Abstract
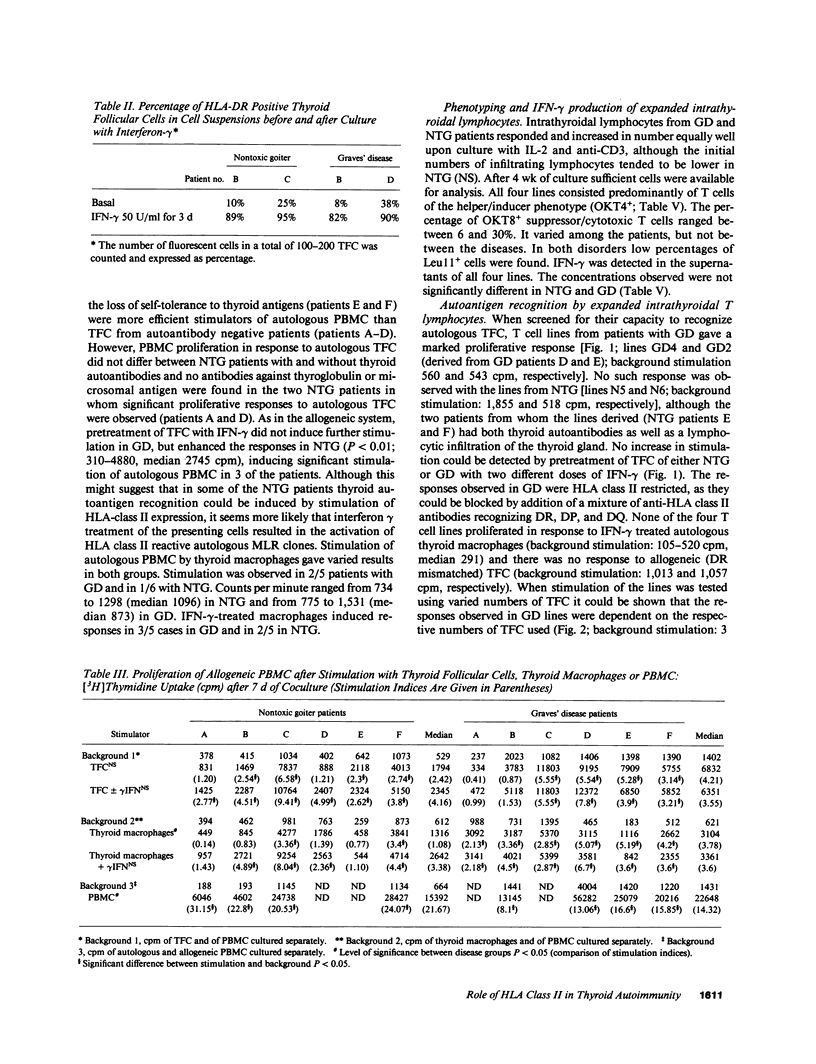
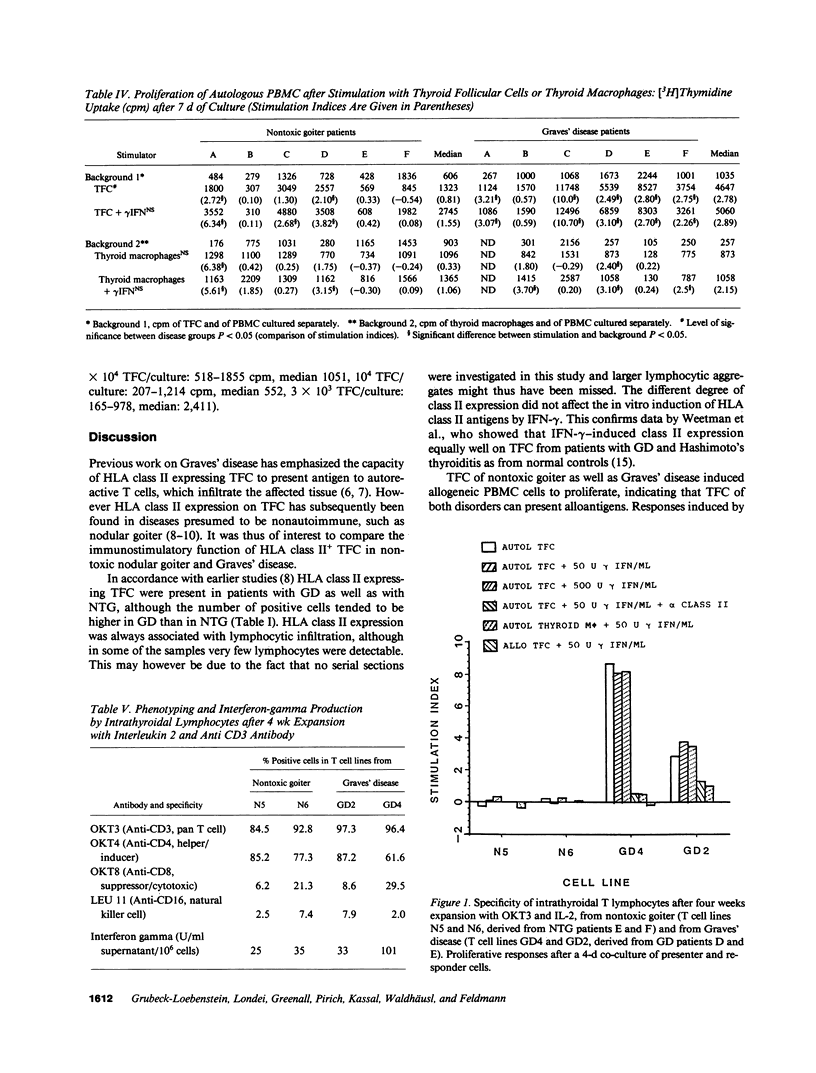
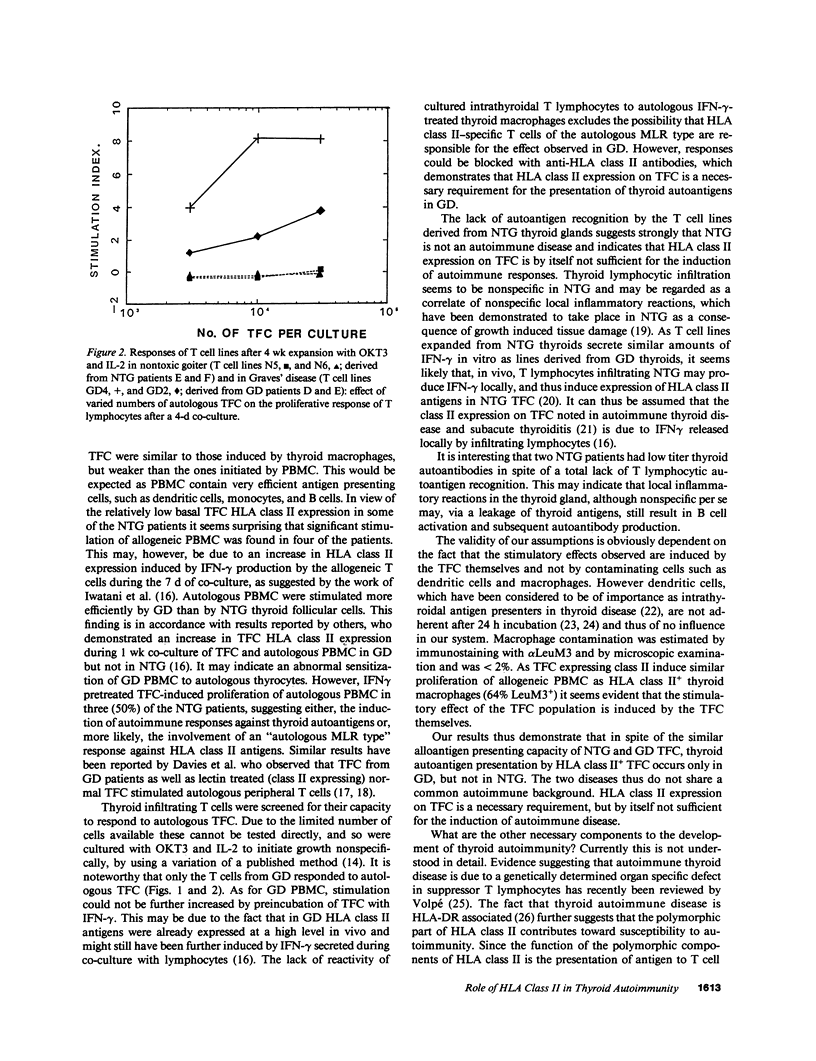
HLA class II expressing thyroid follicular cells are found not only in classical thyroid autoimmune diseases, such as Graves' disease, but also in presumably nonautoimmune thyroid disorders such as nontoxic goiter. In this study the immunostimulatory function of the HLA class II expressing thyroid follicular cells derived from patients with nontoxic goiter and with Graves' disease was compared by assessing their capacity to stimulate allogeneic and autologous peripheral blood mononuclear cells, as well as cultured intrathyriodal T lymphocytes. Proliferation of allogeneic peripheral blood mononuclear cells was stimulated by thyroid follicular cells from both nontoxic goiter and Graves' disease thyroids, thus demonstrating that thyroid follicular cells from both disorders are capable of presenting alloantigens. In contrast the proliferation of autologous peripheral blood mononuclear cells was more efficiently stimulated by thyroid follicular cells from Graves' disease than from nontoxic goiter. Cultured intrathyroidal T lymphocytes proliferated specifically in response to autologous HLA class II+ thyroid follicular cells in Graves' disease, but not in nontoxic goiter. The responses were dose dependent and HLA class II restricted. Thyroid autoantigen presentation by HLA class II expressing thyroid follicular cells thus only occurs in Graves' disease, suggesting that HLA class II expression on thyroid follicular cells is an essential feature, but by itself not sufficient for the induction of autoimmunity. Additional factors, the possible nature of which is discussed must also be involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Bermas B., Platzer M., Roman S. H. T-cell sensitization to autologous thyroid cells and normal non-specific suppressor T-cell function in Graves' disease. Clin Endocrinol (Oxf) 1985 Feb;22(2):155–167. doi: 10.1111/j.1365-2265.1985.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Davies T. F. Cocultures of human thyroid monolayer cells and autologous T cells: impact of HLA class II antigen expression. J Clin Endocrinol Metab. 1985 Sep;61(3):418–422. doi: 10.1210/jcem-61-3-418. [DOI] [PubMed] [Google Scholar]
- Gebel F., Ramelli F., Bürgi U., Ingold U., Studer H., Winand R. The site of leakage of intrafollicular thyroglobulin into the blood stream in simple human goiter. J Clin Endocrinol Metab. 1983 Nov;57(5):915–919. doi: 10.1210/jcem-57-5-915. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Derfler K., Kassal H., Knapp W., Krisch K., Liszka K., Smyth P. P., Waldhäusl W. Immunological features of nonimmunogenic hyperthyroidism. J Clin Endocrinol Metab. 1985 Jan;60(1):150–155. doi: 10.1210/jcem-60-1-150. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Kassal H., Smyth P. P., Krisch K., Waldhäusl W. The prevalence of immunological abnormalities in endemic simple goitre. Acta Endocrinol (Copenh) 1986 Dec;113(4):508–513. doi: 10.1530/acta.0.1130508. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Iwatani Y., Gerstein H. C., Iitaka M., Row V. V., Volpé R. Thyrocyte HLA-DR expression and interferon-gamma production in autoimmune thyroid disease. J Clin Endocrinol Metab. 1986 Sep;63(3):695–708. doi: 10.1210/jcem-63-3-695. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. V., Johnson T. L., Blaivas M., Sisson J. C., Wilson B. S. Detection of HLA-DR antigens in paraffin-embedded thyroid epithelial cells with a monoclonal antibody. Am J Pathol. 1985 Jul;120(1):106–111. [PMC free article] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Padula S. J., Pollard M. K., Lingenheld E. G., Clark R. B. Maintenance of antigen specificity by human interleukin-2-dependent T cell lines. Use of antigen-presenting cells and OKT3 antibody in the absence of antigen. J Clin Invest. 1985 Mar;75(3):788–797. doi: 10.1172/JCI111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROITT I. M., DONIACH D. Thyroid auto-immunity. Br Med Bull. 1960 May;16:152–158. doi: 10.1093/oxfordjournals.bmb.a069816. [DOI] [PubMed] [Google Scholar]
- SCHADE R. O., OWEN S. G., SMART G. A., HALL R. The relation of thyroid auto-immunity to round-celled infiltration of the thyroid gland. J Clin Pathol. 1960 Nov;13:499–501. doi: 10.1136/jcp.13.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. Biology of the human histocompatibility leukocyte antigen (HLA) system and a hypothesis regarding the generation of autoimmune diseases. J Clin Invest. 1986 May;77(5):1411–1415. doi: 10.1172/JCI112451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Volpé R. Autoimmune thyroid disease--a perspective. Mol Biol Med. 1986 Feb;3(1):25–51. [PubMed] [Google Scholar]
- Weetman A. P., Volkman D. J., Burman K. D., Gerrard T. L., Fauci A. S. The in vitro regulation of human thyrocyte HLA-DR antigen expression. J Clin Endocrinol Metab. 1985 Nov;61(5):817–824. doi: 10.1210/jcem-61-5-817. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Volkman D. J., Burman K. D., Margolick J. B., Petrick P., Weintraub B. D., Fauci A. S. The production and characterization of thyroid-derived T-cell lines in Graves' disease and Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986 Apr;39(1):139–150. doi: 10.1016/0090-1229(86)90213-8. [DOI] [PubMed] [Google Scholar]


