Abstract
Background
Studies suggest that oral (OIT) and sublingual (SLIT) immunotherapy for food allergy hold promise; however, the immunologic mechanisms underlying these therapies are not well understood.
Objective
To generate insights into the mechanisms and duration of immunologic suppression to peanut during immunotherapy (IT).
Methods
Blood was obtained from subjects at baseline and at multiple timepoints during a placebo-controlled trial of peanut OIT and SLIT. Immunologic outcomes included spontaneous and stimulated basophil activity by automated fluorometry (histamine) and flow cytometry (activation markers, IL-4), allergen-induced cytokine expression in dendritic cell (DC)-T cell co-cultures by multiplexing technology, and expression of MHC II and costimulatory molecules on DCs by flow cytometry.
Results
Spontaneous and allergen-induced basophil reactivity (histamine release, CD63 expression, and IL-4 production) were suppressed during dose escalation and after 6 months of maintenance dosing. Peanut- and dust mite-induced expression of TH2 cytokines was reduced in DC-T cell co-cultures during IT. This was associated with decreased levels of CD40, HLA-DR, and CD86 expression on DCs, and increased expression of CD80. These effects were most striking in myeloid DC-T cell co-cultures from subjects receiving OIT. Many markers of immunologic suppression reversed following withdrawal from IT, and in some cases during ongoing maintenance therapy.
Conclusion
OIT and SLIT for peanut allergy induce rapid suppression of basophil effector functions, dendritic cell activation, and Th2 cytokine responses during the initial phases of IT in an antigen non-specific manner. While there was some inter-individual variation, in many patients, suppression appeared to be temporary.
Keywords: Peanut allergy, Oral immunotherapy, Sublingual Immunotherapy, Sustained Unresponsivness, Basophil Activation, Dendritic Cells, Food Allergy
INTRODUCTION
Peanut allergy, a public health concern with substantial morbidity, affects 1% of the Western world.1,2 Current clinical management focuses on avoidance and treatment of reactions following accidental exposures.3 However, we and others have recently demonstrated4 that oral (OIT) and sublingual (SLIT) immunotherapy may allow subjects to tolerate increased amounts of peanut compared to their baseline, although clinical reactivity often returns once subjects discontinue treatment, suggesting these therapies more likely induce transient desensitization rather than longer term tolerance.5
The immunologic mechanisms underlying the clinical effects of immunotherapy (IT) continue to be elucidated. Initial studies in peanut OIT and SLIT demonstrated decreases in peanut-specific IgE with concomitant increases in IgG4, as well as reduced TH2 cytokine responses to peanut and upregulation of T regulatory (Treg) cells, especially in OIT.6-10 Hypomethylation of the FOXP3 locus as a result of peanut OIT, and subsequent remethylation with regained sensitivity to peanut, has also been proposed to be associated with clinical desensitization.10 Changes in basophil reactivity during food IT have been another area of interest, as basophils express the high affinity receptor for IgE and are critical effector cells in allergic reactions via their release of histamine, cytokines and leukotrienes upon stimulation.11,12 Decreased peanut-induced expression of basophil activation markers CD63 and CD203c has been demonstrated during peanut OIT.6,13 One study also suggested that IT may induce basophil hyporesponsiveness in a non-antigen-specific manner.14 Studies in milk and egg IT have demonstrated similar findings, although in some cases with less robust basophil suppression.14,15
Dendritic cells (DCs) are professional antigen presenting cells that direct T cell responses to food and other antigens, and therefore likely drive the changes in T cell responses during IT. Two major classes of DCs have been identified in the peripheral blood of humans: plasmacytoid DCs (pDCs) and myeloid DCs (mDCs).16-18 Both subtypes regulate food allergen-driven TH2 cytokine release by CD4+ T cells, and have been shown to exhibit phenotypic changes during the course of venom IT.19,20
In this pilot study, we sought to evaluate the systemic effects of peanut OIT and SLIT4 on the function of immune cells critical in allergic responses and tolerance, including basophils and DCs, in order to gain insight into the immunologic changes exerted by these therapies and to explore whether cellular immune responses qualitatively correlate with the clinical effects of OIT and SLIT.
RESULTS
Spontaneous and peanut-induced basophil histamine release (HR) and CD63 expression
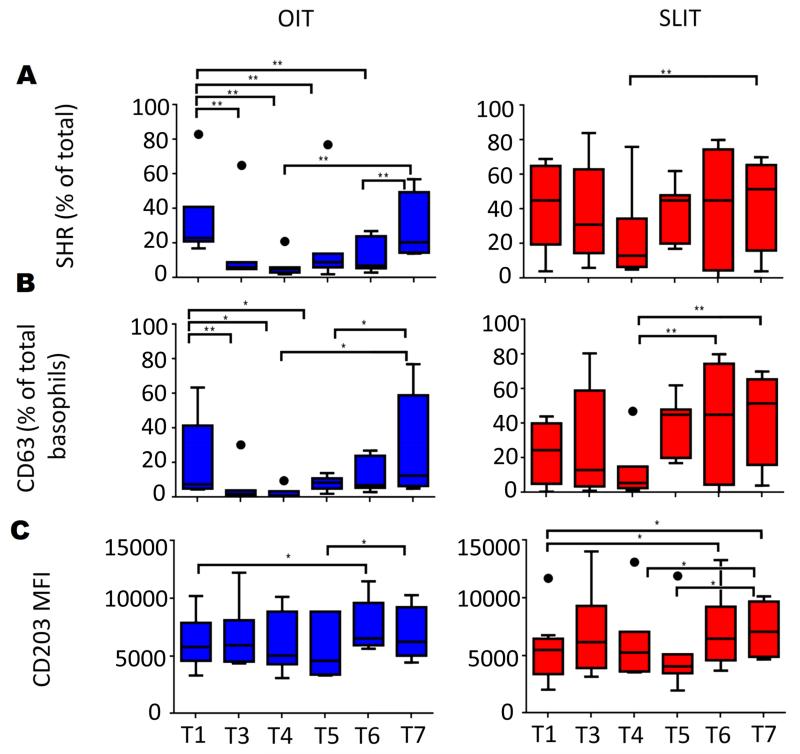
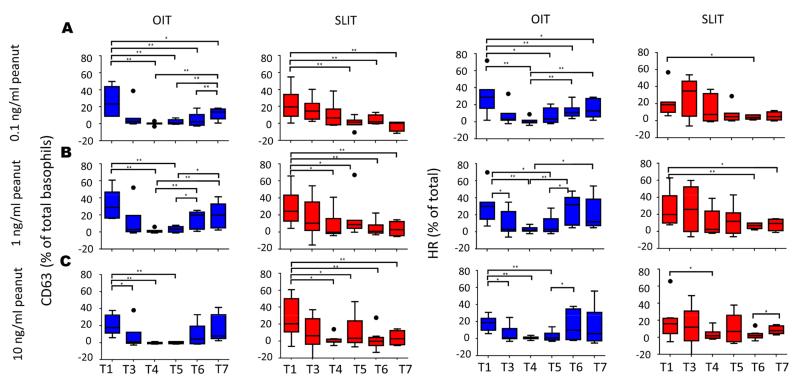
To investigate changes in basophil reactivity during OIT and SLIT, we measured spontaneous histamine release (SHR) and constitutive expression of basophil activation markers CD63 and CD203c in basophil-enriched suspensions (Fig 1 and E3 in the Online Repository). We also examined the same parameters in response to three different doses of peanut (Fig 2 and E4 in the Online Repository). At the end of dose escalation (T3) and after 6 (T4) and 12 (T5) months of maintenance IT, spontaneous CD63 expression and SHR were reduced markedly in the OIT group (p<0.01) compared to baseline (Fig 1A, B). A qualitatively similar decrease was seen in SLIT at T3 and T4, but did not reach significance (Fig 1A, B). Peanut-induced HR and CD63 expression were also suppressed versus baseline by the end of dose escalation in OIT (T3) and in both OIT and SLIT after 6 months of maintenance therapy (T4), especially at the higher doses of peanut (Fig 2).
FIG 1.
Spontaneous histamine release (SHR) and constitutive expression of basophil activation markers during IT. SHR (A) and constitutive CD63 (B) and CD203c mean fluorescence intensity (MFI) (C) were measured in basophil-enriched suspensions from subjects undergoing OIT (left panels) and SLIT (right panels) for peanut. T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 atT7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7. * p<0.05; ** p<0.01
FIG 2.
Effect of OIT and SLIT on basophil CD63 expression and histamine release (HR) in response to peanut. Basophil-enriched suspensions were stimulated with crude peanut extract at 0.1 ng/ml (A), 1 ng/ml (B) and 10ng/ml (C). Spontaneous histamine release and CD63 expression in media alone were subtracted to obtain stimulated values. T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 at T7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7. * p<0.05; ** p<0.01
After this initial suppression, constitutive CD63 expression increased in OIT subjects despite continued maintenance dosing such that CD63 expression was no longer significantly depressed compared to baseline by the end of the maintenance period (T6) (Fig 1B). A qualitatively similar pattern was evident for SHR (Fig 1A), and for both parameters in subjects receiving SLIT (Fig. 1A, B). Peanut-induced HR and CD63 expression also reverted in OIT subjects while they continued maintenance therapy (Fig 2). These parameters remained suppressed in the SLIT cohort (Fig 2). Of note, due to a crossover study design, all subjects in the SLIT group had OIT added between T5 and T6. This did not appear to inhibit SHR or constitutive CD63 expression, while peanut-induced HR and CD63 expression remained suppressed (Fig 1A, B; Fig 2). Additionally, three of seven patients receiving OIT were augmented with SLIT between T5 and T6. This addition of SLIT resulted in qualitatively greater suppression of both constitutive or stimulated basophil reactivity in 2 of 3 subjects, compared to 2 of 4 of those subjects that continued on OIT alone (data not shown).
Increases in both constitutive and peanut-induced CD63 expression and HR were also evident after a short period off therapy (T7), especially when compared to the point of maximal suppression (Fig 1A, B; Fig 2). Constitutive CD203c expression was not suppressed at any timepoint and actually increased at T6 versus baseline in both OIT and SLIT groups (p=0.043 and p=0.018 respectively), and after therapy withdrawal at T7 for SLIT (p=0.011; Fig 1C). CD203c upregulation in response to peanut was generally small and did not show significant changes during OIT or SLIT (Fig E4 in the Online Repository). Finally, CD63 and HR were strongly correlated (p<0.001, r = 0.92), while this relationship was significant but weaker for HR and CD203c (p<0.01, r = 0.11) (Fig. E5 in the Online Repository).
Despite some qualitative differences in spontaneous basophil activity during OIT and SLIT, direct comparisons between these two arms showed no significant differences overall. However, OIT resulted in more rapid suppression of basophil responses to peanut compared to SLIT (e.g. for rate of decrease in HR between T1 and T3: p = 0.001 and p = 0.038 for 0.1ng/ml and 1ng/ml peanut, respectively).
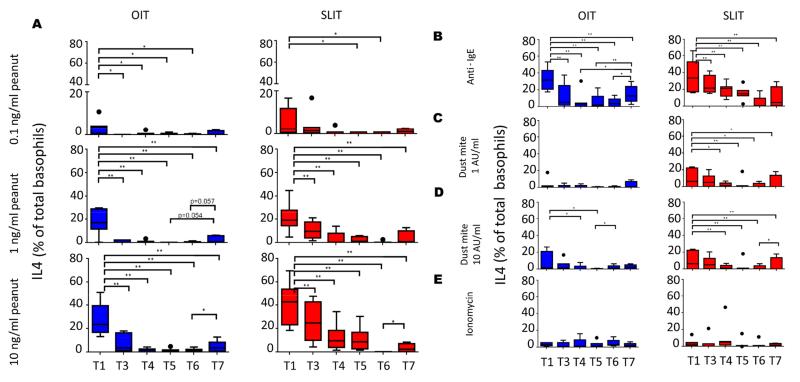
Peanut-induced basophil IL-4 expression
To determine whether changes in basophil IL-4 expression may play a role in desensitization, we incubated whole blood samples with peanut, anti-IgE, dust mite and ionomycin. Peanut-stimulated basophil IL-4 expression was significantly reduced from the end of dose escalation (T3) through maintenance (T4-T6) compared to baseline (T1) in both OIT (T1 vs. T3-T6, p< 0.05 at 0.1ng/ml and p<0.001 at 1ng/ml and 10ng/ml of peanut) and SLIT (T1 vs. T5, p< 0.05 at 0.1ng/ml and p<0.001 for T1 vs. T3-T6 at 1 ng/ml and 10 ng/ml), but unlike CD63 expression and HR, expression of IL-4 did not revert to higher levels during maintenance therapy (Fig 3A). However, IL-4 expression did increase once subjects were taken off therapy (T7 vs. T6) in both cohorts (Fig 3A). Direct comparisons between SLIT and OIT cohorts revealed no significant differences in basophil IL-4 expression.
FIG 3.
Changes in basophil IL-4 expression during peanut OIT and SLIT. Basophil expression of IL-4 was measured by intracellular flow cytometry after incubation of whole blood samples with peanut (A) anti-IgE (B), Dust mite at 1 AU/ml (C) or 10 AU/ml (D), and ionomycin (E). T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 atT7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7. * p<0.05; ** p<0.01
Specificity of basophil suppression during peanut OIT and SLIT
To determine whether the changes we observed in basophil reactivity were specific for peanut, we evaluated responses from basophil-enriched suspensions (CD63, CD203c, HR) and whole blood samples (IL-4) incubated with a polyclonal anti-human IgE crosslinking antibody, two doses of dust mite, or ionomycin, an inducer of FcεRI-independent basophil degranulation. Suppression of basophil IL-4 expression during OIT and SLIT was not peanut-specific, as it was evident following stimulation with both dust mite and anti-IgE (Fig 3B, C, D). In both OIT and SLIT, IL-4 responses to anti-IgE and dust mite increased after the therapy withdrawal period (T7), although these changes did not always reach significance (Fig 3B-D). Basophil CD63 expression and HR to anti-IgE and dust mite were low and did not change significantly with therapy in either OIT or SLIT (Fig E6). No marker of basophil reactivity (IL-4, CD63 or HR) to the IgE-independent stimulus ionomycin changed during the course of OIT or SLIT (Fig. 3, E6). Finally, CD203c expression did not change significantly following stimulation with any of these stimuli in either cohort (data not shown).
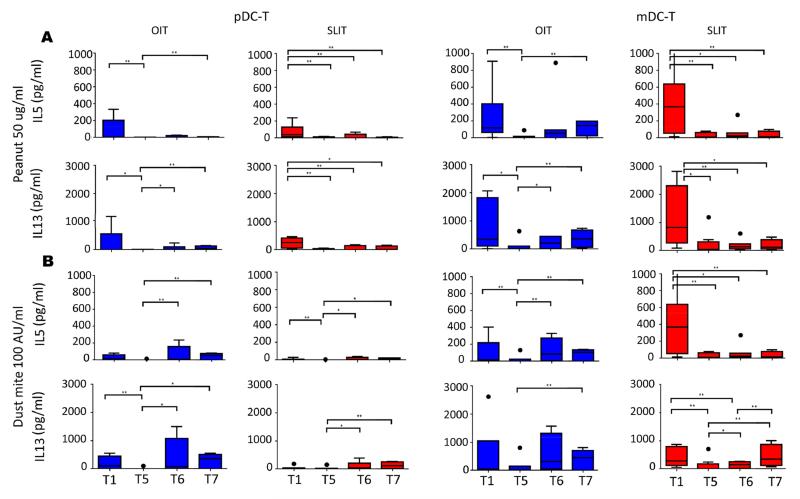
Dendritic cell-driven T cell cytokine responses
Since DCs play a central role in dictating T cell responses to allergens, we explored how SLIT and OIT affected cytokine responses by CD4+ T cells co-cultured with either pDCs or mDCs and stimulated with peanut or dust mite (an allergen for which subjects have not received IT). As seen in Figure 4, TH2 cytokine responses (IL-13 and IL-5) to peanut were robust at baseline (T1), with significantly higher expression in mDC-T than pDC-T co-cultures (IL-13, p = 0.003, IL-5 p = 0.007). After 12 months of maintenance therapy (T5), TH2 cytokine release to peanut as well as dust mite was suppressed in both pDC-T and mDC-T cell co-cultures from OIT and SLIT subjects compared to baseline (T1; Fig 4). However, TH2 cytokine expression subsequently increased despite continued treatment in the OIT cohort such that levels at T6 were no longer significantly depressed compared to baseline (T1; Fig 4). This reversion was less evident in the SLIT group, perhaps because OIT had been added to their treatment regimen between T5 and T6 (Fig 4). Although levels of TH2 cytokines produced in response to PN remained lower at T7 (after subjects discontinued treatment) compared to baseline in the SLIT cohort, neither PN-induced IL-5 nor IL-13 was significantly different between T7 and T1 in the OIT group (Fig 4A). OIT and SLIT also significantly altered peanut-induced expression of IFN-γ, IL-10, and TNF-α in a manner similar to the changes in TH2 cytokine responses, with suppression followed by increased expression (Fig E7), while IL-17 expression was generally unchanged (data not shown). A similar pattern was also seen in cultures stimulated with dust mite (data not shown). For all co-cultures, media alone conditions resulted in low to non-detectable cytokine production (data not shown). Direct comparisons did not show any significant differences in cytokine responses between OIT and SLIT cohorts.
Fig 4.
Effect of peanut OIT and SLIT on TH2 cytokine responses in DC-T cell co-cultures. IL-5 and IL-13 were measured in supernatants from co-cultures of pDCs and mDCs with autologous CD4+ T cells stimulated with 50 ug/ml crude peanut extract (A) or dust mite 100 AU/ml (B). Spontaneous cytokine secretion as measured in media alone was subtracted to obtain allergen-induced values. T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 atT7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7. * p<0.05; ** p<0.01
Expression of HLA-DR and Co-stimulatory molecules
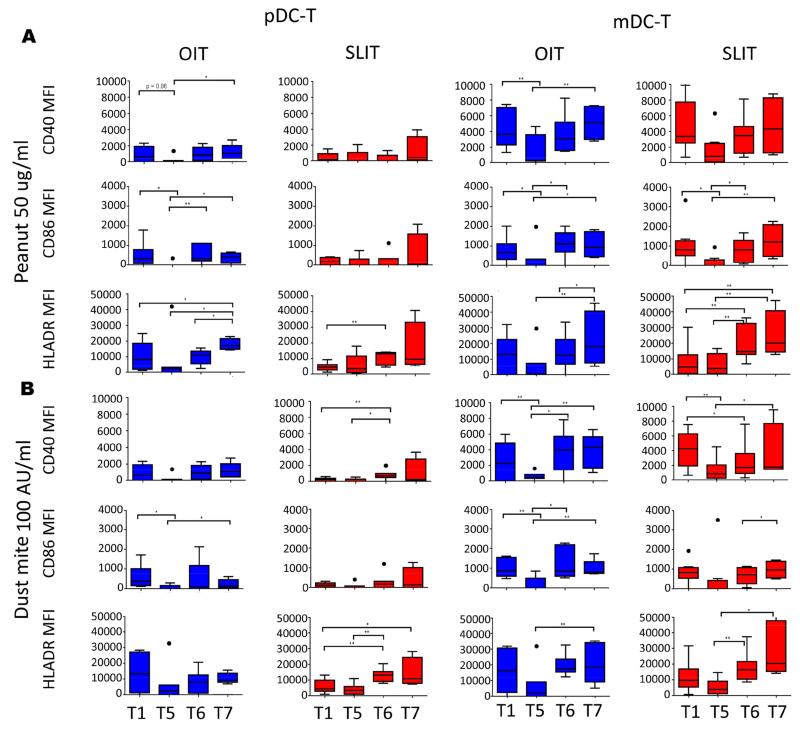
To evaluate whether the changes in DC-driven release of TH2 cytokines were associated with changes in expression of co-stimulatory molecules and HLA-DR by these cells over the course of IT, we stained the co-cultures described above following incubation with media alone, peanut, or dust mite for CD40, CD80, CD86, and HLA-DR. As shown in Figure 5, significant changes were seen in a number of co-stimulatory molecules over the course of OIT and SLIT. CD40 and CD86 were significantly suppressed on mDCs and pDCs from the OIT cohort following peanut and dust mite stimulation after 12 months of maintenance dosing (T5), and a similar trend was seen for HLA-DR (Fig. 5). This suppression was less evident in the SLIT cohort, particularly on pDCs (Fig 5). DCs cultured in media alone showed qualitatively similar trends to cultures treated with allergen, although levels of expression of co-stimulatory molecules were lower than those observed following antigen stimulation (Fig 5, E8).
Fig 5.
Effect of peanut OIT and SLIT on DC expression of co-stimulatory molecules and HLA-DR. Mean fluorescence intensity (MFI) of CD40, CD86, and HLA-DR on pDCs (left two columns) or mDCs (right two columns) following co-culture with CD4+ T cells stimulated with peanut 50ug/ml (A) or dust mite 100 AU/ml (B) T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 atT7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7. * p<0.05; ** p<0.01
The decline in expression of CD40, CD86, and HLA-DR on DCs during IT appeared to only be temporary in many subjects. Following the initial decline after 12 months of maintenance therapy (T5), expression of these markers on mDCs increased while maintenance dosing continued (T6) and following withdrawal of therapy (T7) in OIT (Fig 5, E8). A similar pattern was seen in SLIT (Fig 5, E8). While these changes were most robust with mDCs, the pattern was often visible in pDC cultures as well, but did not always reach significance (Fig. 5, E8). Direct comparisons between OIT and SLIT did not show any significant difference in HLA-DR or co-stimulatory molecule expression.
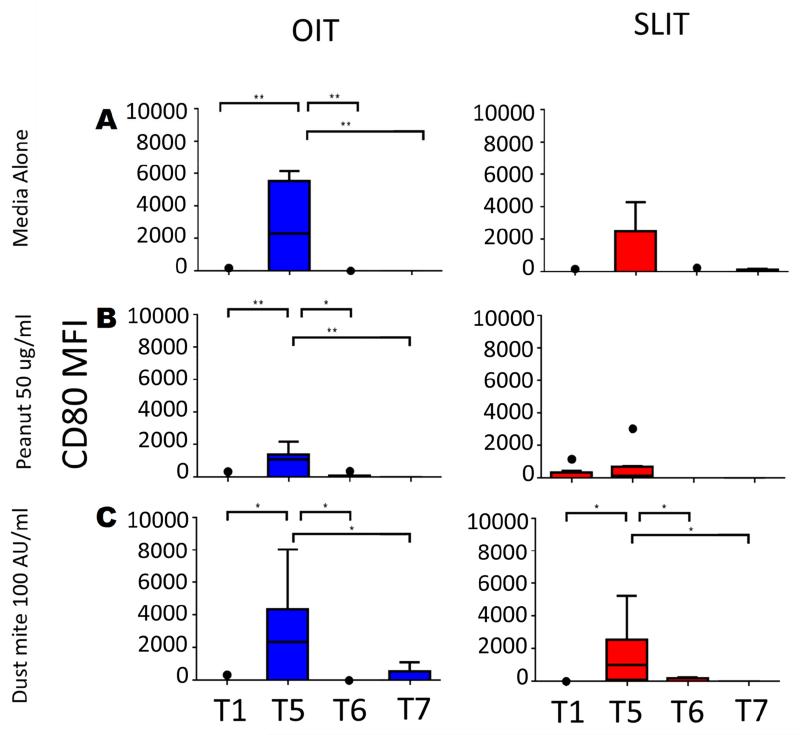
Expression of CD80 showed a very different pattern than HLA-DR and the other co-stimulatory molecules (Fig 6). While essentially not detectable on mDCs at baseline (T1), expression of CD80 was significantly elevated after 12 months of maintenance dosing in OIT, and SLIT to a lesser extent (T1 vs. T5; Fig 6). However, after 18 months of maintenance therapy (T6), expression was nearly completely lost and remained absent after withdrawal of treatment (T7; Fig. 6). Expression of CD80 on pDCs was extremely low and did not change significantly with therapy (data not shown).
Fig 6.
Effect of peanut OIT and SLIT on expression of CD80 on mDCs. CD80 mean fluorescence intensity (MFI) was measured on mDCs following co-culture with CD4+ T cells stimulated with media alone (A), peanut 50ug/ml (B) or dust mite 100 AU/ml (C). T1-T7 correspond to timepoints blood was collected. OIT: N=7 at T1-T5, 5 at T6, and 4 atT7; SLIT: N=8 at T1-T4, 7 at T5, 8 at T6 and 4 at T7.. * p<0.05; ** p<0.01
Correlation between mechanistic and clinical outcomes
Several biomarkers were significantly correlated with certain clinical outcomes (Table 1). A negative correlation was found between achievement of sustained unresponsiveness and baseline basophil CD63 expression, histamine release, and IL-4 production observed at the low dose (0.1ng/ml) of peanut. At baseline, 4 out of 5 patients with basophil CD63 expression of 10% or lower (when incubated with 0.1 ng/ml peanut) achieved sustained unresponsiveness, while all patients with basophil CD63 expression of greater than 10% did not (p=0.002 Fisher’s exact test). Average histamine release and IL-4 production at 0.1ng/ml peanut was lower at baseline in patients who achieved sustained unresponsiveness (28.3% vs. 15.3% of total for HR, and 5.3% vs. 1.7% of total basophils for IL-4) but these differences were not significant. Basophil IL-4 expression in response to all 3 doses of peanut correlated positively with peanut-specific IgE. Additionally, a consistent positive correlation was noted between TH2 cytokine production in mDC-T cell co-cultures, which showed the most robust changes during IT, and peanut-specific IgG4 levels, while peanut-specific IgE/IgG4 levels correlated negatively with this outcome (Table 1). Finally, correlation of specific number of reactions, and the severity of these at the time of DBPCFCs and OFCs, was evaluated for based on expression of certain markers such as expression of CD63. No specific correlations were seen.
Table 1. Correlation of selected biomarkers with clinical outcomes.
| Biomarker | Pass or Fail |
Total dose |
SPT | Log IgE | Log IgG4 |
Log IgE/IgG4 |
|---|---|---|---|---|---|---|
| Spont. CD63 media | 0.476 | 0.757 | 0.272 | 0.393 | 0.018 | 0.434 |
| CD63 Peanut 0.1ng/ml | <0.001 | 0.673 | 0.411 | 0.959 | 0.058 | 0.226 |
| CD63 Peanut 1 ng/ml | 0.089 | 0.727 | 0.064 | 0.318 | 0.174 | 0.836 |
| CD63 Peanut 10ng/ml | 0.949 | 0.638 | 0.092 | 0.576 | 0.082 | 0.927 |
| CD63 anti-IgE | NS | NS | NS | NS | NS | NS |
| CD63 ionomycin | NS | NS | NS | NS | NS | NS |
| CD63 Dust Mite 1AU/ml | NS | NS | 0.041 | NS | NS | NS |
| CD63 Dust Mite 10 AU/ml | 0.017 | 0.305 | 0.075 | NS | 0.872 | NS |
| IL4 Peanut 0.1ng/ml | 0.013 | 0.851 | 0.591 | 0.047 | 0.742 | 0.152 |
| IL4 Peanut 1ng/ml | 0.053 | 0.536 | 0.079 | 0.001 | 0.524 | 0.057 |
| IL4 Peanut 10ng/ml | 0.980 | 0.051 | 0.147 | 0.011 | 0.510 | 0.099 |
| IL4 anti-IgE | 0.018 | 0.076 | 0.936 | 0.116 | 0.849 | 0.879 |
| IL4 ionomycin | NS | NS | NS | NS | NS | NS |
| IL4 Dust Mite 1AU/ml | 0.552 | 0.935 | 0.791 | 0.191 | 0.501 | 0.924 |
| IL4 Dust Mite 10 AU/ml | 0.485 | 0.125 | 0.703 | 0.038 | 0.605 | 0.650 |
| Histamine media (SHR) | 0.332 | 0.548 | 0.749 | 0.280 | 0.915 | 0.344 |
| Histamine Peanut 0.1ng/ml | 0.047 | 0.075 | 0.995 | 0.103 | 0.012 | 0.104 |
| Histamine Peanut 1 ng/ml | 0.233 | 0.556 | 0.262 | 0.330 | 0.083 | 0.739 |
| Histamine Peanut 10ng/ml | 0.892 | 0.930 | 0.248 | 0.332 | 0.090 | NS |
| Histamine anti-IgE | NS | NS | NS | 0.748 | NS | NS |
| Histamine ionomycin | NS | 0.388 | NS | 0.406 | NS | NS |
| Histamine Dust Mite 1AU/ml | NS | 0.116 | 0.018 | NS | NS | NS |
| Histamine Dust Mite 10 AU/ml | NS | NS | NS | 0.042 | NS | NS |
| IL13 pDC Peanut 50ug/ml | 0.412 | NS | 0.221 | 0.581 | 0.978 | 0.739 |
| IL13 mDC Peanut 50ug/ml | 0.492 | 0.745 | 0.667 | 0.209 | 0.049 | 0.029 |
| IL13 pDC Dust Mite 100AU/ml | 0.382 | 0.044 | 0.048 | 0.923 | 0.920 | 0.896 |
| IL13 mDC Dust Mite100AU/ml | 0.845 | 0.872 | 0.885 | 0.345 | 0.007 | 0.016 |
| IL5 pDC Peanut 50ug/ml | 0.409 | 0.660 | 0.458 | 0.225 | 0.861 | 0.377 |
| IL5 mDC Peanut 50ug/ml | 0.486 | 0.974 | 0.771 | 0.450 | 0.008 | 0.033 |
| IL5 pDC Dust Mite 100AU/ml | 0.757 | 0.171 | 0.013 | 0.823 | 0.635 | 0.646 |
| IL5 mDC Dust Mite 100AU/ml | 0.701 | 0.779 | 0.943 | 0.982 | 0.021 | 0.139 |
P values describing significant positive correlations are shown in green and negative correlations in red. NS indicates that the Wald test was > 0.05. Pass or Fail – success or failure in achieving sustained hyporesponsiveness, Total dose – mg of peanut protein ingested during each challenge without symptoms, SPT – peanut skin prick test wheal size in mm, Log IgE – log of peanut-specific IgE (kUa/L), Log IgG4 – log of peanut -specific IgG4 (mGa/L), Log IgE/IgG4 – ratio of the logs of peanut-specific IgE and IgG4. Pass or Fail outcomes were correlated to baseline biomarkers only, while all other outcomes were correlated over the entire course of the clinical trial.
Despite these correlations, a significant amount of inter-individual variation was seen, with some patients maintaining clinical desensitization while immunologic markers increased. For example, subjects who were able to maintain sustained unresponsiveness had generally lower peanut-induced CD63 expression at baseline but not while therapy continued, and in fact, some successful patients had a greater rise in CD63 after an initial suppression than non-successful patients (Fig. E9). A different pattern was evident with IL-4, where subjects who achieved sustained unresponsiveness generally demonstrated lower IL-4 expression to higher doses of peanut as therapy continued (Fig E9). Significant inter-individual variation was also seen in other outcomes including expression of TH2 cytokines and co-stimulatory molecules on DCs (Fig E10).
DISCUSSION
IT trials have generated great excitement that a treatment for food allergy is on the horizon; however, this enthusiasm has been tempered by the knowledge that side effects are common, and only a minority of subjects achieve sustained unresponsiveness. How these clinical observations correlate with the degree and nature of immunologic suppression is not well understood. Here, we demonstrate that OIT (and to a lesser degree SLIT) for peanut allergy effectively suppresses basophil effector cell function and DC-driven TH2 cytokine responses to peanut. However, these parameters reversed in many subjects once they withdrew from therapy, and in some cases, while they continued on maintenance dosing. To our knowledge, this is the first study to demonstrate that systemic immunologic suppression mediated by IT for peanut allergy may not be long-lasting.
Stimulated expression of CD63 and CD203c on basophils has been studied as a potential biomarker of IgE-mediated food allergic responses.22 SLIT and OIT potently suppressed peanut-induced expression of CD63 and HR from basophils, but not CD203c. HR strongly correlated with CD63 and more weakly with CD203c, consistent with reports that CD63 is the best indicator of anaphylactic degranulation.22 Expression of CD203c can be induced by IL-3, exhibits different kinetics than CD63, and may reflect piecemeal rather than anaphylactic degranulation.23 While some previous studies have shown suppression in CD203c over the course of peanut OIT,13 these studies were performed in whole blood with IL-3 stimulation, as opposed to washed basophil suspensions without IL-3 in our study. Basophils additionally support TH2 immune responses by producing IL-4 following activation through FcεRI. This function of basophils was also attenuated during IT, suggesting a novel mechanism by which these cells may contribute to immunologic suppression during IT.
Basophils from the majority of food allergic children have been shown to spontaneously release histamine.24 While the clinical relevance and mechanisms responsible for this phenomenon are not well understood, high SHR appears to be IgE-dependent and may indicate more severe clinical reactivity to cow’s milk.25,26 Consistent with our previous findings in a trial of milk OIT,14 peanut OIT significantly reduced both SHR and constitutive CD63 expression by the end of the dose escalation period. Collectively, these data support an overall decrease in IgE-dependent pathway activation in basophils early in the course of OIT.
Induction of T cell tolerance and/or anergy is purported to be central to the mechanisms of IT. Both pDCs and mDCs direct memory responses by CD4+ T cells to food allergens, but in our study mDCs appeared to promote greater TH2 cytokine responses to peanut than pDCs. Interestingly, Ara h 1, a major peanut allergen, directly binds to Dendritic cell (DC)-specific ICAM-grabbing nonintegrin (DC-SIGN) on mDCs and acts as a TH2 adjuvant to activate T cells.27 Peanut-induced levels of TH2 cytokines declined in DC-T cell co-cultures after 12 months of maintenance dosing, and were associated with reduced expression of co-stimulatory molecules CD86 and CD40 as well as HLA-DR on DCs. On the other hand, expression of CD80 increased on mDCs at the same time point. While the mechanisms of T-cell costimulation are highly complex,28 some studies suggest that CD80 is the preferential ligand for the inhibitory T cell molecule CTLA-4,29 suggesting the increase in CD80 on mDCs during IT may serve to dampen T cell responses. Nearly all of the changes in DC phenotype and function we observed during peanut IT were more prominent with mDCs than pDCs. Consistent with other IT trials for food allergy,9 we did not find a switch from TH2 to TH1 cytokine responses during IT, but rather a generalized suppression of effector cytokine expression. It was recently demonstrated that the increase in IFN-γ during SLIT for grass pollen allergy is not mediated by T cells30; therefore, we cannot exclude the possibility that other cell types did indeed lead to increased Th1 and IL-10 responses in our study that we never detected since we only evaluated the T cell arm.
While peanut IT effectively suppressed basophil, DC and T cell reactivity by the end of dose escalation and after the first 6 months of maintenance dosing, remarkably almost all of the changes were only temporary in a majority of subjects. Peanut-induced and spontaneous HR and CD63 expression by basophils increased despite continued maintenance therapy compared to earlier time points, and IL-4 responses also reverted once subjects withdrew from therapy. IL-4 may have been more persistently suppressed than HR/CD63 as our IL-4 assay was performed in whole blood rather than washed cells, and therefore basophils in this assay had continued exposure to inhibitory serum factors. Even constitutive expression of CD203c, which hadn’t changed during the early stages of IT, significantly increased after 1 year of maintenance dosing. This may have clinical relevance, as patients with lower levels of milk tolerance have been demonstrated to have elevated constitutive CD203C expression at baseline.31 Likewise, the reduction in DC-driven TH2 cytokine responses to peanut, as well as expression of activation markers and HLA-DR on DCs, was often only transient.
The immunologic effects of IT were largely not antigen-specific. DC and T cell responses to dust mite were also reduced, and this suppression appeared to be transient as well in many subjects. This is consistent with previous findings suggesting IT promotes a pathway specific, antigen non-specific basophil anergy.13 While basophil HR and CD63 expression in response to anti-IgE and dust mite did not significantly change during treatment, neither of these stimuli evoked potent responses, perhaps because the doses used were more optimal for IL-4 expression than histamine release. Basophil expression of IL-4 to these stimuli was significantly attenuated, while no change in any measure of basophil reactivity to ionomycin, a non-IgE-dependent stimulant, was observed.
Our study did have several important limitations. First, we did not have a placebo group that received no intervention, although the study was placebo-controlled during the double blind treatment phase. Second, there was a high drop-out rate due to adverse effects, and mechanistic analyses were not performed on subjects who discontinued study participation, which may have excluded subjects with less robust immunologic suppression and skewed toward those better able to tolerate IT. An exception to this was data from histamine release, which was obtained at baseline and at one or two subsequent visits on patients who later dropped out. The addition of these data improves the significance obtained for the predictive value of baseline histamine release for achieving sustained unresponsiveness at 0.1ng/ml from 0.047 to 0.008 (Table 1), but otherwise does not alter the data. Detailed clinical information on the patients who dropped out can be found in Narisety et al.4 Third, the crossover design4 in which all patients on SLIT were augmented with OIT and some patients on OIT were augmented with SLIT created added complexity in analyzing the data, but remarkably revealed that add-on treatments generally did not prevent the increase in immunologic reactivity. For instance, we found that OIT augmentation on SLIT therapy seemed to be associated with continued suppression of stimulated CD63 expression, but most other immunologic markers showed reversion despite OIT augmentation. Finally, only subjects who completed the treatment and had no reaction at their T6 oral food challenge proceeded to T7, which resulted in an N of only 9 subjects at the T7 time point.
The degree of immunologic suppression achieved was qualitatively more pronounced in OIT than SLIT, consistent with our clinical observations that OIT subjects generally experienced greater clinical improvement than those receiving SLIT.4 OIT subjects may have a more pronounced response due to overall larger dose of allergen received. SLIT subjects were more likely in some cases to exhibit persistent immunologic suppression, perhaps because they were augmented with OIT late in the maintenance phase due to the crossover design of the study. The high frequency of reactions and failure of most subjects to achieve sustained unresponsiveness to peanut is consistent with the transient nature of immunologic suppression to peanut that we observed, although it is interesting to note that many subjects remained able to ingest peanut despite an apparent loss of immunologic suppression as mediated by both basophils and dendritic cells. This may be due to small sample size and the considerable inter-individual variation within the sample, or may suggest that other unknown mechanisms may play a role in induction and maintenance of desensitization. Although the study was not powered to identify predictors of clinical outcome, baseline basophil CD63 expression, HR, and Il-4 production at low doses of peanut did correlate significantly with achievement of sustained unresponsiveness, suggesting that those patients with the lowest baseline basophil responsiveness to peanut may have a better clinical outcome. The consistent correlation between TH2 cytokine production in mDC-T cell co-cultures, which showed the most robust changes during IT, and peanut-specific IgG4 levels suggests that further investigation into the role of IgG4 in modulating T cell responses during ITis warranted. A caveat in the interpretation of these data is the inherent risk of increased type 2 error when making multiple comparisons simultaneously. While larger clinical studies are needed to verify our findings and further inform their clinical significance, this pilot study raises the important possibility that current forms of IT for food allergy fail to elicit persistent immunologic suppression.
METHODS
Clinical Protocol Summary
Subjects aged 6–21 years with a diagnosis of peanut allergy (PA) were recruited from the Johns Hopkins Pediatric Allergy Clinic. Participants underwent a baseline evaluation including an oral food challenge (OFC) with up to 1,000 mg of peanut protein, after which eligible subjects were randomized 1:1 to receive either active SLIT with placebo OIT or active OIT with placebo SLIT. Over the next 16 weeks, subjects took daily home doses and returned to the research unit every 1-2 weeks for observed dose increases, with goal maintenance doses of 3.7 mg/day for SLIT and 2000 mg/day for OIT. A 10 gram OFC was completed after 6 months and 12 months of maintenance and, after the 12-month challenge, subjects and investigators were unblinded. Those subjects who completed the 12 month OFC with no more than mild symptoms were taken off treatment for 4 weeks and re-challenged. All other subjects proceeded to the unblinded phase of the study per protocol as follows: (1) those who tolerated between 5 and 10 grams before reacting continued on their prior SLIT or OIT maintenance for an additional 6 months, after which they underwent another 10 gram OFC; (2) those who reacted at < 5 grams were continued on their current active OIT or SLIT and had either active SLIT or OIT added for an additional 6 months. After that period, all subjects underwent a 10 gram OFC and those who tolerated the OFC were taken off therapy for 4 weeks and re-challenged.
Basophil histamine release (HR) and expression of activation markers
Blood was collected in EDTA and subjected to double Percoll (Pharmacia Biotech, Piscataway, NJ) density centrifugation, as previously described.S1 The lower fraction of cells using this protocol consists of basophil-enriched mononuclear cells (BECs) that were washed once in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)/albumin/glucose and then again in column buffer (PIPES containing 1% BSA and 2 mmol/L EDTA). Basophils were counted after staining with Alcian blue. BECs (20,000 basophils per condition) were then cultured in a final volume of 50 μL in AIM V medium (Invitrogen Life Technologies, Carlsbad, Calif). Cells were stimulated with media alone, crude peanut extract (Greer Laboratories) at 0.1ng/mL, 1ng/mL, and 10ng/mL, polyclonal goat anti-human IgE antibody at 10 ng/mL (generated in-house), dust mite (D. Pteronyssinus) extract (Greer Laboratories) at 1AU/mL or 10/AU/mL, or ionomycin (Sigma Aldrich, St. Louis, MO) at 500ng/mL. After a 45-minute incubation at 37°C, 1 mL of PIPES/albumin/glucose was added, and HR was measured in the cell-free culture supernatants by using automated fluorometry.S2 The remaining cell pellet was fixed in 4% buffered paraformaldehyde (Sigma Aldrich, St. Louis, MO) and stored in 10% dimethyl sulfoxide (DMSO)/PBS (Fisher Scientific) at −80°C. Percentage histamine release under each condition was calculated relative to total histamine content, which was determined by treating an identical number of BECs with perchloric acid (1.6% final). Spontaneous HR was subtracted from values reporting HR in response to peanut, dust mite, anti-IgE, and ionomycin.
BECs from all time points were analyzed simultaneously for constitutive expression of CD63 and CD203c using flow cytometry. Cells were first washed in PBS and then blocked with human IgG (1 mg/mL, Sigma Chemical Co). The following antibodies were used: CD63-Phycoerythrin-Cyanine 7 (PE-Cy7; BD Pharmingen, San Jose, CA), CD203c-Phycoerythrin (PE; eBioscience, San Diego, CA), CD123-allophycocyanin (APC; eBioscience), and BDCA2-fluorescin isothiocyanate (FITC; eBioscience). Stained cells were analyzed with a FACSCalibur (BD PharMingen). Basophils were identified by gating on cells that were CD123+ and BDCA2−, and activated CD63 expression was identified by gating on basophils demonstrating CD63high versus CD63low expression, while CD203c mean fluorescence intenstity (MFI) was measured on all basophils. Appropriate isotype controls were used in all assays. Data was analyzed using Flowjo software (Tree Star, Inc., Ashland, OR).
Measurement of basophil IL-4 expression
Heparinized blood was treated with stimulants exactly as described above for HR, with the addition of brefeldin A (Sigma Aldrich, St. Louis, MO) at a final concentration of 5 μg/mL, in a 96 well round bottom plate in a final volume of 0.2 mL per condition. After a 120-minute incubation at 37°C, 0.1 mL of cell suspension was added to a 2 mL eppendorf containing 1.9 mL of pre-warmed Lyse/Fix solution (BD Pharmingen). The cells were incubated for 13 minutes in a 37° water bath, and then immediately spun. The cell pellet was washed in PBS and then stored in 10% DMSO/PBS at −80°C. Samples from all time points were analyzed simultaneously. Measurement of IL-4 protein expression was done by intracellular flow cytometry. Cells were first washed in PBS and then blocked with human IgG (1 mg/mL, Sigma-Aldrich) in PBS-0.1% saponin-5% non-fat milk buffer for 1 hour on ice (saponin; Sigma-Aldrich, non-fat dry milk; Nestle Carnation brand). Cells were then stained with the following antibodies for 30 minutes on ice: BDCA2-FITC (Miltenyi), CD123-PE (BD Pharmingen) and IL-4-APC (eBioscience). Basophils were identified by gating on cells that were CD123+ and BDCA2−. Stained cells were analyzed with a FACSCalibur (BD PharMingen). Instrument variability was corrected for by using CaliBRITE allophycocyanin calibration beads (BD PharMingen).
Preparation and culture of dendritic cells and T cells
Following double Percoll density centrifugation as described above, the upper fraction of cells consisted of basophil-depleted mononuclear cells and were used to isolate pDCs using BDCA4+ magnetic bead selection (Miltenyi, Bergisch Gladbach, Germany). Cells not retained on this column were then used to isolate mDCs with BDCA1+selection (Miltenyi) after depletion of CD19+ B cells. CD4+ T cells were prepared by positive selection (Miltenyi) from remaining cells after DC removal.
DC subtypes (2.5 × 104 cells) were co-cultured with autologous CD4+ T cells (1 × 105 cells) in a final volume of 200 μL of AIM V and stimulated with media alone, 50 ug/ml of crude peanut extract (Greer, Lenoir, NC, USA), or 100 AU/mL of dust mite extract (D. Pteronyssinus) for 5 days in a 96 well round bottom plate. CD4+ T cells were also cultured alone. Cell supernatants were collected and stored at −80°C for cytokine analysis. The cell pellets were combined, fixed in 4% buffered paraformaldehyde, and stored in 10% DMSO at −80°C.
Measurement of HLA-DR and activation markers in DC- T cell co-cultures
Samples from all time points were analyzed simultaneously. Cells were first washed in PBS and then blocked with human IgG (1 mg/mL, Sigma-Aldrich). The pDC-T cell fractions were stained with CD123-APC (eBioscience), and the aforementioned FcεR1-PE, CD3-APC-Cy7, CD80-PerCP-eF710, CD86-AF488, HLA-DR-eF450, and CD40-PE-Cy7. The mDC-T cell fractions were labeled with BDCA1-APC (eBioscience) and CD19-APC-Cy7 (Biolegend) along with the FcεR1-PE, CD3-APC-Cy7, CD80-PerCP-eF710, CD86-AF488, HLA-DR-eF450, and CD40-PE-Cy7. Samples were run on a BD FacsVerse (BD Biosciences, San Josa CA). pDCs were identified as CD123+ CD3−, and mDCs as BDCA-1+ CD3− CD19− cells. After gating on each cell type, the MFI of each activation marker and HLA-DR was determined. Appropriate isotype controls were included in each experiment.
Cytokine measurements
Cytokines were measured using multiplex bead immunoassay (Millipore) according to the manufacturer’s directions. A human x-plex panel consisting of IL-5, IL-13, IFN-γ, IL-10, and IL-17, and TNF-α were used to evaluate supernatants from DC-T co-cultures. Limits of detection for this assay are IL-5 0.6 pg/mL, IL-13 0.5 pg/mL, IFN-γ 0.6 pg/mL, IL-10 0.6 pg/mL, IL-17 0.7 pg/mL, and TNF-α 0.7pg/mL.. Values obtained from culturing T cells alone were subtracted from the values obtained in the co-culture conditions.
Statistical Analysis
Outcomes were evaluated by using linear regression models with generalized estimating equations to account for repeated measures over time or simple regression when appropriate. Difference in an outcome between a study timepoint (T3-T7) and baseline (T1) were considered significant only if the overall p-value for the GEE taking into account multiple comparisons was significant, and the p-value comparing values within the GEE model at one of the time points compared to baseline was also significant. Statistically significant p-values, defined as <0.05, are indicated. When appropriate, variables were log-transformed for analysis. Correlations between HR and CD63/CD203c expression were performed using standard regression. All analyses were performed with STATA/SEv11 software (StataCorp, College Station, Tex). In the graphs, the box defines the 25th and 75th percentile; center line the median; whiskers the adjacent values; individual points the outliers
Supplementary Material
Key messages.
Oral and sublingual immunotherapy for peanut allergy suppress basophil and dendritic cell (DC)-driven T cell effector functions, although this inhibition is often transient.
Although there was significant inter-individual variation and mechanistic outcomes did not always correlate with clinical outcomes, these findings may offer a mechanistic basis as to the relatively low rates of sustained unresponsiveness seen in immunotherapy trials for food allergy.
Acknowledgements
We thank Dr. Xuhang Li and the Johns Hopkins Digestive Diseases Basic and Translational Research Core Center for their assistance with cytokine multiplexing and Dr. Mark Liu for providing dust mite extracts. Assistance with statistics was provided by Carol B. Thompson, Assistant Scientist, Johns Hopkins Biostatistics Center, who was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 101872.
Funding:
Supported by grants from:
Grant # UL1TR001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research
The Research Training in Pediatric Allergy and Immunology, Grant # 5T32AI007007 (R.A.W., M.G)
National Institutes of Health (NIH) K23 Mentored Research Development Award (K23AI091869; P.A.F.-G.)
ARTrust Faculty Development Award (P.A.F.-G.)
Johns Hopkins University Clinician Scientist Award (P.A.F.-G.)
National Institutes of Health (NIH) R21 Research Award (AI079853; J.T.S.)
National Institute of Health Asthma and Allergic Diseases Cooperative Research Centers grant, (U19AI070345-01; J.T.S.)
Food Allergy Research and Education grant (R.A.W)
Eudowood foundation
Winkelstein fellowship (M.G.)
Abbreviations
- OIT
Oral immunotherapy
- SLIT
Sublingual immunotherapy
- DC
Dendritic Cell
- pDC
Plasmacytoid Dendritic Cell
- mDC
Myeloid Dendritic Cell
- DBPCFC
Double-Blind Placebo-Controlled Food Challenge
- OFC
Open Food Challenge
- IT
Immunotherapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, et al. US prevalence self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 3.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010 Dec;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narisety, et al. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of Sublingual versus Oral Immunotherapy for the Treatment of Peanut Allergy. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mousallem T, Burks AW. Immunology in the Clinic Review Series; focus on_allergies: immunotherapy for food allergy. Clin Exp Immunol. 2012 Jan;167(1):26–31. doi: 10.1111/j.1365-2249.2011.04499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300. 300.e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jul;126(1):83–91.e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011 Mar;127(3):654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011 Mar;127(3):640–6.e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014 Feb;133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder JR, MacGlashan DW, Lictenstein LM. Human basophils: mediator release and cytokine production. Adv Immunol. 2001;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 12.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008 Jul;9(7):733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 13.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012 Aug;42(8):1197–205. doi: 10.1111/j.1365-2222.2012.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012 Feb;129(2):448–55. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Consortium of Food Allergy Research (CoFAR). Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012 Jul 19;367(3):233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiter B, Shreffler WG. The role of dendritic cells in food allergy. J Allergy Clin Immunol. 2012 Apr;129(4):921–8. doi: 10.1016/j.jaci.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 17.Ruiter B, Shreffler WG. Innate immunostimulatory properties of allergens and their relevance to food allergy. Semin Immunopathol. 2012 Sep;34(5):617–32. doi: 10.1007/s00281-012-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farkas L, Kvale EO, Johansen FE, Jahnsen FL, Lund-Johansen F. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides. J Allergy Clin Immunol. 2004 Aug;114(2):436–43. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Dreschler K, Bratke K, Petermann S, Bier A, Thamm P, Kuepper M, et al. Impact of immunotherapy on blood dendritic cells in patients with Hymenoptera venom allergy. J Allergy Clin Immunol. 2011;127:487–94. doi: 10.1016/j.jaci.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Frischmeyer-Guerrerio PA, Guerrerio AL, Chichester KL, Bieneman AP, Hamilton RA, Wood RA, et al. Dendritic cell and T cell responses in children with food allergy. Clin Exp Allergy. 2011 Jan;41(1):61–71. doi: 10.1111/j.1365-2222.2010.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder JT, Saini SS. Assay methods for measurement of mediators and markers of allergic inflammation. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. 7th ed ASM Press; Washington (DC): 2006. [Google Scholar]
- 22.Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006 Jun;6(3):226–33. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 23.MacGlashan D., Jr. Expression of CD203c and CD63 in human basophils:relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010 Sep;40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989 Jul 27;321(4):228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 25.Ford LS, Bloom KA, Nowak-Węgrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol. 2013 Jan;131(1):180–6.e1-3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder JT, Bieneman AP, Chichester KL, Keet CA, Hamilton RG, MacGlashan DW, Jr, et al. Spontaneous basophil responses in food-allergic children are transferable by plasma and are IgE-dependent. J Allergy Clin Immunol. 2013 Dec;132(6):1428–31. doi: 10.1016/j.jaci.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006 Sep 15;177(6):3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011 Apr 29;332(6029):600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004 Mar 1;172(5):2778–84. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 30.Suárez-Fueyo A, Ramos T, Galán A, Jimeno L, Wurtzen PA, Marin A, de Frutos C, Blanco C, Carrera AC, Barber D, Varona R. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014 Jan;133(1):130–8.e1-2. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Ford LS, Bloom KA, Nowak-Węgrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol. 2013 Jan;131(1):180–6.e1-3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Schroeder JT, Saini SS. Assay methods for measurement of mediators and markers of allergic inflammation. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. 7th ed. ASM Press; Washington (DC): 2006. [Google Scholar]
- E2.Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.