Abstract
Obesity and its associated metabolic diseases present a major public health problem around the world. The discovery that thermogenic fat is active in adult humans has sparked a renewal of interest in the study of its development and function and in the feasibility of using modulators of thermogenesis to work against obesity. In recent years it has been shown that there are at least two distinct types of thermogenic fat cells; brown and beige fat. In this review we discuss the transcriptional mediators of thermogenesis and the signaling molecules that regulate thermogenic cells. We also review the effects of thermogenic fat activation on whole body metabolic parameters and evaluate the increasing evidence that activating thermogenesis in humans can be a viable method of ameliorating obesity. In these discussions we highlight targets that can potentially be stimulated or modified in anti-obesity treatments.
Keywords: Obesity, Diabetes, Brown adipocyte, Beige adipocyte, Adaptive thermogenesis
Introduction
Obesity is a major health problem in the United States and around the world. Over one third of adults in the United States (Ogden, et al. 2014) and 11% percent of adults worldwide are obese (WHO 2014). A number of conditions including heart disease, type 2 diabetes, and some cancers are more prevalent in obese individuals and worldwide approximately 3.4 million deaths each year are tied to obesity (WHO 2014). Obesity is characterized by an excessive amount of lipid accumulation in fat cells; as a result there has been a continual effort to find cellular processes and molecular targets in fat that can be manipulated in anti-obesity treatments.
Three types of fat cells have been identified to date (Rosen and Spiegelman 2014). White adipocytes are primarily used for energy storage; they contain a large lipid droplet and few cellular organelles. In contrast, brown adipocytes are primarily a site for adaptive thermogenesis which, unlike the obligatory thermogenesis that is a natural byproduct of metabolic processes, is activated in response to cold stimulation (Himms-Hagen 1989). Brown adipocytes contain multiple small lipid droplets and a number of mitochondria, which express uncoupling protein 1 (UCP1), a major component of the thermogenic program and a specific marker of thermogenic adipocytes (Cannon and Nedergaard 2004). UCP1 is activated by long chain fatty acids and increases the conductance of the inner mitochondrial membrane, causing the mitochondria to produce heat at the expense of ATP production efficiency (Fedorenko, et al. 2012). While overabundance of white fat is the defining characteristic of obesity and contributes to the development of metabolic disease, brown fat in fact works to counteract obesity by converting chemical energy into heat as opposed to storing it as lipid (Rosen and Spiegelman 2014). Brown fat is primarily found interscapularly in rodents and arises from MYF5+ stem cells that can also differentiate into skeletal myocytes (Seale, et al. 2008). “Beige” or “brite” fat is a newly identified type of fat that is located in white adipose tissue and arises from MYF5− stem cells but has an inducible thermogenic program (Petrovic, et al. 2010; Wu, et al. 2012). We will refer to thermogenic cells in white fat depots as beige cells and to the process of the activation of thermogenic fat cells in white fat depots as “beiging” in this review. It is conceivable that other types of fat exist in addition to white, brown, and beige fat. Recent work on marrow adipose tissue, for example, has begun to characterize the distinctive functions of these unique fat cells that are not seen in currently identified types of fat (Cawthorn, et al. 2014; Scheller and Rosen 2014).
Adaptive thermogenesis in fat is activated by cold exposure mainly through the signaling of catecholamines secreted by the sympathetic nervous system (Cannon and Nedergaard 2004). The sympathetic nervous system primarily signals through the α and β1-3 adrenergic receptors (AR), consequently in β-less mice, which lack all isoforms of the β adrenergic receptor, the brown fat depot is comprised of cells with large lipid droplets and blunted UCP1 expression (Bachman, et al. 2002). The β ARs are G-protein coupled receptors that, upon stimulation, activate adenylyl cyclase and increase levels of intracellular cAMP. This leads to the phosphorylation of protein kinase A (PKA), which in turn activates the p38 MAP kinase (MAPK) pathway and induces cAMP response-element binding protein (CREB) mediated upregulation of UCP1 (Cannon and Nedergaard 2004). Studies have begun to show that therapeutics that act centrally can affect thermogenesis, for example a Glucagon-like peptide-1 (GLP1) receptor agonist works in the central nervous system to activate brown adipose tissue and may increase resting energy expenditure in humans (Beiroa, et al. 2014). β-adrenergic signaling may not be the only pathway for cold induced activation of thermogenic fat. Ye et al. showed that cultured, mature, adipocytes can upregulate thermogenesis in response to cold exposure, suggesting that there is also a cell autonomous cold sensing mechanism in fat cells (Ye, et al. 2013). This implies that there may be unexplored pathways that function in parallel with β AR signaling to activate cold induced thermogenesis.
This review focuses on the transcriptional control of thermogenic genes and the signaling beyond the sympathetic nervous system that regulates both brown and beige fat function. We also discuss the effects that thermogenic fat activation may have on systemic metabolism in humans and highlight molecules that have begun to be tested as drug targets.
Transcriptional control of thermogenic fat
The cascades mediated by a number of transcriptional factors and cofactors tightly control the adipogenic process. The unique mechanisms that regulate thermogenic fat are much less well understood. In the following section, we discuss the factors that contribute to the development of thermogenic cells and the activation of the thermogenic program (Figure 1).
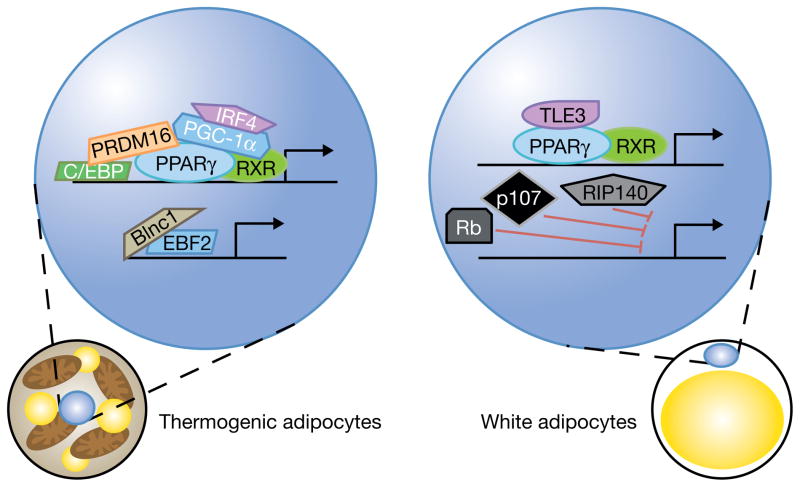
Figure 1. Transcriptional regulators of the thermogenic program.
The PPARγ/RXR heterodimer plays a key role in regulating the development of all adipocytes. In thermogenic adipocytes (left) it also plays a major role in the regulation of the thermogenic program. A number of coactivators interact with PPARγ, including PGC1α which is regulated in part by IRF4, and PRDM16 which interacts with both PPARγ and C/EBPβ to drive thermogenesis. Other transcriptional regulators of thermogenesis include EBF2, which forms a ribonucleoprotein complex with Blinc1 to upregulate thermogenic genes. In white adipocytes (right) the PPARγ/RXR heterodimer instead interacts with TLE3, leading to the expression of white fat selective genes. Rb, p107, and RIP140 also work in white adipocytes to inhibit the transcription of thermogenic genes.
PPARγ
Peroxisome proliferator-activated receptor γ (PPARγ) is the master regulator of adipogenesis; ectopic expression of PPARγ stimulates the differentiation of fibroblasts into adipocytes (Tontonoz, et al. 1994). PPARγ is a nuclear receptor that heterodimerizes with the retinoid x receptor (RXR) to induce transcription of genes related to the adipogenic program (Ahmadian, et al. 2013). In addition to its central role in adipogenesis, PPARγ has been shown to be important in the regulation of thermogenesis. Chronic stimulation of adipocyte cultures with PPARγ agonists results in an induction of the thermogenic program (Fukui, et al. 2000; Petrovic et al. 2010; Wilson-Fritch, et al. 2004). Ongoing research is beginning to elucidate the molecular mechanisms by which PPARγ regulates thermogenesis. A mouse model with a point mutation in PPARγ was found to have normal development of adipose tissue but has defective thermogenesis (Gray, et al. 2006) and more recently it was shown that SIRT1-dependent deacetylation of PPARγ plays a role in the upregulation of thermogenic genes (Qiang, et al. 2012). It has also been proposed that stabilization of PRD1-BF1-RIZ1 homologous domain containing 16 (PRDM16) through the action of PPARγ agonists may contribute to the induction of thermogenesis (Ohno, et al. 2012). These studies have begun to provide mechanistic insight into our understanding of how PPARγ regulates the function of thermogenic fat.
PGC1α
The peroxisome proliferator-activated receptor γ coactivator 1 (PGC1) family of proteins are coactivators that are key inducers of mitochondrial biogenesis (Puigserver 2005). The first PGC1 protein to be identified is PGC1α, which was isolated in a yeast two-hybrid screen for PPARγ interacting proteins in brown fat (Puigserver, et al. 1998). Both PGC1α and a closely related family member, PGC1β, are regulators of the thermogenic program. Brown fat cells from PGC1α knockout animals have a decrease in cAMP induced thermogenesis and loss of both PGC1α and PGC1β in brown fat cells reduces basal levels of thermogenesis (Uldry, et al. 2006). Additionally, while PGC1β knockout mice have a compensatory increase in PGC1α expression, there is a reduction of thermogenic gene expression in the brown fat of those animals (Lelliott, et al. 2006). Recently, interferon regulatory factor 4 (IRF4) has been shown to interact with PGC1α to mediate thermogenesis. In the model of IRF4 overexpression in UCP1 positive cells, thermogenesis is activated in brown fat of the transgenic animals compared to the controls. Additionally, a Ucp1-CRE driven IRF4 knockout results in cold intolerance and a reduction in thermogenic gene expression (Kong, et al. 2014). PGC1α has also been shown to induce the expression of Cell death-inducing DFFA-like effector (CIDEA), a regulator of UCP1 function. This interaction is inhibited through direct interaction of PGC1α with the corepressor receptor interacting protein 140 (RIP140) (Hallberg, et al. 2008). RIP140 had previously been reported to inhibit mitochondrial biogenesis and the expression of thermogenic genes such as UCP1 (Christian, et al. 2005; Powelka, et al. 2006). PGC1α is also negatively regulated by retinoblastoma protein (Rb) and its closely related family member p107. Rb has been shown to suppress PPARγ signaling and promote osteogenic differentiation and p107 knockout mice have increased expression of PGC1α and UCP1 in both brown and white fat depots (Calo, et al. 2010; Scime, et al. 2005). These studies show that various interacting proteins can regulate PGC1α function in mediating thermogenic gene expression.
PRDM16
PRDM16 is a zinc finger protein that is an important regulator of thermogenic fat. Ectopic expression of PRDM16 in cultured fibroblasts and in vivo results in thermogenic adipocyte differentiation (Seale, et al. 2007). It was subsequently found that PRDM16 expression in precursor cells determines cell fate, PRDM16 knockdown in primary brown fat precursor cells results in the differentiation of those cells into skeletal myotubes while overexpression of PRDM16 in skeletal muscle precursor cells results in brown adipocyte differentiation (Seale et al. 2008). The mechanism by which PRDM16 determines precursor cell fate was later shown to be controlled in part by a transcription complex consisting of PRDM16 and CCAAT/enhancer binding protein β (C/EBPβ) (Kajimura, et al. 2009). Further in vivo models showed that fat specific PRDM16 overexpression results in improved metabolic function and less weight gain in high fat diet fed mice (Seale, et al. 2011) and knocking out PRDM16 results in a loss of the thermogenic program in both brown and beige fat (Cohen, et al. 2014; Harms, et al. 2014). Ongoing work is beginning to characterize the transcriptional complex that interacts with PRDM16 to promote thermogenesis (Dempersmier, et al. 2015).
EBF2
Early B-cell factor 2 (EBF2) is a helix-loop-helix transcription factor that regulates B lymphocytes and neuronal genes (Hagman, et al. 1993) and has been shown to regulate adipogenesis (Akerblad, et al. 2002; Jimenez, et al. 2007). More recently, EBF2 has also been shown to play a role in regulating the thermogenic program in brown and beige adipocytes. A 2013 study used models of EBF2 knockout and overexpression to demonstrate that EBF2 helps to recruit PPARγ to the promoter regions of thermogenic target genes and, with PPARγ, activates the transcription of PRDM16. EBF2 knockout mice present defective brown fat development (Rajakumari, et al. 2013) and recent studies have indicated that EBF2 may also be involved in the regulation of beige fat function (Wang, et al. 2014b). The identification of Brown fat long noncoding RNA 1 (Blnc1) has provided insight into the mechanisms of EBF2 action. Blnc1 was shown to be an important component of the EBF2 ribonucleoprotein complex (Zhao, et al. 2014). Adipocytes that overexpress Blnc1 express thermogenic genes at a higher level than controls at both basal and stimulated states, underlining the role of the EBF2 transcription complex in regulating thermogenesis (Zhao et al. 2014).
TLE3
Transducin-like enhancer of split 3 (TLE3) is a groucho family co-repressor that was identified as a modulator of adipogenesis in a high throughput cDNA screen (Villanueva, et al. 2011). It was found that PPARγ directly drives Tle3 expression and that the TLE3 protein binds to PPARγ and uncharacteristically acts as a co-activator to promote differentiation (Villanueva et al. 2011). Subsequent work has shown that TLE3 is actually a white fat selective protein; overexpression of TLE3 in fat leads to a decrease in thermogenic gene expression and a fat specific TLE3 knockout has an increase in the thermogenic response to cold exposure (Villanueva, et al. 2013). Co-expression experiments revealed that TLE3 and PRDM16 “compete” to bind to PPARγ and the resulting distinct transcription complexes determine the expression of either lipid storage “white fat specific” genes or thermogenic fat specific genes (Villanueva et al. 2013).
SMAD
Transforming growth factor β (TGFβ) signaling through SMAD proteins has been shown to negatively regulate adipogenesis (Zamani and Brown 2011). A SMAD3 global knockout mouse is resistant to diet induced obesity and there is increased UCP1 expression in the adipose tissue of these animals compared to controls (Yadav, et al. 2011). Similarly, treating wild type animals with exogenous TGFβ1 reduced thermogenic gene expression in fat (Yadav et al. 2011). Zinc finger protein 423, a transcriptional regulator of SMAD proteins, has been identified as playing a key role in preadipocyte fate commitment (Gupta, et al. 2010). The therapeutic potential of targeting TGFβ signaling has been explored using a dominant negative activin receptor type IIB fusion protein that promotes thermogenesis through binding of TGFβ and inhibition of downstream signaling (Koncarevic, et al. 2012).
Secreted molecules and signaling in thermogenic fat
While sympathetic signaling is the most understood pathway for the activation of adaptive thermogenesis, new research has focused on identifying other secreted factors that can activate thermogenic fat, both to gain a greater understanding of the regulation of the thermogenic program and to identify potential targets for drug discovery (Figure 2).
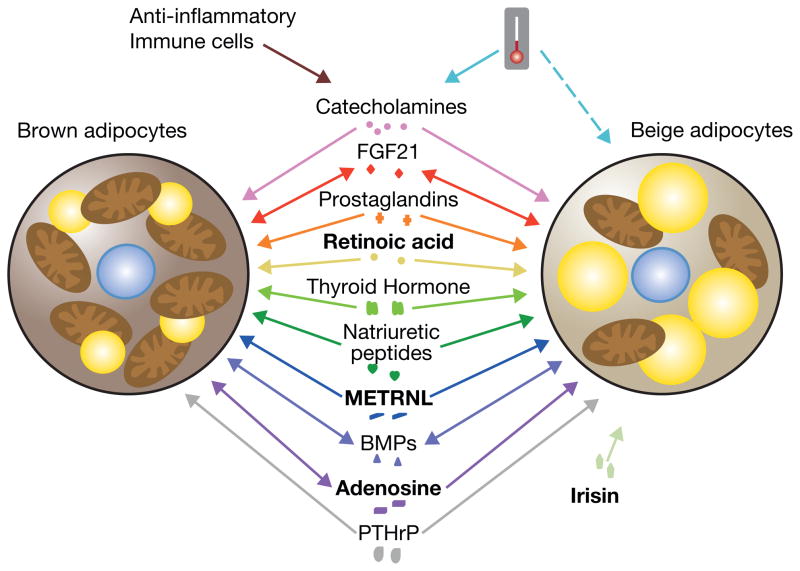
Figure 2. The regulation of thermogenic fat cells by secreted factors.
A number of secreted factors regulate the thermogenic program in brown and beige cells. Cold exposure induces production of catecholamines from the sympathetic nervous system (solid teal line) and may also activate beige fat through a cell autonomous mechanism (dashed teal line). Anti-inflammatory macrophages can also produce catecholamines. FGF21, PG, RA, NPs, thyroid hormone, METRNL, BMPs, adenosine, and PTHrP regulate thermogenesis in brown and beige cells. Additionally, the myokine irisin regulates thermogenesis in beige fat. FGF21, some BMPs, and adenosine have also been shown to be secreted by thermogenic adipocytes. To differentiate the factors that work in concert with central activation of thermogenesis and through beta-adrenergic signaling from those that work through independent pathways, these independent factors, RA, adenosine, Irisin, and METRNL, have been bolded.
FGF family
Fibroblast growth factor (FGF) family members such as FGF1 and FGF15/19, have been implicated in contributing to the regulation of glucose homeostasis and the beiging of white fat (Fu, et al. 2004; Jonker, et al. 2012). FGF21 is the family member most well studied in metabolism; it has been shown to regulate glucose homeostasis, lipid metabolism, insulin sensitivity, ketogenesis, and the prevention of cardiovascular disease (Badman, et al. 2009; Itoh 2014; Kharitonenkov, et al. 2005; Lin, et al. 2013; Patel, et al. 2014). FGF21 is mostly produced in and released from the liver; however, thermogenic activation also increases FGF21 expression in subcutaneous and brown adipose tissue (Fisher, et al. 2012; Hondares, et al. 2011). Ongoing research is investigating the differential roles of liver- and adipose tissue-derived FGF21 in regulating energy homeostasis (Markan, et al. 2014). Systemic administration of FGF21 increases the expression of UCP1 (Coskun, et al. 2008) and genetic ablation of FGF21 impairs the ability of animals to adapt to cold exposure (Fisher et al. 2012). Though FGF21 is not expressed in the central nervous system, it can cross the blood-brain barrier to induce sympathetic nerve activity and thus centrally increase thermogenic gene expression and energy expenditure (Owen, et al. 2014). Recent studies have revealed that adiponectin at least partially mediates the effects of FGF21 on energy expenditure and insulin action (Holland, et al. 2013; Lin et al. 2013). Due to the beneficial effects of FGF21 in metabolism, there has been considerable interest in the development of an FGF21 analog drug, the successful production of which could potentially provide new strategies to improve metabolic health in humans (Kharitonenkov and Adams 2014).
COX2 and Prostaglandins
Cyclooxygenase-2 (COX2) is an enzyme that synthesizes prostaglandins (PG) in response to stimuli such as inflammatory signaling (Ricciotti and FitzGerald 2011). In 2010, two independent studies reported the induction of COX2 in white fat depots upon cold-exposure or β-adrenergic stimulation (Madsen, et al. 2010; Vegiopoulos, et al. 2010). Pharmacological inhibition or genetic ablation of COX2 diminishes the cold or β-AR activation induced beiging of white adipose tissue (Madsen et al. 2010; Vegiopoulos et al. 2010). Overexpression of COX2 has been shown to increase UCP1 expression in adipose tissue, elevate energy expenditure, and reduce weight gain (Vegiopoulos et al. 2010). More recently, prostaglandin E2 (PGE2) and the enzyme that synthesizes it, microsomal synthase-1 (mPGES-1), were shown to play a role in the development of beige fat (Garcia-Alonso, et al. 2013).
Retinoic acid
Retinoic acid (RA), the active derivative form of vitamin A, is mainly synthesized intracellularly from retinaldehyde (Rald) by retinaldehyde dehydrogenases (RALDHs) (Niederreither and Dolle 2008). Studies have suggested that RA, Rald and RALDHs all play functional roles in regulating thermogenic gene expression. It has long been recognized that RA induces UCP1 expression both in cultured brown adipocytes and in brown adipose tissue (Puigserver, et al. 1996). In mouse embryonic fibroblast-derived adipocytes, UCP1 expression is highly elevated upon all-trans RA stimulation in a p38 MAPK-dependent manner (Mercader, et al. 2010). Rald has also been implicated in the induction of the thermogenic program; Plutzky and colleagues have shown that RALDH1 knockout mice have elevated levels of Rald and exhibit increased energy expenditure, improved insulin sensitivity, and resistance to diet-induced obesity (Ziouzenkova, et al. 2007). Later studies demonstrated that the beneficial metabolic effects of Rald signaling likely act through a PGC1α mediated pathway (Kiefer, et al. 2012).
Thyroid Hormone
Thyroid hormones are produced by the thyroid and bind to thyroid hormone receptors (TR) α1-2 and β1-2 to affect to growth and metabolism in target tissues throughout the body, including bone, liver, heart, and fat (Yen 2001). Thyroxine, or T4 thyroid hormone, comprises the majority of thyroid output and deiodinases at peripheral tissues, such as the liver and kidney, remove the 5′ iodine on T4 to form the metabolically active form of thyroid hormone, triiodothyronine (T3) (Yen 2001). T3 is responsible for increasing the metabolic rate and it has been shown to work in concert with norepinephrine to induce transcription of UCP1 in the brown adipose tissue of rats in vivo (Bianco, et al. 1988). Later studies showed that the treatment of primary fetal brown adipocytes from rats with T3 increases Ucp1 transcription and stabilizes Ucp1 mRNA (Guerra, et al. 1996). The ability of T3 to induce thermogenesis was shown to be dependent on the TR isoform that it signals through, the TRβ1 specific agonist GC-1 stimulates UCP1 expression but a TRα1 agonist does not (Martinez de Mena, et al. 2010; Ribeiro, et al. 2001). While thyroid hormone can directly activate the thermogenic program in fat cells, T3 signaling in the hypothalamus through AMPK also works to activate the central nervous system to induce thermogenesis via β3 AR signaling (Lopez, et al. 2010).
Natriuretic Peptides
The natriuretic peptides (NPs) are a family of cardiac and vascular derived hormones that regulate sodium homeostasis in blood and urine. There are three main types of NPs; atrial natriuretic peptide (ANP), and B- and C-type natriuretic peptides (BNP and CNP) (Zois, et al. 2014). Additionally, there are two major classes of NP receptors; NP receptors A and B mediate an intracellular cyclic guanosine monophosphate-dependent signaling cascade, while the NP receptor C (NPRC) facilitates the removal of NPs from circulation (Anand-Srivastava 2005). The discovery that NP receptors are expressed in the adipose tissue of rats and humans opened an area of inquiry into the actions of NPs in fat (Sarzani, et al. 1996; Sarzani, et al. 1993) and it was subsequently shown that ANP and BNP stimulate lipolysis in human adipocytes (Lafontan, et al. 2008). Recent work on NPRC global knockout mice shows that these animals have increased ANP, reduced fat depot size, and increased thermogenic gene expression (Bordicchia, et al. 2012). Further studies implicated both ANP and BNP in the regulation of thermogenesis, ANP was shown to mediate the induction of thermogenic genes through the stimulation of a cGMP/p38 MAPK pathway and the constant delivery of BNP to mice resulted in increased energy expenditure and beiging of white adipose tissue (Bordicchia et al. 2012).
Signaling from Immune Cells
One consequence of obesity is a change of the macrophage populations seen in adipose tissue from anti-inflammatory M2 macrophages to pro-inflammatory M1 macrophages (Lumeng and Saltiel 2011). This switch in macrophage populations may contribute to the decrease in thermogenesis because catecholamines produced by M2 macrophages can signal through the β ARs to induce thermogenesis (Nguyen, et al. 2011; Qiu, et al. 2014). Recently, it has been shown that other cells associated with the type 2 immune response, specifically type 2 lymphoid cells, can also contribute to the beiging of fat (Brestoff, et al. 2015; Lee, et al. 2015). The development of chronic inflammation during obesity leads to upregulation of the non-canonical NF-κB target, IκB kinase ε (IKKε) (Chiang, et al. 2009). The increase of IKKε results in catecholamine resistance in adipose tissue, which in turn suppresses the induction of UCP1 (Mowers, et al. 2013). This is consistent with the observation that an IKKε global knockout mouse has less inflammation, increased energy expenditure, and upregulation of thermogenic gene expression in the visceral depot compared to wild type animals (Chiang et al. 2009). Treating high fat diet fed animals with a specific inhibitor of both IKKε and the related kinase TBK1 results in reduced lipid deposition in brown adipose tissue and an increase in thermogenic gene expression (Reilly, et al. 2013). These studies provide a model in which the development of obesity leads not only to the loss of a thermogenic signal from type 2 inflammatory cells but also to the development of chronic inflammation and subsequent resistance to thermogenic signals.
Myokines (Irisin and METRNL)
Induction of PGC1α in skeletal muscle has systematic benefits including an increase in energy expenditure and prevention of age-related obesity (Puigserver and Spiegelman 2003; Wenz, et al. 2009). Elevated expression of the protein fibronectin type III domain containing 5 (FNDC5) was seen in a model of skeletal muscle specific overexpression of PGC1α. Irisin, the cleaved form of FNDC5, is a secreted hormone released after exercise that stimulates the beiging of white fat (Bostrom, et al. 2012; Huh, et al. 2012). Recently, irisin was demonstrated to be not only a myokine but also an adipokine that can be secreted from white fat tissue under certain physiological and pathological conditions (Roca-Rivada, et al. 2013). It has been shown that cold exposure increases levels of circulating irisin, suggesting that shivering may result in irisin release from muscle and therefore providing another potential physiological mechanism by which irisin stimulates beiging (Lee, et al. 2014a). In addition to irisin, meteorin-like (METRNL) has also recently been implicated as playing a role in metabolism. METRNL is a hormone that is released from muscle after exercise and from adipose tissue upon cold exposure. Intravenous injections of an adenoviral METRNL construct or direct injection of recombinant protein into mice induces thermogenesis in fat, increases whole-body energy expenditure and improves glucose tolerance through eosinophil-dependent IL4/IL13 signaling (Rao, et al. 2014). The identification and characterization of irisin and METRNL provide a model in which myokines released during exercise influence metabolism. The extent to which myokine signaling contributes to the overall metabolic benefits of exercise and how these signals interact with other exercise regulated pathways await further study.
Bone morphogenetic proteins
Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily and are involved in multiple biological processes in adipose tissue including enhancing preadipocyte proliferation (Stewart, et al. 2010), inducing adipogenesis (Jin, et al. 2006), influencing adipocyte lineage commitment (Huang, et al. 2009), and regulating thermogenesis (Tseng, et al. 2008; Whittle, et al. 2012). BMP7 has been shown to promote brown adipogenesis through the induction of PRDM16 and PGC1α (Tseng et al. 2008). Another family member, BMP8b, functions in the central nervous system to increase sympathetic output and therefore increases the response of thermogenic fat to cold exposure through p38 MAPK and CREB signaling (Whittle et al. 2012). A BMP8b global knockout mouse has reduced thermogenic gene expression in adipose tissue compared to controls (Whittle et al. 2012).
Newly discovered factors
More recently, additional secreted factors important to thermogenic fat biology have been reported (Crane, et al. 2015; Fang, et al. 2015; Gnad, et al. 2014; Kir, et al. 2014; Wang, et al. 2014a). For want of space, we will briefly discuss only a few of them here.
It has been observed that cachexia, the wasting of adipose and skeletal muscle tissues seen in diseases such as cancer, is associated with the activation of brown fat (Shellock, et al. 1986). A recent study found that the thermogenic program is activated in fat cells treated with conditioned medium from Lewis lung carcinoma (LLC) cells, a well-characterized model of cachexia (Kir et al. 2014). Global gene expression analysis of LLC cells identified parathyroid-hormone-related protein (PTHrP) as regulating the activation of thermogenesis, likely through the cAMP/PKA pathway (Kir et al. 2014). This discovery has begun to provide mechanistic insight into the etiology of the development of cachexia and further studies may suggest treatments that can prevent tissue wasting during disease.
Adenosine has recently been shown to increase lipolysis in primary human and mouse adipocytes (Gnad et al. 2014). Adenosine is released from brown fat upon sympathetic stimulation and can signal brown adipocytes to stimulate thermogenesis. The adenosine receptor A2A is not highly expressed in white adipose tissue, however, pharmacological activation of A2A and viral delivery of A2A into the subcutaneous depot of mice both significantly increase beiging (Gnad et al. 2014).
Neuregulin 4 (NRG4), a member of the epidermal growth factor (EGF) family, was recently discovered to be a secreted factor that is released from brown fat (Wang et al. 2014a). NRG4 binds to ERBB receptors in the liver and its activation inhibits the SREBP1c-lipogenic pathway through trans-repression of the liver X receptor by STAT5. In vivo gain and loss of function studies have shown that NRG4 helps to ameliorate diet-induced obesity and insulin resistance (Wang et al. 2014a). These studies suggest that factors released from brown fat can play a role in regulating energy expenditure and systemic metabolism.
Human Thermogenic Fat
While originally it was believed that the only brown fat in humans was found in newborns and was rapidly lost postnatally, analysis of 18F-fluorodeoxyglucose positron-emission tomographic and computed tomographic (PET-CT) scans showed that there is active thermogenic fat in some adults (Nedergaard, et al. 2007). Biopsies of “hot” areas indicated by PET-CT scans reveal that the fat tissue in the supraclavicular region, as well as in the neck and paraspinal regions, expresses UCP1 (Cypess, et al. 2009; Saito, et al. 2009; van Marken Lichtenbelt, et al. 2009; Virtanen, et al. 2009). The identity of the thermogenic fat in adults remains uncertain. While the thermogenic fat found in babies has the characteristics of classical brown fat, gene expression analyses performed on adult thermogenic fat have shown the presence of genes that are thought to be beige specific (Lee, et al. 2014c; Lidell, et al. 2013; Sharp, et al. 2012; Wu et al. 2012), suggesting that they might be beige fat. Other studies have suggested that human thermogenic fat tissue may be a mixture of brown and beige cells (Cypess, et al. 2013; Jespersen, et al. 2013).
Recent work has indicated that thermogenic fat may play an important, active, metabolic role in humans (Table 1). Multiple studies have shown that the presence of thermogenic fat is negatively correlated with age and BMI (Cypess et al. 2009; Lee, et al. 2010; Ouellet, et al. 2011; Saito et al. 2009; van Marken Lichtenbelt et al. 2009). There is an increase in the amount of detectable thermogenic fat in patients who underwent significant weight loss after gastric bypass surgery, suggesting that the decrease in the amount of thermogenic fat seen during obesity can be reversed (Vijgen, et al. 2012). It has also been shown that environmental temperature modulates the amount of detectable thermogenic fat in adults (Saito et al. 2009; van Marken Lichtenbelt et al. 2009; Virtanen et al. 2009). Furthermore, acute cold exposure increases resting metabolic rate more in individuals that have visible thermogenic fat on PET-CT scans compared to individuals without detectable thermogenic fat (Chen, et al. 2013; Ouellet, et al. 2012; van der Lans, et al. 2013; Yoneshiro, et al. 2011a; Yoneshiro, et al. 2013), this is in line with reports that there is increased glucose and fatty acid uptake in supraclavicular fat depots in response to cold exposure (Orava, et al. 2011; Ouellet et al. 2012). Most excitingly, it has recently been shown that the accumulation of thermogenic fat in response to cold exposure results in improvements in insulin sensitivity and glucose homeostasis (Chondronikola, et al. 2014; Lee, et al. 2014b) as well as a decrease in body weight (Yoneshiro et al. 2013). These studies indicate that human thermogenic fat is a viable target for anti-obesity and anti-diabetic treatments.
Table 1.
Thermogenic fat in humans correlates with physiological and environmental factors and influences metabolic parameters.
Concluding remarks
In the long pursuit of better understanding and more effective therapeutics for metabolic disease, we have become aware that many of these disorders are polygenic and multifactorial, suggesting the ultimate solution demands a thorough knowledge of all cell types involved, and of both cell autonomous regulation and intercellular communication. Increasing appreciation has been directed towards the role of adipose tissue in this complicated network. For two decades, since the cloning of leptin (Zhang, et al. 1994) and the discovery that fat tissue can generate inflammatory cytokines in obesity (Hotamisligil, et al. 1993), the endocrine function of adipose tissue has been studied in detail and is relatively well understood. It has only been in the last decade that researchers and clinicians in the metabolic field have begun to recognize the potential influence of thermogenic fat cells on whole body metabolism. We have made great advances in our understanding of how these cells are regulated both transcriptionally and by circulating factors and our knowledge of how these cells contribute to human metabolism is growing. Ongoing research is continually uncovering new methods to target these cells and recent studies have begun to show that some therapeutics already in clinical use, like the mineralocorticoid receptor antagonist spirolactone and the GLP1 receptor agonist liraglutide, may also be able to stimulate thermogenic fat (Armani, et al. 2014; Beiroa et al. 2014). With this knowledge we can hopefully soon develop treatments that target thermogenic fat to fight against obesity and associated conditions.
Acknowledgments
Funding
Work in the Wu laboratory is supported by grants K01DK094824 and R03DK100698 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, a Young Investigator Grant RGY0082/14 from the Human Frontier Science Program, and a Pilot and Feasibility Grant from the Michigan Diabetes Research Center (NIH Grant 2P30-DK020572).
We thank the members of the Wu lab for valuable discussions and apologize to those whose work we were not able to cite due to space limitations.
Footnotes
Declaration of Interest
The authors have declared that no conflict of interest exists.
References
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-Cell Factor (O/E-1) Is a Promoter of Adipogenesis and Involved in Control of Genes Important for Terminal Adipocyte Differentiation. Mol Cell Biol. 2002;22:8015–8025. doi: 10.1128/MCB.22.22.8015-8025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014;28:3745–3757. doi: 10.1096/fj.13-245415. [DOI] [PubMed] [Google Scholar]
- Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Ferno J, Salvador J, Escalada J, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Sheng X, Silva JE. Triiodothyronine Amplifies Norepinephrine Stimulation of Uncoupling Protien Gene Transcription by a Mechanism Not Requiring Protein Synthesis. Journal of Biological Chemistry. 1988;263:18168–18175. [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blumer RM, Fullerton MD, Yabut JM, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21:166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF, Kwan HY, Kang C, Wong RH, Sul HS. Cold-Inducible Zfp516 Activates UCP1 Transcription to Promote Browning of White Fat and Development of Brown Fat. Mol Cell. 2015;57:235–246. doi: 10.1016/j.molcel.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Masui S, Osada S, Umesono K, Motojima K. A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes. 2000;49:759–767. doi: 10.2337/diabetes.49.5.759. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso V, Lopez-Vicario C, Titos E, Moran-Salvador E, Gonzalez-Periz A, Rius B, Parrizas M, Werz O, Arroyo V, Claria J. Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor gamma (PPARgamma) in the conversion of white-to-brown adipocytes. J Biol Chem. 2013;288:28230–28242. doi: 10.1074/jbc.M113.468603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- Gray SL, Dalla Nora E, Backlund EC, Manieri M, Virtue S, Noland RC, O’Rahilly S, Cortright RN, Cinti S, Cannon B, et al. Decreased brown adipocyte recruitment and thermogenic capacity in mice with impaired peroxisome proliferator-activated receptor (P465L PPARgamma) function. Endocrinology. 2006;147:5708–5714. doi: 10.1210/en.2006-0684. [DOI] [PubMed] [Google Scholar]
- Guerra C, Roncero C, Porras A, Fernandez M, Benito M. Triiodothyronine Induces the Transcription of the Uncoupling Protein Gene and Stabilizes Its mRNA in Fetal Rat Brown Adipocyte Primary Cultures. Journal of Biological Chemistry. 1996;271:2076–2081. doi: 10.1074/jbc.271.4.2076. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J, Belanger C, Travis A, Turck CW, Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993;7:760–773. doi: 10.1101/gad.7.5.760. [DOI] [PubMed] [Google Scholar]
- Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, Dilworth SM, White R, Parker MG, Christian M. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol. 2008;28:6785–6795. doi: 10.1128/MCB.00504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Role of thermogenesis in the regulation of energy balance in relation to obesity. Can J Physiol Pharmacol. 1989;67:394–401. doi: 10.1139/y89-063. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G, Shargill N, Spiegelman B. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front Endocrinol (Lausanne) 2014;5:107. doi: 10.3389/fendo.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006;10:461–471. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Adams AC. Inventing new medicines: The FGF21 story. Mol Metab. 2014;3:221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW, Vernochet C, O’Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18:918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA, et al. A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology. 2012;153:3133–3146. doi: 10.1210/en.2012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Lee M, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–606. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014a;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014b;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 2014c;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, et al. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Mena R, Scanlan TS, Obregon MJ. The T3 receptor beta1 isoform regulates UCP1 and D2 deiodinase in rat brown adipocytes. Endocrinology. 2010;151:5074–5083. doi: 10.1210/en.2010-0533. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2014;38:812–817. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- Mercader J, Palou A, Bonet ML. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity (Silver Spring) 2010;18:655–662. doi: 10.1038/oby.2009.330. [DOI] [PubMed] [Google Scholar]
- Mowers J, Uhm M, Reilly SM, Simon J, Leto D, Chiang SH, Chang L, Saltiel AR. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKK{varepsilon} and TBK1. Elife. 2013;2:e01119. doi: 10.7554/eLife.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerback S, Rissanen A, Pietilainen KH, Virtanen KA. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 2013;21:2279–2287. doi: 10.1002/oby.20456. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, Hillhouse EW, Tan BK, Randeva HS. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS One. 2014;9:e87102. doi: 10.1371/journal.pone.0087102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29(Suppl 1):S5–9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Vazquez F, Bonet ML, Pico C, Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem J. 1996;317(Pt 3):827–833. doi: 10.1042/bj3170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer Stephen R, et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone--sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform--specific. J Clin Invest. 2001;108:97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzani R, Dessi-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996;19:581–585. doi: 10.1007/BF03349021. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Paci VM, Dessi-Fulgheri P, Espinosa E, Rappelli A. Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J Hypertens Suppl. 1993;11:S214–215. [PubMed] [Google Scholar]
- Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: Possible cause of cancer-induced cachexia. Journal of Cancer Research and Clinical Oncology. 1986;111:82–85. doi: 10.1007/BF00402783. [DOI] [PubMed] [Google Scholar]
- Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010;223:658–666. doi: 10.1002/jcp.22064. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva CJ, Waki H, Godio C, Nielsen R, Chou WL, Vargas L, Wroblewski K, Schmedt C, Chao LC, Boyadjian R, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 2011;13:413–427. doi: 10.1016/j.cmet.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106–3113. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014a;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014b;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Fact Sheet on Obesity and Overweight. who.int 2014 [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011a;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011b;19:1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- Zamani N, Brown CW. Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell. 2014;55:372–382. doi: 10.1016/j.molcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11:403–412. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]