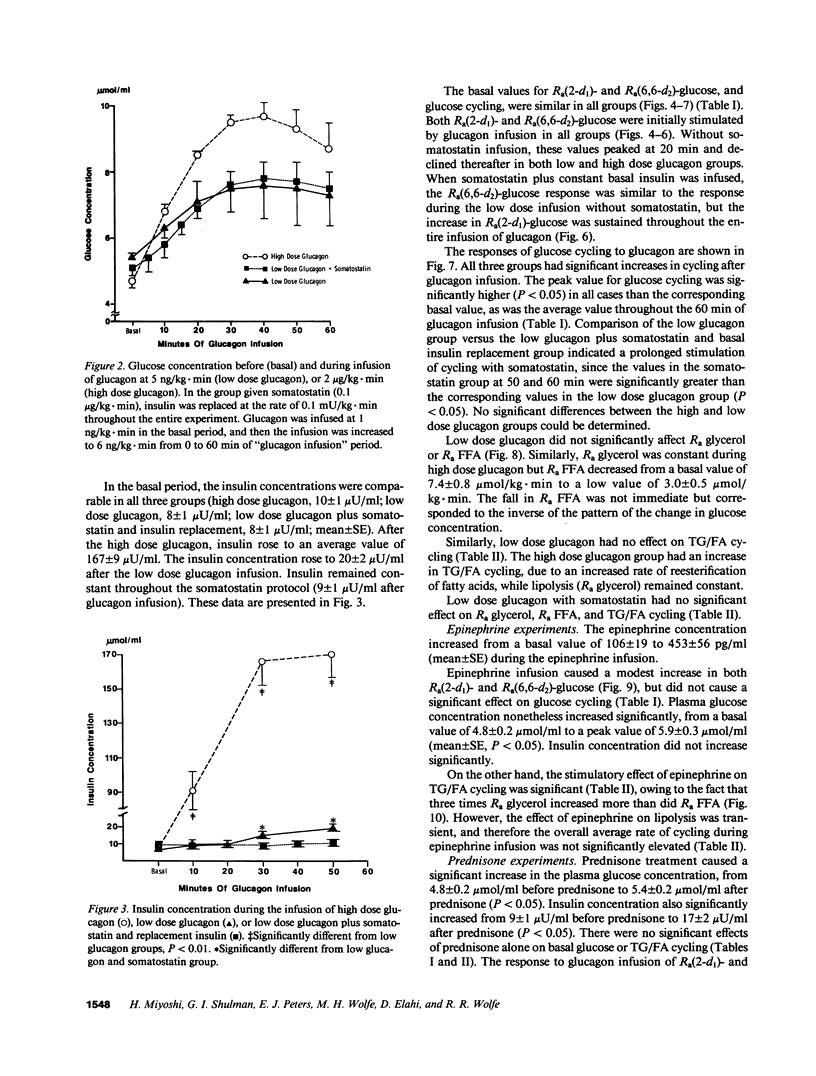
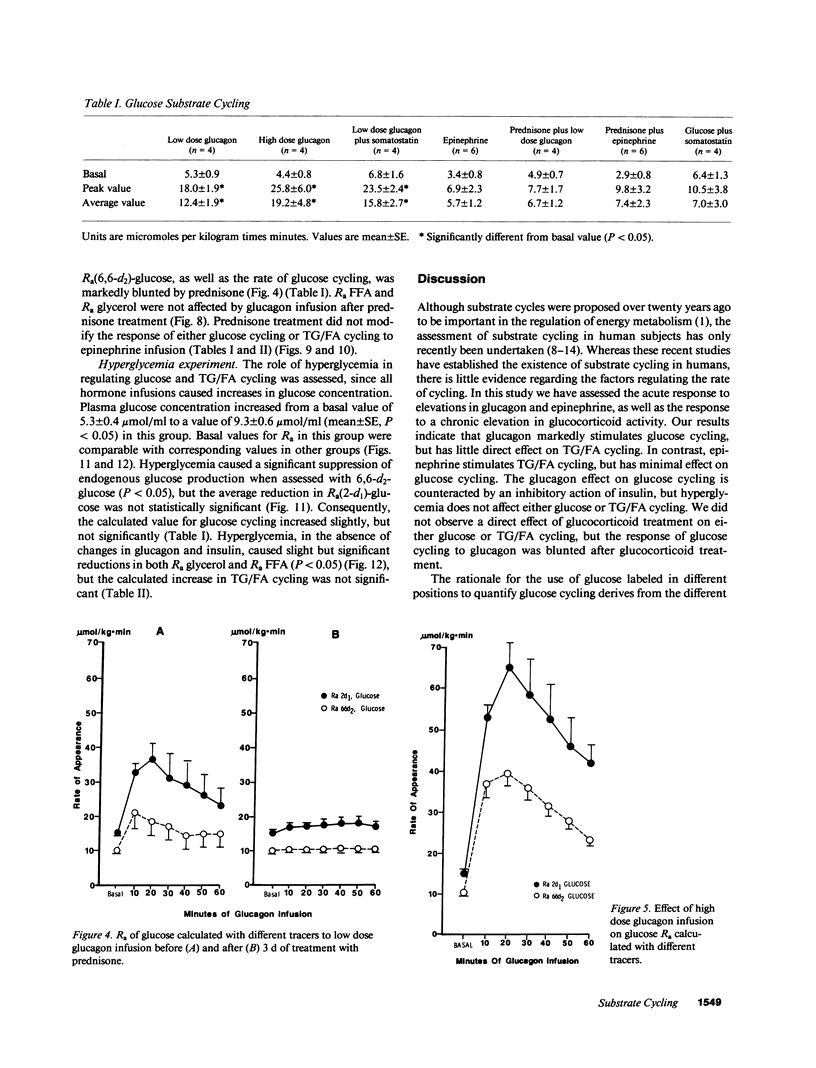
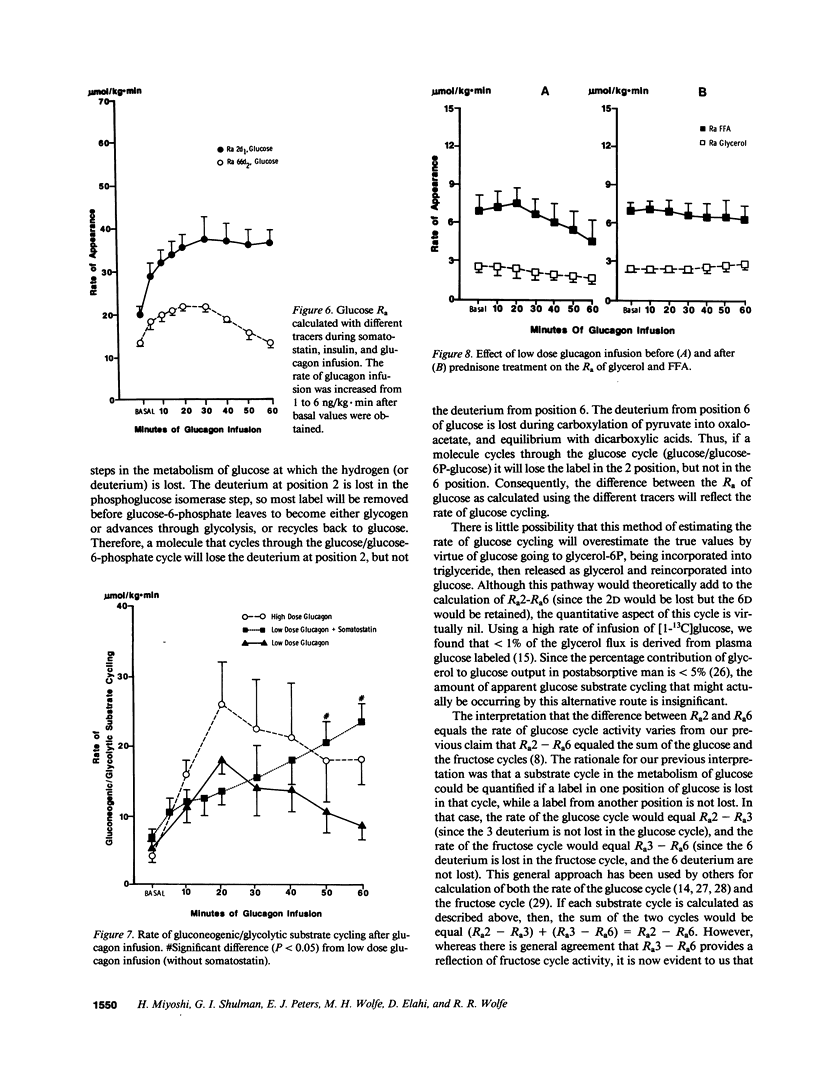
Abstract
Recent studies have established the existence of substrate cycles in humans, but factors regulating the rate of cycling have not been identified. We have therefore investigated the acute response of glucose/glucose-6P-glucose (glucose) and triglyceride/fatty acid (TG/FA) substrate cycling to the infusion of epinephrine (0.03 microgram/kg.min) and glucagon. The response to a high dose glucagon infusion (2 micrograms/kg.min) was tested, as well as the response to a low dose infusion (5 ng/kg.min), with and without the simultaneous infusion of somatostatin (0.1 microgram/kg.min) and insulin (0.1 mU/kg.min). Additionally, the response to chronic prednisone (50 mg/d) was evaluated, both alone and during glucagon (low dose) and epinephrine infusion. Finally, the response to hyperglycemia, with insulin and glucagon held constant by somatostatin infusion and constant replacement of glucagon and insulin at basal rates, was investigated. Glucose cycling was calculated as the difference between the rate of appearance (Ra) of glucose as determined using 2-d1- and 6,6-d2-glucose as tracers. TG/FA cycling was calculated by first determining the Ra glycerol with d5-glycerol and the Ra FFA with [1-13C]palmitate, then subtracting Ra FFA from three times Ra glycerol. The results indicate that glucagon stimulates glucose cycling, and this stimulatory effect is augmented when the insulin response to glucagon infusion is blocked. Glucagon had minimal effect on TG/FA cycling. In contrast, epinephrine stimulated TG/FA cycling, but affected glucose cycling minimally. Prednisone had no direct effect on either glucose or TG/FA cycling, but blunted the stimulatory effect of glucagon on glucose cycling. Hyperglycemia, per se, had no direct effect on glucose or TG/FA cycling. Calculations revealed that stimulation of TG/FA cycling theoretically amplified the sensitivity of control of fatty acid flux, but no such amplification was evident as a result of the stimulation of glucose cycling by glucagon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Effects of hyperglycemia on glucose production and utilization in humans. Measurement with [23H]-, [33H]-, and [614C]glucose. Diabetes. 1986 Jun;35(6):642–648. doi: 10.2337/diab.35.6.642. [DOI] [PubMed] [Google Scholar]
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B. J., Arch J. R., Newsholme E. A. Effect of some hormones on the rate of the triacylglycerol/fatty-acid substrate cycle in adipose tissue of the mouse in vivo. Biosci Rep. 1983 Mar;3(3):263–267. doi: 10.1007/BF01122458. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Arch J. R., Crabtree B., Newsholme E. A. Measurement of the rate of substrate cycling between fructose 6-phosphate and fructose 1,6-bisphosphate in skeletal muscle by using a single-isotope technique. Biochem J. 1984 Nov 1;223(3):849–853. doi: 10.1042/bj2230849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzipanteli K., Saggerson D. Streptozotocin diabetes results in increased responsiveness of adipocyte lipolysis to glucagon. FEBS Lett. 1983 May 2;155(1):135–138. doi: 10.1016/0014-5793(83)80225-7. [DOI] [PubMed] [Google Scholar]
- Chenoweth M., Dunn A. Fructose-6-phosphate substrate cycling and hormonal regulation of gluconeogenesis in vivo. Am J Physiol. 1978 Sep;235(3):E295–E303. doi: 10.1152/ajpendo.1978.235.3.E295. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Isotopic evidence for futile cycles in liver cells. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1141–1148. doi: 10.1016/0006-291x(73)90811-5. [DOI] [PubMed] [Google Scholar]
- Clutter W. E., Bier D. M., Shah S. D., Cryer P. E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980 Jul;66(1):94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Vranić M. Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes. Proc Natl Acad Sci U S A. 1985 May;82(9):2965–2969. doi: 10.1073/pnas.82.9.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckemeyer C. M., Barker J., Duckworth W. C., Solomon S. S. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Endocrinology. 1983 Jul;113(1):270–276. doi: 10.1210/endo-113-1-270. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Hussain M. N., Benedict C. R. Radioenzymatic microassay for simultaneous estimations of dopamine, norepinephrine, and epinephrine in plasma, urine, and tissues. Clin Chem. 1985 Nov;31(11):1861–1864. [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Karlander S., Roovete A., Vranić M., Efendić S. Glucose and fructose 6-phosphate cycle in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E530–E536. doi: 10.1152/ajpendo.1986.251.5.E530. [DOI] [PubMed] [Google Scholar]
- Karlander S., Vranić M., Efendić S. Increased glucose turnover and glucose cycling in acromegalic patients with normal glucose tolerance. Diabetologia. 1986 Nov;29(11):778–783. doi: 10.1007/BF00873216. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Nair K. S. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987 May;64(5):896–901. doi: 10.1210/jcem-64-5-896. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Foster D. W., McGarry J. D. Evidence for suppression of hepatic glucose-6-phosphatase with carbohydrate feeding. Diabetes. 1984 Feb;33(2):192–195. doi: 10.2337/diab.33.2.192. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes. 1982 Aug;31(8 Pt 1):663–669. doi: 10.2337/diab.31.8.663. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Ladenson P. W., Wolfe M. H., Ridgway E. C., Wolfe R. R. Substrate cycling between gluconeogenesis and glycolysis in euthyroid, hypothyroid, and hyperthyroid man. J Clin Invest. 1985 Aug;76(2):757–764. doi: 10.1172/JCI112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Williams P. E., Liljenquist J. E., Lacy W. W., Keller U., Cherrington A. D. Effect of hyperglycemia independent of changes in insulin or glucagon on lipolysis in the conscious dog. Metabolism. 1980 Apr;29(4):317–320. doi: 10.1016/0026-0495(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Pitot H. C. Maintenance of glucokinase activity in primary hepatocyte cultures. J Cell Physiol. 1980 May;103(2):173–178. doi: 10.1002/jcp.1041030202. [DOI] [PubMed] [Google Scholar]
- Vaughan G. M., Becker R. A., Unger R. H., Ziegler M. G., Siler-Khodr T. M., Pruitt B. A., Jr, Mason A. D., Jr Nonthyroidal control of metabolism after burn injury: possible role of glucagon. Metabolism. 1985 Jul;34(7):637–641. doi: 10.1016/0026-0495(85)90091-5. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Evans J. E., Mullany C. J., Burke J. F. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980 Apr;7(4):168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Herndon D. N., Jahoor F., Miyoshi H., Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987 Aug 13;317(7):403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Peters E. J. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987 Feb;252(2 Pt 1):E218–E223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]


