Abstract
The prognosis of adolescent and young adult patients battling metastatic Ewing Sarcoma Family of Tumours (ESFT) remains less than 30% despite the development of systemic therapies. In the era of personalized medicine, novel molecular targets have been tested in preclinical or clinical settings in ESFT. In this review, we focus on early clinical and translational research that identified multiple molecular targets, including IGF-1R; mTOR; tyrosine kinase inhibitors; EWS-FLI1-related targets, and others. Overall, novel targeted therapies demonstrated modest efficacy; however pronounced and durable antineoplastic responses have been observed in small subsets of treated patients, for example with IGF-1R antibodies. Identifying outcome-predicting biomarkers and overcoming treatment resistance remain major challenges. Due to the rarity of ESFT, multi-institutional collaboration efforts of clinicians, basic and translational scientists are needed in order to understand biology of therapeutic response or resistance, which can lead to development of novel therapeutic methods and improved patient outcomes.
Introduction
Ewing sarcoma family tumours (ESFT), heretofore simply referred to as Ewing’s sarcoma (ES), are bone or soft tissue sarcomas that are found primarily in adolescents and young adults, with peak occurrence between ages 10 and 20 1. ES as a malignant entity is genetically characterized by chromosomal translocation involving the Ewing sarcoma breakpoint region 1 (EWSR1) gene. Translocation of EWSR1 on chromosome 22 to chromosome 11 occurs in 85% of ES cases, forming the fusion protein product EWS-FLI12,3. In addition, fusion product EWS-ERG is identified in 10% of cases, whereas several other translocation types are rarely identified 4–9 (Table 1). The EWSR1 breakpoint appears to be a hot spot for genetic translocations and can promiscuously bind other C-terminal genes in other sarcoma subtypes such as clear cell sarcoma, extraskeletal myxoid chondrosarcoma and others10–12. FLI1, ERG and other ETS genes contain the DNA-binding domain 13. Consequently, EWS-FLI1 protein functions as an aberrant transcription factor regulating malignant transformation to ES.
Table 1.
ES translocation types and fusion products
Of all ES cases, approximately 26%-28% are metastatic diseases at diagnosis with the remainder being localized disease 14. Instituting a systemic chemotherapy regimen in combination with surgery and/or radiotherapy has significantly increased the survival of patients with localized disease. The 5-year survival rate was less than 15% before chemotherapy became available, 44% for patients in the decade between 1973–1982, while for the decade between 1993–2004, survival rates from recently completed large cooperative groups trials (such as AWES-0031 and EURO-Ewing 99) report survival rates of approximately 70% 14,15. Unfortunately, the prognosis of patients with metastatic ES remains dismal, with 5-year survival rates of approximately 20%-30%16. In addition to standard of care treatment modalities, which will clearly continue to have value, novel therapies have been tested in clinical trials with the hopes of increasing survival and clinical benefits have been achieved in some patients.
Compared to conventional chemotherapies, targeted therapies are specifically directed to molecules associated with tumorigenesis and tumour progression of ES. These include insulin-like growth factor 1 receptor (IGF-1R), mammalian target of rapamycin (mTOR), tyrosine kinases such as platelet-derived growth factor receptor (PDGFR), KIT, epidermal growth factor receptor (EGFR), vascular growth factor receptors (VEGFRs), Aurora A, poly ADP ribose polymerase 1 (PARP1), and GD2, all of which are in phase I and II clinical testing (Tables 2 and 3) 17–32. Therapies targeting other proteins such as EWS-FLI1 and CD99 are in preclinical testing and may be promising targets for novel therapies. In addition, new molecules have been identified in mechanistic studies and may be clinically applicable. A better understanding of the underlying mechanism of ES and associated molecular aberrations will greatly aid in the discovery of new molecular targets and the development of targeted therapies.
Table 2.
Reported clinical trials of targeted therapies for ES.
| Study | Phase | Enrichment of Molecular target |
Treatment Arms | No. of Patients |
RR (%) | Median PFS (mo) |
Median OS (mo) |
|---|---|---|---|---|---|---|---|
| IGF-1R | |||||||
| Kurzrock et al.17 | I | No | R1507 | 9 | 22.2% | N/R | N/R |
| Pappo et al.18 | II | No | R1507 | 115 | 9.6% | 1.3mo | 7.6mo |
| Tolcher et al.19 | I | No | AMG 479 | 12 | 16.7% | N/R | N/R |
| Tap et al.20 | II | No | AMG 479 | 19 | 5.3% | 7.9mo | N/R |
| Olmos et al.21 | I | No | figitumumab | 16 | 12.5% | N/R | N/R |
| Juergens et al.22 | I II |
No No |
figitumumab figitumumab |
16 106 |
6.3% 14.2% |
N/R 1.9mo |
N/R 8.9mo |
| Malempati et al.23 | I/II | No | cixutumamab | 35 | 8.6% | N/R | N/R |
| mTOR | |||||||
| Mita et al.24 | I | No | deforolimus | 1 | 100.0% | N/R | N/R |
| Bagatell et al.25 | I | No | temsirolimus +irinotecan +temozolomide |
7 | 0% | N/R | N/R |
| mTOR combination therapy | |||||||
| Naing et al.26 | I | No | Cixutumumab +temsirolimus |
17 | 11.8% | N/R | 12.3mo |
| Schwartz et al.27 | II | No | cixutumumab+ temsirolimus |
27 | 14.8% | 7.5weeks | 16.2mo |
| Kit/PDGFR | |||||||
| Bond et al.28 | II | No | imatinib mesylate | 24 | 4.2% | N/R | N/R |
| Chao et al.29 | II | IHC level | imatinib mesylate | 5 | 20.0% | N/R | N/R |
| EGFR | |||||||
| Daw et al.30 | I | No | gefitinib | 3 | 33.3% | N/R | N/R |
| VEGFR | |||||||
| Fox et al.31 | I | cediranib | 3 | 33.3% | N/R | N/R | |
N/R: not reported
Table 3.
A list of selected ongoing Ewing’s sarcoma trials (from clinicaltrials.gov, accessed on 06/10/2014)
| Study Drugs | Phase |
clinicaltrial.org identifier |
Sponsor/lead organizations |
Targeted molecule |
Number of recruites |
|---|---|---|---|---|---|
|
Cyclophosphamide, Topotecan, and Bevacizumab (CTB) |
II | NCT01492673 | Memorial Sloan- Kettering Cancer Center |
VEGFR | 29 |
| Dasatinib | II | NCT00464620 | Sarcoma Alliance for Research through Colloboration |
KIT | 502 |
| Dasatinib and ipilimumab | I | NCT01643278 | National Cancer Institute |
KIT, CTLA-4 | 30 |
| Regorafenib | II | NCT02048371 | Sarcoma Alliance for Research through Collaboration |
RTKs | 126 |
| Aflac ST0901 CHOANOME - Sirolimus | I | NCT01331135 | Emory University | mTOR | 24 |
| CC-115 | I | NCT01353625 | Celgene Corporation | mTOR | 144 |
| Sorafenib and Irinotecan | I | NCT01518413 | Children’s Research Institute |
VEGFR | 24 |
| Pazopanib | II | NCT01956669 | GlaxoSmithKline | VEGFR | 154 |
| Alisertib | II | NCT01154816 | Children’s Research Institute |
Aurora A | 228 |
| BMN-673 and temozolomide | I/II | NCT02116777 | National Cancer Institute |
PARP | 172 |
| BMN 673 | I | NCT01286987 | BioMarin Pharmaceutical |
PARP | 85 |
| Olaparib | II | NCT01583543 | Massachusetts General Hospital |
PARP | 24 |
| Olaparib and Temozolomide | I | NCT01858168 | Massachusetts General Hospital |
PARP | 34 |
| Niraparib and Temozolomide | I | NCT02044120 | Sarcoma Alliance for Research through Collaboration |
PARP | 30 |
| RO4929097 | I/II | NCT01154452 | National Cancer Institute |
SMO; γ secretase |
120 |
| humanized anti-GD2 antibody | I | NCT00743496 | St. Jude Children’s Research Hospital |
GD2 | 75 |
|
T cells expressing an anti-GD2 chimeric antigen receptor |
I | NCT02107963 | National Cancer Institute |
GD2 | 72 |
| Iodine I 131 monoclonal antibody 3F8 | II | NCT00445965 | Memorial Sloan- Kettering Cancer Center |
GD2 | 77 |
| Mithramycin | I/II | NCT01610570 | National Cancer Institute |
EWS-FLI1 | 44 |
Molecular targets for directed therapy
IGF-1R
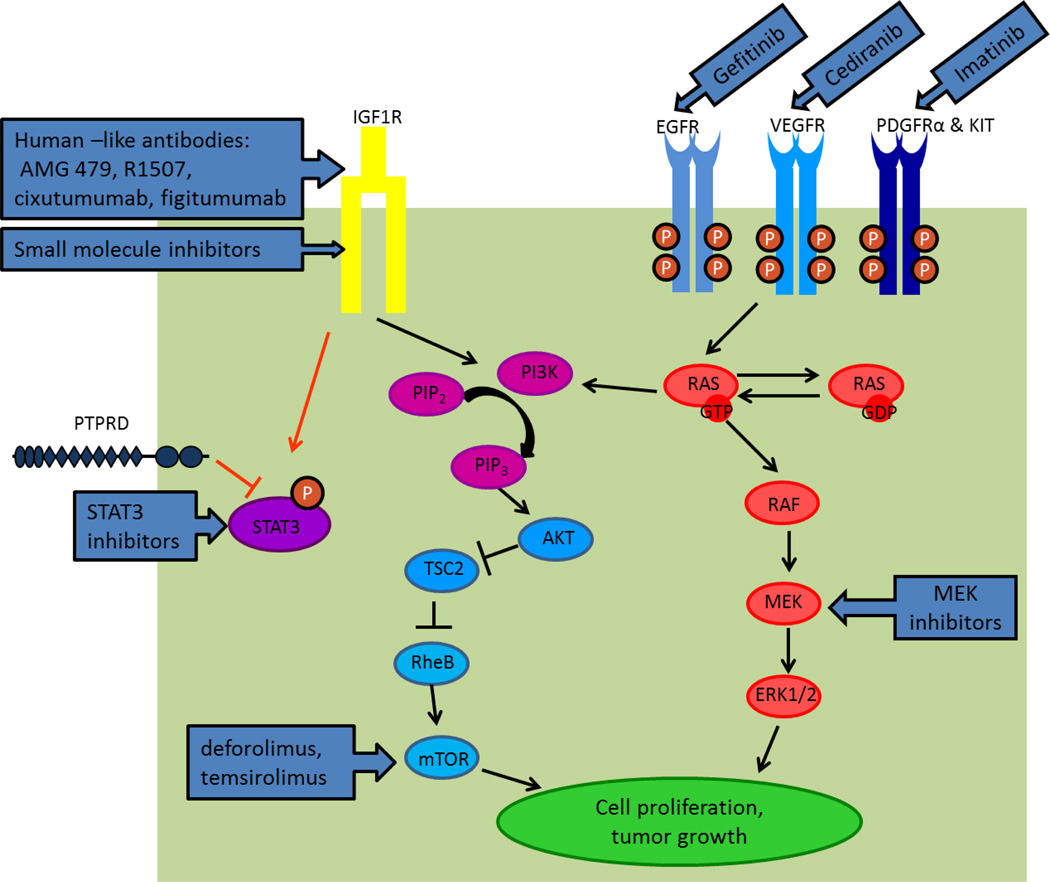
When bound to IGF1 (and with less affinity to IGF2), IGF-1R autophosphorylation initiates several cancer-related pathways known to regulate cell growth and tumorigenesis 33. The best characterized include PI3K/AKT/mTOR and MEK/ERK/MAPK, though other pathways are also affected (Figure 1) 34. Not only do most, if not all, ES cell lines and clinical samples express IGF-1R, an activated IGF-1R pathway is a prerequisite for malignant transformation by the EWS-FLI1 translocation 35,36. As occurs in patients, IGF-1R inhibition induces cell death and tumour regression in some ES cell lines and xenograft models 37–39. Therefore, IGF-1R is one of the most important targets for novel ES therapies. At least a half dozen IGF-1R targeted monoclonal antibodies produced partial or complete responses in small subsets of patients with ES (Table 2). These antibodies include human-like IgG1 antibodies AMG 479 40, R1507 17 and cixutumumab 41 as well as the human-like IgG2 antibody figitumumab 42.
Figure 1.
Schematic figure of targeted molecules in ES-related pathways and drugs used in clinical and preclinical testing.
In a phase I trial using R1507, two (22.2%) of nine ES patients achieved partial responses (PR), and one (11.1%) patient had stable disease (SD) for more than 6 months and no dose-limiting toxicities were identified 17. The subsequent phase II study with R1507 demonstrated responses in 11 (9.6%) of 115 patients with ES, including one complete response (CR) and 10 PRs with a median progression-free survival (PFS) of 1.3 months and median overall survival (OS) of 7.6 months 18. In a phase I trial using AMG 479, two (16.7%) of 12 ES patients responded to treatment, including one CR and one unconfirmed PR19. Using AMG 479, one (5.3%) of 19 ES patients achieved a PR and one patient has had SD for more than 24 months with a median PFS of 7.9 months20. In a phase I trial of figitumumab, two (12.5%) of 16 ES patients responded, including one CR and one PR and six (37.5%) patients had SD longer than 4 months21. In a different phase I/II study of figitumumab, one (6.3%) of 16 ES patients had a PR in the phase I portion of the study and in the phase 2 portion of the study, 15 (14.2%) of 106 patients had a PR with a median PFS and OS of 1.9 months and 8.9 months, respectively 22. In a phase I/II trial of cixutumumab in pediatric patients with refractory solid tumours, three (8.6%) of 35 ES patients had a PR 23.
In addition to the IGF-1R antibodies that have already been clinically tested, several small molecule inhibitors of IGF-1R have been evaluated preclinically. OSI-906, a dual inhibitor of IGF-1R and insulin receptor (IR), displayed antiproliferative effects in a variety of tumour cell lines as well as in vivo antitumor activity in xenograft models43. A phase I study using OSI-906 in combination with erlotinib was conducted in patients with advanced solid tumours and one ES patient had SD for at least 12 weeks 44. In addition, BMS-754807, a reversible ATP-competitive antagonist of the IGF-1R kinase domain demonstrated moderate growth inhibition in in vitro and in vivo ES models. Another small molecule IGF-1R inhibitor, ADW742, has been shown to induce dose-dependent G1 phase blockade and apoptosis in ES cell lines, which demonstrated synergy with the KIT/PDFGR and BCR-ABL tyrosine kinase inhibitor imatinib45–47. Despite the modest activity of small IGF-1R inhibitors in preclinical studies, further investigation is needed to elucidate their utility and translation to the clinic.
Collectively, clinical trials demonstrated that anti-IGF-1R targeting therapies can produce striking anticancer activity in small subsets of patients with ES, ranging up to 22%. Unfortunately, there were no biomarkers identified to predict response to therapies. The total IGF-1R level did not correlate with response. IR isoform IR-A, which is responsible for somatic growth, is the only IR expressed in ES and some studies suggested that IGF-1R-resistant cells are able to switch from IGF1/IGF-1R to IGF-2/IR-A signaling to maintain levels of phosphorylated (p-) Akt and other downstream regulators 33,48. Garofalo et al. have suggested that the IGF-1R to IR-A ratio may be used as a biomarker for identifying the subset of patients that may respond to IGF-1R-related therapies. Patients with higher IGF-1R : IR-A ratios are most likely to benefit 33. The mechanisms of resistance to IGF-1R therapies are complicated due to their involvement in relevant downstream pathways. Further investigation is warranted to identify biomarkers that can contribute to predicting outcomes of IGF-1R therapies.
mTOR
Genetic and epigenetic aberrations of the PI3K/AKT/mTOR pathway play a critical role in tumorigenesis and cancer progression for many cancer types, and ES is no exception (Figure 1) 34,49. Activation of the PI3K/AKT/mTOR pathway is characterized by upregulated phosphorylated (p-) Akt levels50, and has been observed frequently in ES samples 51. Among the components of the PI3K/AKT/mTOR pathway, mTOR is one of the most frequently targeted molecules in ES-related clinical trials.
In a nonselective phase I trial in multiple tumour types treated with the mTOR complex 1 (mTORC1) inhibitor, deforolimus 24, the only patient with ES enrolled in the study achieved a PR. In a phase I trial using the mTOR inhibitor temsirolimus, irinotecan and temozolomide, one (14%) of seven ES patients achieved SD and continued on therapy for more than five months with no evidence of disease progression 25. However, this response is likely due to the known activity of irinotecan and temozolomide52.
Inhibitors of mTOR have been shown more effective in combinations such as with IGF-1R than as single agents by our institution and others24,26,27,53. mTOR inhibition releases the inhibitory feedback loop on the insulin receptor substrate 1 (IRS-1) and, therefore, upregulates PI3K and Akt in an IGF-1/IGF-1R dependent manner 54,55. Additionally, mTOR inhibition can lead to autocrine release of IGF-1, a cancer promoting effect that can be successfully blocked by IGF-1R antibodies56. Just as mTORi has counter-regulatory effects upon the IGF-1R/Akt/mTOR pathway, morphoproteomic profiling of ES tumour samples ES suggest that resistance to IGF-1R monotherapy is driven by the downstream activation of the PI3K/mTOR pathway, which can be plausibly abrogated by mTOR inhibitors57. Proving the synergy of IGF-1R and mTOR inhibition to maximally blunt proximal and distal pathway components, a phase I trial conducted by Naing et al. combined cixutumumab with temsirolimus; two (11.8%) of 17 ES patients achieved a CR and three (17.6%) patients had SD lasting for 8, 15 and 18 months, respectively 26. Interestingly, one of two patients with the CR had a history of a previous PR when treated with the single-agent IGF-1R antibody R1507 alone, which lasted for nearly 30 months17. A number of interesting conclusions can be drawn from a confirmatory trial that used the same drug combination in diverse sarcoma subtypes. First, the IGF-1R/mTOR inhibitors combination did, in fact, lead to considerably higher response rates than had been observed when either agent was used alone; four (14.8%) of 27 ES patients achieved a PR 27. Second, the duration of response among ES patients was significantly less than the prior study, a result likely attributable to the mTOR inhibitor temsirolimus dose reductions that were mandated in the nearly half of patients that exhibited mild transaminitis. Last, prospective patient stratification by immunohistochemical staining of IGF-1R expression did not predict response to therapy.
Despite an abundance of data and strong rationale that IGF-1R and/or mTOR targeted therapies are most effective when used together or combined with other biologically targeted therapies, no clinical trials were available for ES patients at the time of this publication. In communication with Pharma, one explanation is their concern that IGF-1R inhibitors would not receive FDA approval as a single-agent activity and, therefore, would not be allowed conditional approval in combination with mTOR inhibitors or other agents (personal communication). Though some sarcoma subtypes are dependent upon single oncogenic targets (gastrointestinal stromal tumour’s reliance upon KIT or PDGFR, for example), this is the exception rather than the norm. It is much more likely that multiple biologically targeted therapies must be used together to prevent rapid drug-induced signaling changes that counteract the intended drug effects and, ultimately, leads to treatment failure.
Tyrosine kinases
PDGFRα and KIT
PDGFRα and KIT are members of the class III receptor tyrosine kinases (RTKs) 58. Both PDGFRα and KIT are expressed and activated in ES samples 59,60. Accordingly, the specific tyrosine kinase inhibitor imatinib was used to target PDGFRα and KIT in preclinical and clinical studies. Though in vitro preclinical experiments demonstrated proof of concept, in ES the IC50 values of imatinib (10–12 µM) markedly exceeded levels achievable in the clinic 28,29,61,62. In a phase II study of patients with refractory or relapsed pediatric solid tumours, one (4.2%) of 24 ES patients had a PR28. Despite this low response rate, the therapy may benefit a small subset of patients. Because imatinib primarily targets PDGFRα and KIT, a high protein expression level in tumour could be considered as one criterion for trial enrollment. In another phase II clinical trial, immunohistochemical evidence of expression ≥2+/4+ for either KIT or PDGFRα was, in fact, applied as one of the required criteria for patient enrollment29. One (20%) out of five ES patients had a PR after eight months of treatment. Of interest, the only patients responding to the therapy had the highest expression level of PDGFRα and KIT (3+/4+ PDGFRα and 3+/4+ KIT) 29. The low response rates of the clinical trials thus far suggests that stricter selection criteria for PDGFRα and KIT levels in combination with novel biomarkers should be required to enhance the efficacy of imatinib treatment in future clinical trials. As mentioned previously, one could hypothesize that imatinib activity could be enhanced if it were combined with other biologically targeted therapies, though this has not yet been demonstrated in clinical practice.
EGFR
EGFR is a tyrosine kinase receptor that modulates cell proliferation, tumour growth and angiogenesis through downstream activation of the RAS/RAF/MEK/ERK and PI3K/AKT/mTOR pathways (Figure 1)63. Gefitinib is a small molecule inhibitor targeting the intracellular kinase domain of EGFR64,65. In a phase I study in patients with pediatric solid tumours, one (33.3%) out of three ES patients had a PR that lasted 10 weeks; however, the mechanism of action remains unclear30. Somatic EGFR mutations, which are associated with a salutary response to EGFR inhibitors in non-small cell lung cancer patients, have not been reported in ES66–68. In addition, an analysis of biomarkers from a phase I trial of gefitinib in pediatric patients with solid tumours showed no correlation between baseline levels of plasma EGFR and VEGF and antitumor activity69. There was also no significant alteration of EGFR or VEGF levels in responding patients69. Tumours with increased expression of p-Akt were associated with a better response to gefitinib in non-small cell lung cancer patients with unknown mutational status70. Though it is plausible that the baseline Akt phosphorylation level could serve as a biomarker of EGFR activity in ES, this remains just an untested hypothesis.
VEGFR
VEGFR signaling is stimulated by the binding of VEGF and promotes angiogenesis 71. Cediranib is an ATP competitive, small molecule inhibitor targeting the tyrosine kinase domains of VEGFR272. In a phase I trial using cediranib in children and adolescents with refractory solid tumours, one (33.3%) out of three ES patients achieved a PR with an overall 77% reduction in tumour size31. Other inhibitors targeting VEGF/VEGFR signaling, such as the anti-VEGF antibody bevacizumab, multi tyrosine kinase inhibitors (including VEGFR inhibitor) sorafenib and pazopanib were used in ongoing studies of ES (Table 3)32.
No validated biomarkers are available for selecting patients for anti-angiogenic treatment. One interesting study to date does, however, suggest that specific VEGF germline single nucleotide polymorphisms (VEGF-2578 AA and VEGF-1154 AA) were associated with superior median OS in breast cancer patients treated with a bevacizumab containing therapy, whereas other genotypes (VEGF-634 CC and VEGF-1498 TT) were associated with significantly fewer side effects such as hypertension73. Recent studies have suggested that among the VEGFRs, VEGFR1 is a kinase-defective receptor tyrosine kinase and negatively modulates angiogenesis by acting as a decoy receptor, whereas VEGFR2 is the major mediator that promotes downstream angiogenesis activity74. A study in patients with locally advanced rectal cancer showed that individuals with high concentrations of plasma VEGFR1 did not benefit as much from a bevacizumab-based therapy as patients with lower concentrations 75. In addition to VEGF polymorphisms and VEGFR1 levels, some VEGFR mutations also contribute to drug response. For example, a VEGFR1 Y1053D mutation was found to be associated with sorafenib resistance76. Taken together, VEGF polymorphism, VEGFR1 level and VEGFR somatic mutations can be further investigated as promising biomarkers for drug response in therapies targeting VEGF/VEGFR signaling.
EWS-FLI1 and related molecular targets
EWS-FLI1 is specifically expressed in ES cells and is theoretically considered an optimal drug target for ES (Figure 2) 77,78. EWS-FLI1 contains a DNA-binding domain at the C-terminus, which regulates DNA transcription activities79, serving as a transcriptional activator as well as contributing to down-regulation and up-regulation of multiple genes in different transcriptional machinery settings80. One microarray study reported that EWS-FLI1 upregulated 320 and downregulated 1,151 genes at a 95% confidence level81.
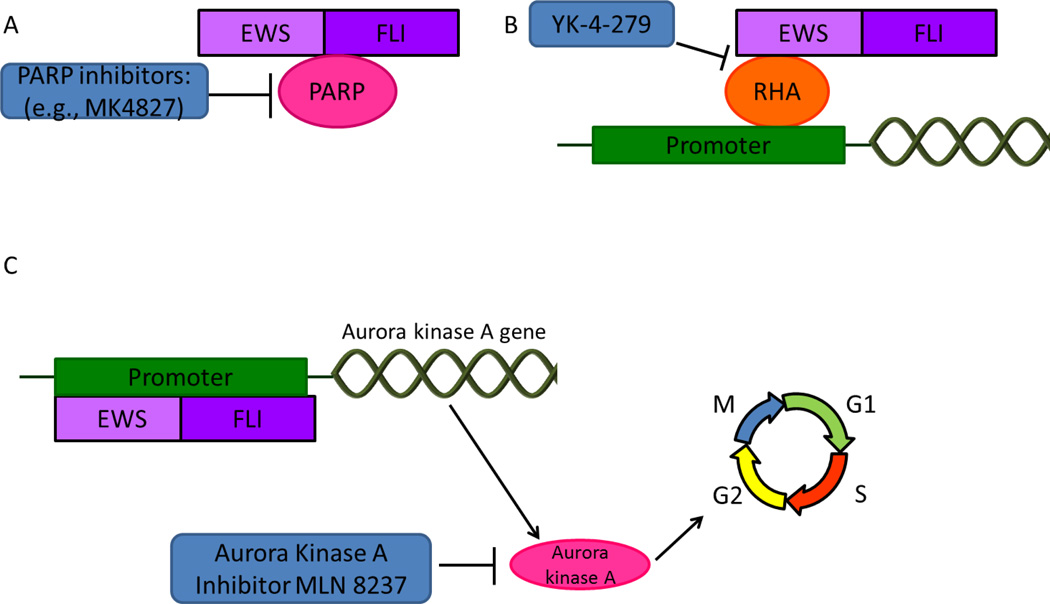
Figure 2.
Indirect targeting of EWS-FLI1. A) Interaction between EWS-FLI1 and PARP. PARP inhibitors are used to directly target EWS-FLI1. B) EWS-FLI1 regulates gene expression by binding to RHA, a transcription modulator. YK-4-279, interrupts of the binding of RHA to EWS-FLI1. C) EWS-FLI1 regulates transcription of aurora kinase A, which is a cell cycle regulator. Aurora kinase inhibitors are being used to indirectly target EWS-FLI1.
Theoretically, one could target EWS-FLI1 in several ways. The first is to interfere with the transcriptional modulators to which EWS-FLI1 binds. Second, the genes dysregulated by EWS-FLI1 expression might be targeted. Finally, the EWS-FLI1 protein itself may serve as a valid therapeutic target.
RHA
RNA helicase A (RHA) is a highly expressed transcription modulator in ES cell lines and patient samples82. Binding of EWS-FLI1 to RHA stimulates the transcription activity of EWS-FLI1 (Figure 2)82. A small molecule inhibitor of RHA, YK-4-279, has been shown to interrupt the binding of EWS-FLI1 to RHA, inducing apoptosis in ES cell lines 83. In vivo, a rat xenograft model treated with the active (S)-enantimer of YK-4-279 resulted in a sustained CR in two of six (33.3%) models 84. Other than the FLI1 ETS gene, YK-4-279 also inhibited ERG and ETV1 in ETS-expressing prostate cancer, likely through inhibiting RHA 85. Decreased tumour growth and metastasis inhibition was also observed in in vivo mouse xenografts of prostate cancer 86. Our experience using an oral formulation of YK-4-279 in mouse xenografts bearing ES explants demonstrated significant clinical activity and early phase clinical trials using YK-4-279 or a close analog are in the concept stage (personal communication).
Aurora kinase A
Aurora kinase A is a serine threonine kinase that associates with the spindle poles to regulate the entry for cell mitosis (Figure 2)87. A screening with 200 small molecule kinase inhibitors in two different ES cell lines as well as additional validation by RNA interference revealed that inhibition of aurora kinases A and B lead to specific vulnerability to ES cells 88. Furthermore, Wakahara et al. reported that EWS-FLI1 up-regulates levels of aurora kinase A and B by directly binding to their promoter regions89. Preclinical testing using an aurora kinase A inhibitor MLN8237 showed maintained CRs in pediatric cancer xenograft models including ES90. MLN8237 is currently being evaluated in an ongoing phase II trial sponsored by the Children’s Oncology Group for young patients with recurrent or refractory solid tumours or leukemia and results are expected soon (Table 3) 32.
EWS-FLI1 downstream signatures
Grohar et al. reported that trabectedin could reverse induced downstream targets of EWS-FLI1, and ES cells lines are more sensitive to the drug than other sarcoma types such as osteosarcoma, rhabdomyosarcoma, and others91. In addition, a high-throughput screen of compounds potentially capable of reversing consequences of downstream activation of EWS-FLI1 downstream activation and other preclinical studies led to identification of mithramycin, which is now being tested in clinical trials at the National Institute of Health (Table 3) 32,92.
Though targeting the downstream signatures of EWS-FLI1 may eventually prove to be effective, the shear number of downstream targets affected by EWS-FLI1 raises challenges of their own. As an example, cytarabine was recently identified from a drug library enriched for FDA-approved drugs as an agent able to reverse a EWS-FLI1 gene signature. While effective in vitro, the subsequent phase II human clinical trial was disappointing. Of ten ES patients enrolled, minimal activity and considerable hematologic toxicity were seen 93.
PRKCB
The protein kinase PKC-β (PRKCB) was shown to phosphorylate histone H3T6, which leads to increased cell survival in vitro and tumour growth in vivo in ES cell lines94. In addition, there is a strong overlap between genes modulated by the EWS-FLI1 fusion protein and PRKCB94. Inhibiting PRKCB may counteract gene transcription alteration caused by the EWS-FLI1 fusion protein. PRKCB could thus be a promising target for ES therapy.
PARP
Poly (ADP-ribose) polymerase (PARP) plays a role in repairing single-strand DNA breaks95. A recent study showed that PARP-1 interacts with ES fusion proteins EWS-FLI1 and EWS-ERG96. In ES cell lines expressing EWS-FLI1 or EWS-ERG, inhibition of PARP-1 leads to reduced DNA damage following lowered expression level of the fusion proteins96. In a screening of PARP inhibitor olaparib, ES cells demonstrated higher sensitivity compared to cells of other tumour types, including bone and soft tissue sarcoma97. In addition, olaparib in combination with temozolomide resulted in CR in a mouse xenograft model of ES96. Preclinical studies using ES cell lines showed that the combination of olaparib and radiation amplifies the DNA damage level caused by radiation therapy, synergistically increasing lethal DNA damage 98. In addition to the indirect targeting against ES fusion targets, a preclinical study also found that PARP inhibitors could reduce the viability of human cells depleted for cohesin complexes99. Three recently published comprehensive studies reported STAG2, the gene encoding one of the cohesin subunits SA2, as a secondary mutation in about 15–20% of the ES tumours100–102. Mutation in STAG2 can lead to the truncation of SA2, which causes the structural disruption of the cohesin complex, resulting in chromosomal instability and aneuploidy 103. Because STAG2 mutation is frequently observed in ES tumours, targeting the cohesin complex using PARP inhibitors may benefit this population of ES patients.
Phase I clinical trials using olaparib for recurrent/metastatic ES are being conducted (Table 3) 104.
Targeted immunotherapy
Targeted immunotherapy requires the use of antibodies to specifically identify tumour cells. Several molecules have been identified in ES as potential targets for immunotherapy.
Targeting EWS-FLI1 with vaccine therapy
The tumour-specific fusion protein EWS-FLI1 can be used as an optimal target in ES. However, a pilot vaccination study using peptides derived from the breakpoint region of the fusion proteins had minimal antitumor activity105. Preclinical data suggested that native peptides from the breakpoint region of EWS-FLI1 had a weak affinity to HLA-A2.1, resulting in poor stability of the peptide/MHC complex on cell surface and was thus unable to induce cytotoxic T-lymphocytes (CTL) that recognize and kill ES cells106. Another EWS-FLI1-modified peptide induced CTL and cell death in several ES in vitro and in vivo models 106.
Vaccine therapy might be a promising approach for ES treatment. However, the utility of such an approach needs to be further studied, and novel targets for cancer vaccines including EWS-FLI1 need to be explored.
CD99
Cluster of differentiation 99 (CD99) is a membrane protein expressed in most cases of ES 107. Because it is expressed specifically across the membranes of tumour cells, it can be investigated as an antigen for targeted immunotherapy. Studies have suggested that CD99 inhibits neural differentiation of ES cell lines through the MAPK pathway, contributing to cell proliferation and tumour growth108. A 64Cu-labeled anti-CD99 antibody was successfully used for targeted imaging in ES murine xenografts; however, the therapeutic utility remains unknown109.
GD2
Ganglioside antigen G (D2) (GD2) is found on the surface of many cancer cells including ES110–112. It is not widely expressed in normal cells, which makes it a possible target for immunotherapy113,114. GD2-related therapies have shown promising results in preclinical and clinical studies115,116. GD2-specific T cells demonstrated activity in ES xenografts115. Immunotherapy using a GD2 antibody combined with GM-CSF and IL-2 significantly improved PFS and OS in high-risk neuroblastoma patients116. Clinical trials using anti-GD2 antibodies or T cells expressing anti-GD2 chimeric antigen receptors are being conducted (Table 3).
TRAIL receptors
Tumour necrosis factor (TNF)-related apoptosis–inducing ligand (TRAIL) is a member of TNF super family and was shown to specifically induce apoptosis in tumour cells including ES but not in normal cells117,118. HGS-ETR2 is a TRAIL receptor 2 antibody that agonistically binds to TRAIL receptor 2 and induces apoptosis119. In phase I clinical trial using HGS-ETR2 to treat pediatric patients with solid tumour120, no CR or PR was achieved in any of the four patients with ES, although minor tumour shrinkage was observed. Further studies are warranted to explicate the efficacy of TRAIL receptor antibodies.
NY-ESO-1
The expression of cancer testis antigen NY-ESO-1 (also known as CTAG1) is limited to germ cells but is frequently identified in cancer cells. Individual cases of the NY-ESO-1 expression in ES have been reported121. A phase I clinical trial using the vaccine in combination with sirolimus is being conducted (Table 2). However, a phase I trial with the combination of decitabine and dendritic cell vaccine targeting cancer testis antigens MAGE-A1, MAGE-3 and NY-ESO-1 demonstrated no clinical benefits in two ES patients 122.
Other molecular targets
STAT3
Signal transducer and activator transcriptor 3 (STAT3) is a transcription factor, activated upon phosphorylation and essential in cell growth123,124. However, enhanced STAT3 phosphorylation may lead to tumorigenesis and it is observed in approximately 50% of ES samples125, but with an unknown underlying mechanism. Protein tyrosine phosphatase receptor type D (PTPRD) regulates STAT3 through dephosphorylating Y705. A PTPRD mutation W775 stop was identified in a patient with ES 126. This mutation results in a truncated PTPRD protein, causing accumulation of phosphorylated STAT3, which likely explains the enhanced level of activated STAT3 found in some of the ES samples125. A phase 0 trial has been reported using a STAT3 decoy agent, which is an oligonucleotide that binds specifically to STAT3 and inhibits its downstream transcription regulation127. Two (20%) out of the ten tumour xenograft models of head and neck cancer treated with the STAT3 decoy achieved a CR 127. Because STAT3 phosphorylation is frequently observed in ES, it is reasonable to target STAT3 as a novel therapy. Another study has suggested that recruitment of STAT3 to IGF-1R was required for STAT3 phosphorylation123. Therefore, in addition to directly targeting STAT3, an IGF-1R inhibitor might also be used to downregulate STAT3 phosphorylation.
MEK
The GTPase KRAS and NRAS are upstream regulators of the MEK/ERK pathway. Enhanced GTPase function may lead to oncogenic stimulation 128. There is anecdotal evidence from early phase clinical trials in ESFT that resistance to IGF1R and mTOR targeting therapies can be mediated through KRAS mutation and MAPK pathway activation129. In addition, an NRAS mutation, which activates the MAPK pathway, has been anecdotally reported in patients with ES and the biological implication remains unclear130. MEK inhibitors are being investigated as a means to overcome the deleterious effects of MAPK activation.
Challenges of Targeted therapies
Biologically targeted therapies have shown promise in some patients with advanced ES and some drug combinations—notably IGF-1R antibodies with mTOR inhibitors—may offer significant synergy. However, no specific therapies for this patient population have yet been approved by the US Food and Drug Administration, which is likely the result of disinterested pharmaceutical companies because of the relative rarity of ES. Targeted therapies for ES face several challenges.
The first challenge stems from the disconnection between preclinical studies and human clinical trial results. Frequently, clinical trials showed low efficacy despite promising results in preclinical trials. Several factors are behind this. First, the origin of ES is not precisely known, which adds difficulty in understanding the transformation from normal cells to tumour 131. Second, it is difficult to build in vitro and animal models for preclinical testing. Two-dimensional monolayer cells have been used predominantly in in vitro studies while several studies have shown phenotype and drug sensitivity changes in three-dimensional cultures using the same cell line132. In addition, there are no spontaneous ES animal models and genetically-engineered ones fail to result in ES-like tumours, limiting the predictive value of preclinical animal studies133. Third, most of the tested targetable proteins play a role in initial tumor growth and hence their inhibition may be clinically helpful in early disease. However, clinical trials are usually performed on advanced stage tumours, in which targeting these proteins may not be sufficient to inhibit the tumorigenesis process. Last but not least, potential and attractive targets, such as AKT, still do not have a clinically useful and stable inhibitor.
The second challenge is to identify biomarkers that accurately forecast treatment outcome 134. Our institutional experience from several advanced cancers suggests that therapies matching underlying actionable somatic mutations can improve outcomes compared to unmatched therapies135. Unfortunately, this strategy has not yet been successfully adapted for the treatment of ES. Recently published studies utilizing next-generation sequencing technologies have shown that significant fraction of ES patients have recurrent genetic mutations other than EWSR1-ETS fusion gene product, particularly STAG2 mutations, which may lead to chromosomal structural defect and aneuploidy 100–102. Crompton et al. suggested that relapsed disease is genetically different from disease at diagnosis, which increases the genomic complexity of the disease101.
The third challenge is to understand the mechanisms of drug resistance. There are several different mechanisms of ES tumour to develop drug resistance. First, cancer stem cells are capable of proliferate and generate tumor cells with new sets of mutations which may harbor different protein targets 136. Second, drug resistance may rise from altered modulation of related cellular signalling pathway as a result of targeted therapies. For example, anti-IGF-1R therapies may lead to activation of downstream pathways and thus result in tumour drug resistance through a bypass pathway26,57. In order to improve long term treatment outcomes, resistance mechanisms need to be elucidated. This may require serial blood and tumor tissue collections for systematic molecular and other correlative studies.
Conclusion
Targeted therapies for ES have shown promising results in a small subset of patients with advanced disease. However, disconnection between preclinical studies and clinical trials, identification of outcome-predicting biomarkers, and understanding drug resistance mechanisms remain challenging. Due to the rarity and complexity of ES, a multi-institutional global collaboration is warranted in better understanding the genomic/proteomic landscape of ES and development of new, targeted therapies.
Highlights.
The prognosis of patients with metastatic Ewing sarcoma remains dismal and 5-year survival usually does not exceed 30%.
Targeting IGF1-R was found effective in preclinical models and small subsets of patients with advanced Ewing sarcoma.
Other targeted therapies such as therapies against EWS-FLI1-related targets are in clinical development.
Biomarkers predicting efficacy of novel targeted therapies remain to be identified.
Acknowledgements
We thank Ms. Joann Aaron for scientific review and editing of this article. This work was supported by NCATS grant UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support grant (P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Burchill SA. Molecular abnormalities in Ewing's sarcoma. Expert Rev Anticancer Ther. 2008;8:1675–1687. doi: 10.1586/14737140.8.10.1675. [DOI] [PubMed] [Google Scholar]
- 2.Zucman J, et al. Cloning and characterization of the Ewing's sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes, chromosomes & cancer. 1992;5:271–277. doi: 10.1002/gcc.2870050402. [DOI] [PubMed] [Google Scholar]
- 3.de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:204–213. doi: 10.1200/JCO.2000.18.1.204. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen PH, et al. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994;6:146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- 5.Jeon IS, et al. A variant Ewing's sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene. 1995;10:1229–1234. [PubMed] [Google Scholar]
- 6.Ishida S, et al. The genomic breakpoint and chimeric transcripts in the EWSR1-ETV4/E1AF gene fusion in Ewing sarcoma. Cytogenetics and cell genetics. 1998;82:278–283. doi: 10.1159/000015119. [DOI] [PubMed] [Google Scholar]
- 7.Peter M, et al. A new member of the ETS family fused to EWS in Ewing tumors. Oncogene. 1997;14:1159–1164. doi: 10.1038/sj.onc.1200933. [DOI] [PubMed] [Google Scholar]
- 8.Shing DC, et al. FUS/ERG gene fusions in Ewing's tumors. Cancer research. 2003;63:4568–4576. [PubMed] [Google Scholar]
- 9.Ng TL, et al. Ewing sarcoma with novel translocation t(2;16) producing an in-frame fusion of FUS and FEV. The Journal of molecular diagnostics : JMD. 2007;9:459–463. doi: 10.2353/jmoldx.2007.070009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura Y, et al. The EWS-ATF-1 gene involved in malignant melanoma of soft parts with t(12;22) chromosome translocation, encodes a constitutive transcriptional activator. Oncogene. 1996;12:159–167. [PubMed] [Google Scholar]
- 11.Clark J, et al. Fusion of the EWS gene to CHN, a member of the steroid/thyroid receptor gene superfamily, in a human myxoid chondrosarcoma. Oncogene. 1996;12:229–235. [PubMed] [Google Scholar]
- 12.Bode-Lesniewska B, et al. Relevance of translocation type in myxoid liposarcoma and identification of a novel EWSR1-DDIT3 fusion. Genes, chromosomes & cancer. 2007;46:961–971. doi: 10.1002/gcc.20478. [DOI] [PubMed] [Google Scholar]
- 13.Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing's family tumors. Oncogene. 2001;20:5747–5754. doi: 10.1038/sj.onc.1204598. [DOI] [PubMed] [Google Scholar]
- 14.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 15.Subbiah V, et al. Ewing's sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–140. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 16.Cotterill SJ, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 17.Kurzrock R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 18.Pappo AS, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolcher AW, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 20.Tap WD, et al. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1849–1856. doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 21.Olmos D, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. The lancet oncology. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juergens H, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malempati S, et al. Phase I/II Trial and Pharmacokinetic Study of Cixutumumab in Pediatric Patients With Refractory Solid Tumors and Ewing Sarcoma: A Report From the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mita MM, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 25.Bagatell R, et al. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: A children's oncology group study. Pediatric blood & cancer. 2014;61:833–839. doi: 10.1002/pbc.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naing A, et al. Insulin Growth Factor-Receptor (IGF-1R) Antibody Cixutumumab Combined with the mTOR Inhibitor Temsirolimus in Patients with Refractory Ewing's Sarcoma Family Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GK, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. The lancet oncology. 2013;14:371–382. doi: 10.1016/S1470-2045(13)70049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bond M, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2008;50:254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 29.Chao J, et al. Phase II clinical trial of imatinib mesylate in therapy of KIT and/or PDGFRalpha-expressing Ewing sarcoma family of tumors and desmoplastic small round cell tumors. Anticancer Res. 2010;30:547–552. [PubMed] [Google Scholar]
- 30.Daw NC, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a Children's Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- 31.Fox E, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health", U.S.N.I.o. clinicaltrials.gov. 2014;2014 [Google Scholar]
- 33.Garofalo C, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–2740. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 34.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 36.Scotlandi K, et al. Insulin-like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res. 1996;56:4570–4574. [PubMed] [Google Scholar]
- 37.Kang HG, et al. Inhibition of the insulin-like growth factor I receptor by epigallocatechin gallate blocks proliferation and induces the death of Ewing tumor cells. Molecular cancer therapeutics. 2010;9:1396–1407. doi: 10.1158/1535-7163.MCT-09-0604. [DOI] [PubMed] [Google Scholar]
- 38.Manara MC, et al. Preclinical in vivo study of new insulin-like growth factor-I receptor--specific inhibitor in Ewing's sarcoma. Clin Cancer Res. 2007;13:1322–1330. doi: 10.1158/1078-0432.CCR-06-1518. [DOI] [PubMed] [Google Scholar]
- 39.Scotlandi K, et al. Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing's sarcoma in athymic mice. Cancer Res. 1998;58:4127–4131. [PubMed] [Google Scholar]
- 40.Beltran PJ, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Molecular cancer therapeutics. 2009;8:1095–1105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 41.Rowinsky EK, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 42.Cohen BD, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 43.Mulvihill MJ, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future medicinal chemistry. 2009;1:1153–1171. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- 44.Macaulay VM, Middleton MR, Eckhardt SG, Juergens RA, Stephens AW, Poondru S, McCarthy SP, Gadgeel SM. Phase I study of OSI-906, dual tyrosine kinase inhibitor of insulinlike growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in combination with erlotinib (E) in patients with advanced solid tumors. ASCO. 2010;28:15s. J Clin Oncol. [Google Scholar]
- 45.Kolb EA, et al. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;56:595–603. doi: 10.1002/pbc.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins AS, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:3532–3540. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 47.Rousselot P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:424–430. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]
- 48.Garofalo C, et al. Identification of common and distinctive mechanisms of resistance to different anti-IGF-IR agents in Ewing's sarcoma. Mol Endocrinol. 2012;26:1603–1616. doi: 10.1210/me.2012-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janku F. Bringing target-matched PI3King from the bench to the clinic. Cell cycle. 2013;12:1817–1818. doi: 10.4161/cc.25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 51.Scotlandi K, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer research. 2005;65:3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 52.Wagner LM, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatric blood & cancer. 2007;48:132–139. doi: 10.1002/pbc.20697. [DOI] [PubMed] [Google Scholar]
- 53.Demetri GD, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 54.Dancey J. mTOR signaling and drug development in cancer. Nature reviews. Clinical oncology. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 55.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R–dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 56.Kurmasheva RT, et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer research. 2009;69:7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbiah V, et al. Targeted morphoproteomic profiling of Ewing's sarcoma treated with insulinlike growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PloS one. 2011;6:e18424. doi: 10.1371/journal.pone.0018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nature reviews. Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 59.Scotlandi K, et al. C-kit receptor expression in Ewing's sarcoma: lack of prognostic value but therapeutic targeting opportunities in appropriate conditions. J Clin Oncol. 2003;21:1952–1960. doi: 10.1200/JCO.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 60.Bozzi F, et al. Evidence for activation of KIT, PDGFRalpha, and PDGFRbeta receptors in the Ewing sarcoma family of tumors. Cancer. 2007;109:1638–1645. doi: 10.1002/cncr.22587. [DOI] [PubMed] [Google Scholar]
- 61.Heinrich MC, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- 62.Buchdunger E, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–145. [PubMed] [Google Scholar]
- 63.Steelman LS, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baselga J. Targeting the epidermal growth factor receptor with tyrosine kinase inhibitors: small molecules, big hopes. J Clin Oncol. 2002;20:2217–2219. doi: 10.1200/JCO.2002.20.9.2217. [DOI] [PubMed] [Google Scholar]
- 65.Wakeling AE, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 66.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 67.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 68.Pao W, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jimeno A, et al. Analysis of biologic surrogate markers from a Children's Oncology Group Phase I trial of gefitinib in pediatric patients with solid tumors. Pediatr Blood Cancer. 2007;49:352–357. doi: 10.1002/pbc.20753. [DOI] [PubMed] [Google Scholar]
- 70.Cappuzzo F, et al. Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:1133–1141. doi: 10.1093/jnci/djh217. [DOI] [PubMed] [Google Scholar]
- 71.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer research. 2012;72:1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wedge SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 73.Schneider BP, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer RD, Mohammadi M, Rahimi N. A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. The Journal of biological chemistry. 2006;281:867–875. doi: 10.1074/jbc.M506454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duda DG, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. The oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Bubnoff N, et al. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlapping profile of resistance mutations in vitro. Cancer research. 2009;69:3032–3041. doi: 10.1158/0008-5472.CAN-08-2923. [DOI] [PubMed] [Google Scholar]
- 77.Bonin G, Scamps C, Turc-Carel C, Lipinski M. Chimeric EWS-FLI1 transcript in a Ewing cell line with a complex t(11;22;14) translocation. Cancer research. 1993;53:3655–3657. [PubMed] [Google Scholar]
- 78.May WA, et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magnaghi-Jaulin L, Masutani H, Robin P, Lipinski M, Harel-Bellan A. SRE elements are binding sites for the fusion protein EWS-FLI-1. Nucleic Acids Res. 1996;24:1052–1058. doi: 10.1093/nar/24.6.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siligan C, et al. EWS-FLI1 target genes recovered from Ewing's sarcoma chromatin. Oncogene. 2005;24:2512–2524. doi: 10.1038/sj.onc.1208455. [DOI] [PubMed] [Google Scholar]
- 81.Smith R, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's sarcoma. Cancer cell. 2006;9:405–416. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Toretsky JA, et al. Oncoprotein EWS-FLI1 activity is enhanced by RNA helicase A. Cancer Res. 2006;66:5574–5581. doi: 10.1158/0008-5472.CAN-05-3293. [DOI] [PubMed] [Google Scholar]
- 83.Barber-Rotenberg JS, et al. Single Enantiomer of YK-4-279 Demonstrates Specificity in Targeting the Oncogene EWS-FLI1. Oncotarget. 2012;3:172–182. doi: 10.18632/oncotarget.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hong SH, et al. Pharmacokinetic modeling optimizes inhibition of the ‘undruggable' EWS-FLI1 transcription factor in Ewing Sarcoma. Oncotarget. 2013 doi: 10.18632/oncotarget.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahim S, et al. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PloS one. 2011;6:e19343. doi: 10.1371/journal.pone.0019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahim S, Justvig S, Hong S, Kong Y, Brown ML, Morrissey C, Toretsky JA, Uren A. YK-4-279 inhibits ETS-positive prostate cancer cell metastasis in a mouse xenograft model. AACR. 2013;73 Cancer Research. [Google Scholar]
- 87.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Current opinion in cell biology. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winter GE, et al. An integrated chemical biology approach identifies specific vulnerability of Ewing's sarcoma to combined inhibition of Aurora kinases A and B. Molecular cancer therapeutics. 2011;10:1846–1856. doi: 10.1158/1535-7163.MCT-11-0100. [DOI] [PubMed] [Google Scholar]
- 89.Wakahara K, et al. EWS-Fli1 up-regulates expression of the Aurora A and Aurora B kinases. Molecular cancer research : MCR. 2008;6:1937–1945. doi: 10.1158/1541-7786.MCR-08-0054. [DOI] [PubMed] [Google Scholar]
- 90.Maris JM, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grohar PJ, et al. Ecteinascidin 743 interferes with the activity of EWS-FLI1 in Ewing sarcoma cells. Neoplasia. 2011;13:145–153. doi: 10.1593/neo.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grohar PJ, et al. Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. Journal of the National Cancer Institute. 2011;103:962–978. doi: 10.1093/jnci/djr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DuBois SG, et al. Phase II study of intermediate-dose cytarabine in patients with relapsed or refractory Ewing sarcoma: a report from the Children's Oncology Group. Pediatric blood & cancer. 2009;52:324–327. doi: 10.1002/pbc.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Surdez D, et al. Targeting the EWSR1-FLI1 oncogene-induced protein kinase PKC-beta abolishes ewing sarcoma growth. Cancer research. 2012;72:4494–4503. doi: 10.1158/0008-5472.CAN-12-0371. [DOI] [PubMed] [Google Scholar]
- 95.Molinete M, et al. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. The EMBO journal. 1993;12:2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brenner JC, et al. PARP-1 inhibition as a targeted strategy to treat Ewing's sarcoma. Cancer Res. 2012;72:1608–1613. doi: 10.1158/0008-5472.CAN-11-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee HJ, et al. Combining PARP-1 inhibition and radiation in ewing sarcoma results in lethal DNA damage. Molecular cancer therapeutics. 2013;12:2591–2600. doi: 10.1158/1535-7163.MCT-13-0338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.McLellan JL, et al. Synthetic lethality of cohesins with PARPs and replication fork mediators. PLoS genetics. 2012;8:e1002574. doi: 10.1371/journal.pgen.1002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brohl AS, et al. The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS genetics. 2014;10:e1004475. doi: 10.1371/journal.pgen.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crompton BD, et al. The Genomic Landscape of Pediatric Ewing Sarcoma. Cancer discovery. 2014 doi: 10.1158/2159-8290.CD-13-1037. [DOI] [PubMed] [Google Scholar]
- 102.Tirode F, et al. Genomic Landscape of Ewing Sarcoma Defines an Aggressive Subtype with Co-Association of STAG2 and TP53 Mutations. Cancer discovery. 2014 doi: 10.1158/2159-8290.CD-14-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annual review of genetics. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 104.Health, U.S.N.I.o. 2013. [Google Scholar]
- 105.Dagher R, et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Medical and pediatric oncology. 2002;38:158–164. doi: 10.1002/mpo.1303. [DOI] [PubMed] [Google Scholar]
- 106.Evans CH, et al. EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5341–5351. doi: 10.1158/1078-0432.CCR-12-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovar H, et al. Overexpression of the pseudoautosomal gene MIC2 in Ewing's sarcoma and peripheral primitive neuroectodermal tumor. Oncogene. 1990;5:1067–1070. [PubMed] [Google Scholar]
- 108.Rocchi A, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J Clin Invest. 2010;120:668–680. doi: 10.1172/JCI36667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Neill AF, et al. Targeted Imaging of Ewing Sarcoma in Preclinical Models Using a 64Cu-Labeled Anti-CD99 Antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:678–687. doi: 10.1158/1078-0432.CCR-13-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheung NK, et al. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer research. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 111.Lipinski M, et al. Phenotypic characterization of Ewing sarcoma cell lines with monoclonal antibodies. Journal of cellular biochemistry. 1986;31:289–296. doi: 10.1002/jcb.240310406. [DOI] [PubMed] [Google Scholar]
- 112.Chang HR, Cordon-Cardo C, Houghton AN, Cheung NK, Brennan MF. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer. 1992;70:633–638. doi: 10.1002/1097-0142(19920801)70:3<633::aid-cncr2820700315>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 113.Lammie G, Cheung N, Gerald W, Rosenblum M, Cordoncardo C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas - an immunohistochemical study. International journal of oncology. 1993;3:909–915. doi: 10.3892/ijo.3.5.909. [DOI] [PubMed] [Google Scholar]
- 114.Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS letters. 2014;588:288–297. doi: 10.1016/j.febslet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 115.Kailayangiri S, et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. British journal of cancer. 2012;106:1123–1133. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. The New England journal of medicine. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 118.Picarda G, et al. Preclinical evidence that use of TRAIL in Ewing's sarcoma and osteosarcoma therapy inhibits tumor growth, prevents osteolysis, and increases animal survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2363–2374. doi: 10.1158/1078-0432.CCR-09-1779. [DOI] [PubMed] [Google Scholar]
- 119.Marini P, et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145–5154. doi: 10.1038/sj.onc.1209516. [DOI] [PubMed] [Google Scholar]
- 120.Merchant MS, et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4141–4147. doi: 10.1200/JCO.2012.44.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai JP, et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:854–858. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.George R, Krishnadas DK, Bai F, Diller R, Shusterman S, Sullivan JE, Lucas KG. Phase 1 trial of decitabine and CT antigen-specific vaccine in relapsed pediatric solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 32:5s. 2014 [Google Scholar]
- 123.Zhang W, et al. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Molecular and cellular biology. 2006;26:413–424. doi: 10.1128/MCB.26.2.413-424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ruggiero RA, et al. Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer research. 2012;72:1043–1050. doi: 10.1158/0008-5472.CAN-11-2964. [DOI] [PubMed] [Google Scholar]
- 125.Lai R, et al. STAT3 is activated in a subset of the Ewing sarcoma family of tumours. J Pathol. 2006;208:624–632. doi: 10.1002/path.1941. [DOI] [PubMed] [Google Scholar]
- 126.Jiang Y, et al. Germline PTPRD mutations in Ewing sarcoma: biologic and clinical implications. Oncotarget. 2013;4:884–889. doi: 10.18632/oncotarget.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sen M, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zuber J, et al. A genome-wide survey of RAS transformation targets. Nat Genet. 2000;24:144–152. doi: 10.1038/72799. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Y, et al. Novel secondary somatic mutations in Ewing's sarcoma and desmoplastic small round cell tumors. PloS one. 2014;9:e93676. doi: 10.1371/journal.pone.0093676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shukla N, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res. 2012;18:748–757. doi: 10.1158/1078-0432.CCR-11-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ordonez JL, Osuna D, Herrero D, de Alava E, Madoz-Gurpide J. Advances in Ewing's sarcoma research: where are we now and what lies ahead? Cancer research. 2009;69:7140–7150. doi: 10.1158/0008-5472.CAN-08-4041. [DOI] [PubMed] [Google Scholar]
- 132.Fong EL, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monument MJ, Bernthal NM, Randall RL. Salient features of mesenchymal stem cells-implications for Ewing sarcoma modeling. Frontiers in oncology. 2013;3:24. doi: 10.3389/fonc.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nature reviews Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 135.Tsimberidou AM, et al. Personalized Medicine for Patients with Advanced Cancer in the Phase I Program at MD Anderson: Validation and Landmark Analyses. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]