Abstract
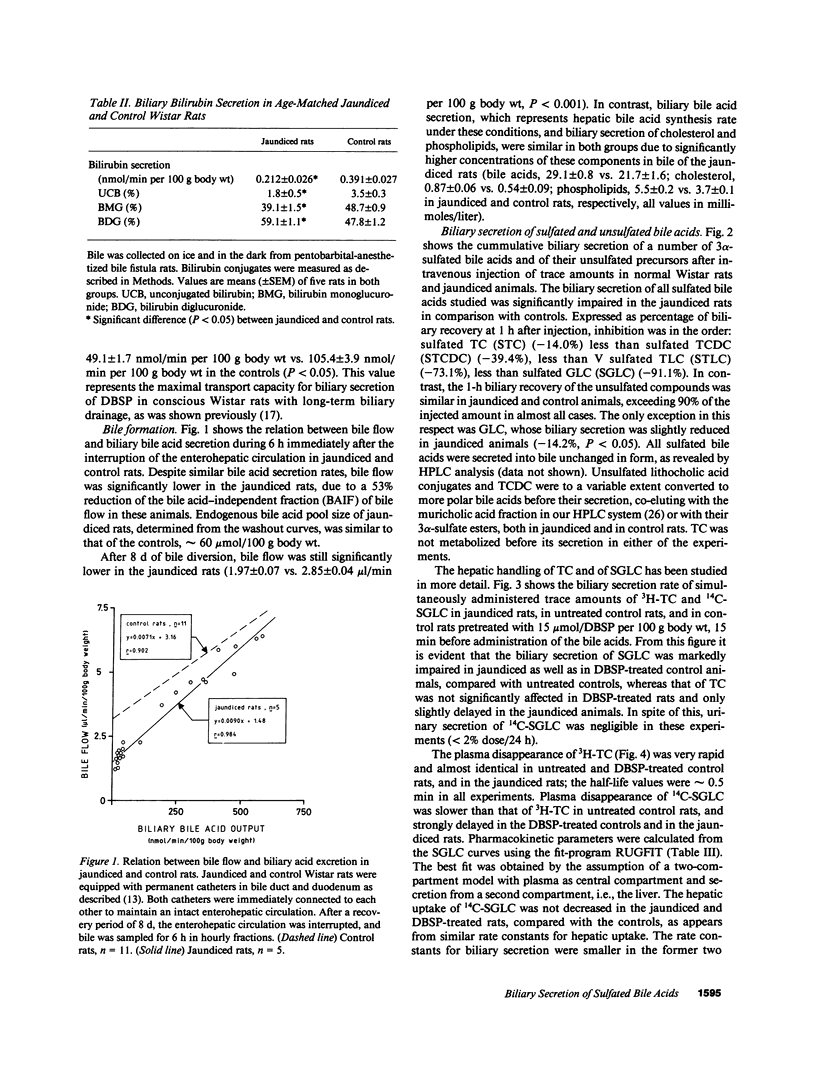
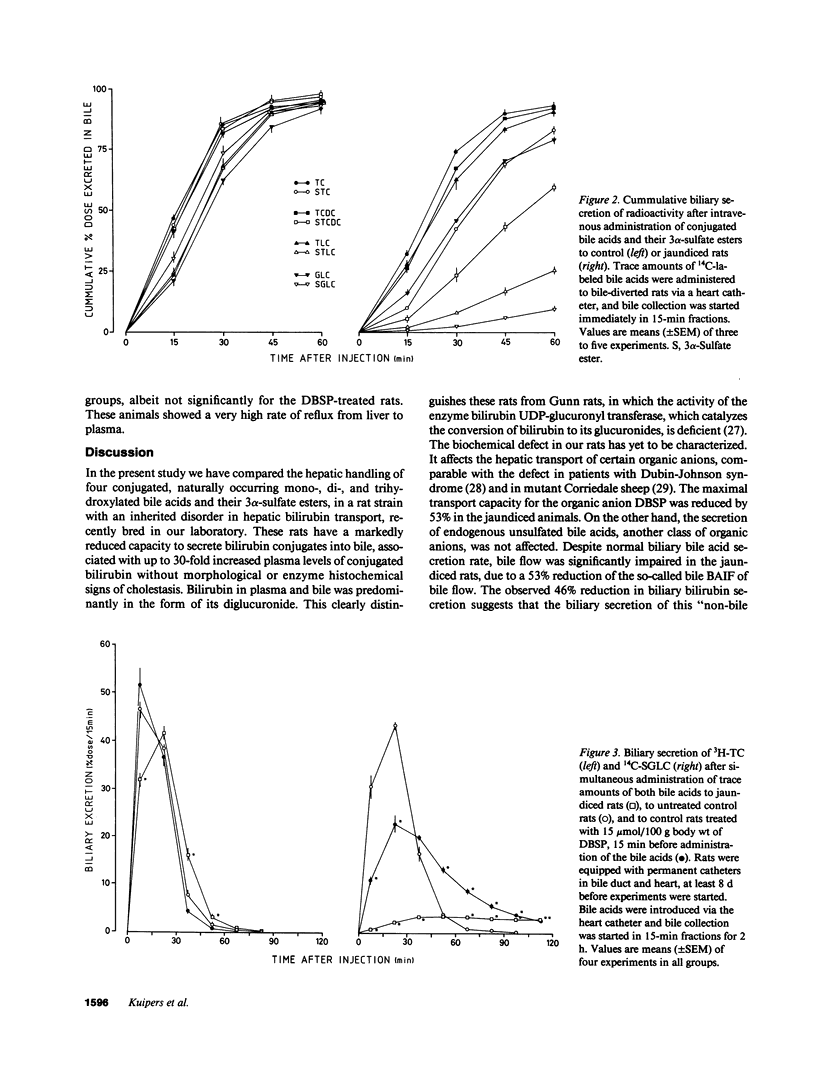
Biliary secretion of 3 alpha-sulfated bile acids has been studied in Wistar rats with an autosomal recessive defect in the hepatic transport of bilirubin. Liver function, established by measurement of various enzymes in plasma, by enzyme histochemical methods, and by electron microscopy, appeared to be normal in these rats. Serum levels of unconjugated, monoglucuronidated, and diglucuronidated bilirubin were 0.62, 1.62, and 6.16 mumol/liter, respectively, compared with 0.17, 0.08, and 0.02 mumol/liter in control rats. Biliary bilirubin secretion was strongly reduced in the mutant animals: 0.21 +/- 0.03 vs. 0.39 +/- 0.03 nmol/min per 100 g body wt in control rats. Despite normal biliary bile acid output, bile flow was markedly impaired in the mutant animals, due to a 53% reduction of the bile acid-independent fraction of bile flow. The transport maximum for biliary secretion of dibromosulphthalein (DBSP) was also drastically reduced (-53%). Biliary secretion of intravenously administered trace amounts of the 3 alpha-sulfate esters of 14C-labeled taurocholic acid (-14%), taurochenodeoxycholic acid (-39%), taurolithocholic acid (-73%), and glycolithocholic acid (-91%) was impaired in the jaundiced rats compared with controls, in contrast to the biliary secretion of the unsulfated parent compounds. Hepatic uptake of sulfated glycolithocholic acid was not affected in the jaundiced animals. Preadministration of DBSP (15 mumol/100 g body wt) to normal Wistar rats significantly impaired the biliary secretion of sulfated glycolithocholic acid, but did not affect taurocholic acid secretion. We conclude that separate transport systems in the rat liver exist for biliary secretion of sulfated and unsulfated bile acids; the sulfates probably share secretory pathways with the organic anions bilirubin and DBSP. The described genetic defect in hepatic transport function is associated with a reduced capacity to secrete sulfated bile acids into bile; this becomes more pronounced with a decreasing number of hydroxyl groups on the sulfated bile acid's molecule.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Anwer M. S., Kroker R., Hegner D. Effect of albumin on bile acid uptake by isolated rat hepatocytes. Is there a common bile acid carrier? Biochem Biophys Res Commun. 1976 Nov 8;73(1):63–71. doi: 10.1016/0006-291x(76)90497-6. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bartholomew T. C., Billing B. H. The effect of 3-sulphation and taurine conjugation on the uptake of chenodeoxycholic acid by rat hepatocytes. Biochim Biophys Acta. 1983 Nov 1;754(1):101–109. doi: 10.1016/0005-2760(83)90086-3. [DOI] [PubMed] [Google Scholar]
- Bartholomew T. C., Summerfield J. A., Billing B. H., Lawson A. M., Setchell K. D. Bile acid profiles of human serum and skin interstitial fluid and their relationship to pruritus studied by gas chromatography-mass spectrometry. Clin Sci (Lond) 1982 Jul;63(1):65–73. doi: 10.1042/cs0630065. [DOI] [PubMed] [Google Scholar]
- Blanckaert N. Analysis of bilirubin and bilirubin mono- and di-conjugates. Determination of their relative amounts in biological samples. Biochem J. 1980 Jan 1;185(1):115–128. doi: 10.1042/bj1850115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNELIUS C. E., ARIAS I. M., OSBURN B. I. HEPATIC PIGMENTATION WITH PHOTOSENSITIVITY: A SYNDROME IN CORRIEDALE SHEEP RESEMBLING DUBIN-JOHNSON SYNDROME IN MAN. J Am Vet Med Assoc. 1965 Apr 1;146:709–713. [PubMed] [Google Scholar]
- Carey M. C., Wu S. F., Watkins J. B. Solution properties of sulfated monohydroxy bile salts. Relative insolubility of the disodium salt of glycolithocholate sulfate. Biochim Biophys Acta. 1979 Oct 26;575(1):16–26. doi: 10.1016/0005-2760(79)90126-7. [DOI] [PubMed] [Google Scholar]
- Cleland D. P., Bartholomew T. C., Billing B. H. Hepatic transport of sulfated and nonsulfated bile acids in the rat following relief of bile duct obstruction. Hepatology. 1984 May-Jun;4(3):477–485. doi: 10.1002/hep.1840040321. [DOI] [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W. Metabolism of lethocholate in healthy man. I. Biotransformation and biliary excretion of intravenously administered lithocholate, lithocholylglycine, and their sulfates. Gastroenterology. 1975 Jul;69(1):59–66. [PubMed] [Google Scholar]
- DUBIN I. N., JOHNSON F. B. Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore) 1954 Sep;33(3):155–197. doi: 10.1097/00005792-195409000-00001. [DOI] [PubMed] [Google Scholar]
- Dooley J. S., Bartholomew C., Summerfield J. A., Billing B. H. The biliary excretion of sulphated and non-sulphated bile acids and bilirubin in patients with external bile drainage. Clin Sci (Lond) 1984 Jul;67(1):61–68. doi: 10.1042/cs0670061. [DOI] [PubMed] [Google Scholar]
- Eklund A., Norlander A., Norman A. Bile acid synthesis and excretion following release of total extrahepatic cholestasis by percutaneous transhepatic drainage. Eur J Clin Invest. 1980 Oct;10(5):349–355. doi: 10.1111/j.1365-2362.1980.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Eng C., Javitt N. B. Chenodeoxycholic acid-3-sulfate. Metabolism and excretion in the rat and hamster and effects on hepatic transport systems. Biochem Pharmacol. 1983 Dec 1;32(23):3555–3558. doi: 10.1016/0006-2952(83)90302-7. [DOI] [PubMed] [Google Scholar]
- Eyssen H., Van Eldere J., Parmentier G., Huijghebaert S., Mertens J. Influence of microbial bile salt desulfation upon the fecal excretion of bile salts in gnotobiotic rats. J Steroid Biochem. 1985 Apr;22(4):547–554. doi: 10.1016/0022-4731(85)90176-1. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Hardonk M. J., Dijkhuis F. W., Haarsma T. J., Koudstaal J., Huijbers W. A. Application of enzymehistochemical methods to isolated subcellular fractions and to sucrose-ficoll density gradients. A contribution to the comparison of histochemical and biochemical data. Histochemistry. 1977 Aug 1;53(2):165–181. doi: 10.1007/BF00498491. [DOI] [PubMed] [Google Scholar]
- Hardonk M. J., Harms G., Koudstaal J. Zonal heterogeneity of rat hepatocytes in the in vivo uptake of 17 nm colloidal gold granules. Histochemistry. 1985;83(5):473–477. doi: 10.1007/BF00509211. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Lamers W. H. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985 Jul-Aug;5(4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Watkins J. B., 3rd Mechanisms of bile formation, hepatic uptake, and biliary excretion. Pharmacol Rev. 1984 Mar;36(1):1–67. [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. Enterohepatic circulation in the rat. Gastroenterology. 1985 Feb;88(2):403–411. doi: 10.1016/0016-5085(85)90499-8. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Vonk R. J. Cholestasis induced by sulphated glycolithocholic acid in the rat: protection by endogenous bile acids. Clin Sci (Lond) 1985 Feb;68(2):127–134. doi: 10.1042/cs0680127. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Heslinga H., Havinga R., Vonk R. J. Intestinal absorption of lithocholic acid sulfates in the rat: inhibitory effects of calcium. Am J Physiol. 1986 Aug;251(2 Pt 1):G189–G194. doi: 10.1152/ajpgi.1986.251.2.G189. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Spanjer H. H., Havinga R., Scherphof G. L., Vonk R. J. Lipoproteins and liposomes as in vivo cholesterol vehicles in the rat: preferential use of cholesterol carried by small unilamellar liposomes for the formation of muricholic acids. Biochim Biophys Acta. 1986 May 21;876(3):559–566. doi: 10.1016/0005-2760(86)90044-5. [DOI] [PubMed] [Google Scholar]
- Little J. M., Chari M. V., Lester R. Excretion of cholate glucuronide. J Lipid Res. 1985 May;26(5):583–592. [PubMed] [Google Scholar]
- Makino I., Hashimoto H., Shinozaki K., Yoshino K., Nakagawa S. Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology. 1975 Mar;68(3):545–553. [PubMed] [Google Scholar]
- Marks J. W., Sue S. O., Pearlman B. J., Bonorris G. G., Varady P., Lachin J. M., Schoenfield L. J. Sulfation of lithocholate as a possible modifier of chenodeoxycholic acid-induced elevations of serum transaminase in patients with gallstones. J Clin Invest. 1981 Nov;68(5):1190–1196. doi: 10.1172/JCI110364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. J., St Meier-Abt A., Barrett C., Boyer J. L. Mechanisms of taurocholate transport in canalicular and basolateral rat liver plasma membrane vesicles. Evidence for an electrogenic canalicular organic anion carrier. J Biol Chem. 1984 Aug 25;259(16):10614–10622. [PubMed] [Google Scholar]
- Muraca M., Blanckaert N. Liquid-chromatographic assay and identification of mono- and diester conjugates of bilirubin in normal serum. Clin Chem. 1983 Oct;29(10):1767–1771. [PubMed] [Google Scholar]
- Oelberg D. G., Chari M. V., Little J. M., Adcock E. W., Lester R. Lithocholate glucuronide is a cholestatic agent. J Clin Invest. 1984 Jun;73(6):1507–1514. doi: 10.1172/JCI111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. H. Bile acid sulfates. II. Formation, metabolism, and excretion of lithocholic acid sulfates in the rat. J Lipid Res. 1971 Nov;12(6):680–687. [PubMed] [Google Scholar]
- Palmer R. H., Bolt M. G. Bile acid sulfates. I. Synthesis of lithocholic acid sulfates and their identification in human bile. J Lipid Res. 1971 Nov;12(6):671–679. [PubMed] [Google Scholar]
- Palmer R. H. The formation of bile acid sulfates: a new pathway of bile acid metabolism in humans. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1047–1050. doi: 10.1073/pnas.58.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., AXELROD J., HAMMAKER L., SWARM R. L. Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest. 1958 Aug;37(8):1123–1130. doi: 10.1172/JCI103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D., Matsui A. Serum bile acid analysis. Clin Chim Acta. 1983 Jan 7;127(1):1–17. doi: 10.1016/0009-8981(83)90070-0. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Becker M., Czygan P., Fröhling W., Kommerell B., Rotthauwe H. W., Senn M. Bile acids and their sulphated and glucuronidated derivatives in bile, plasma, and urine of children with intrahepatic cholestasis: effects of phenobarbital treatment. Eur J Clin Invest. 1980 Aug;10(4):307–316. doi: 10.1111/j.1365-2362.1980.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Raedsch R., Rudolph G., Gundert-Remy U., Senn M. Biliary and urinary excretion of sulfated, glucuronidated and tetrahydroxylated bile acids in cirrhotic patients. Hepatology. 1985 May-Jun;5(3):492–495. doi: 10.1002/hep.1840050325. [DOI] [PubMed] [Google Scholar]
- Tiribelli C., Lunazzi G. C., Sottocasa G. L. Mechanisms of hepatic uptake of organic anions. Clin Sci (Lond) 1986 Jul;71(1):1–8. doi: 10.1042/cs0710001. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen W., Fevery J. Maximal biliary secretion of bilirubin in the anaesthetized rat: dependence on UDP-glucuronosyltransferase activity. Clin Sci (Lond) 1982 May;62(5):521–528. doi: 10.1042/cs0620521. [DOI] [PubMed] [Google Scholar]
- Vonk R. J., Danhof M., Coenraads T., van Doorn A. B., Keulemans K., Scaf A. H., Meijer D. K. Influence of bile salts on hepatic transport of dibromosulphthalein. Am J Physiol. 1979 Dec;237(6):E524–E534. doi: 10.1152/ajpendo.1979.237.6.E524. [DOI] [PubMed] [Google Scholar]
- Vonk R. J., Scholtens E., Strubbe J. H. Biliary excretion of dibromosulphthalein in the freely moving unanaesthetized rat: circadian variation and effects of deprivation of food and pentobarbital anaesthesia. Clin Sci Mol Med. 1978 Oct;55(4):399–406. doi: 10.1042/cs0550399. [DOI] [PubMed] [Google Scholar]
- Wolthers B. G., Volmer M., van der Molen J., Koopman B. J., de Jager A. E., Waterreus R. J. Diagnosis of cerebrotendinous xanthomatosis (CTX) and effect of chenodeoxycholic acid therapy by analysis of urine using capillary gas chromatography. Clin Chim Acta. 1983 Jun 30;131(1-2):53–65. doi: 10.1016/0009-8981(83)90352-2. [DOI] [PubMed] [Google Scholar]


