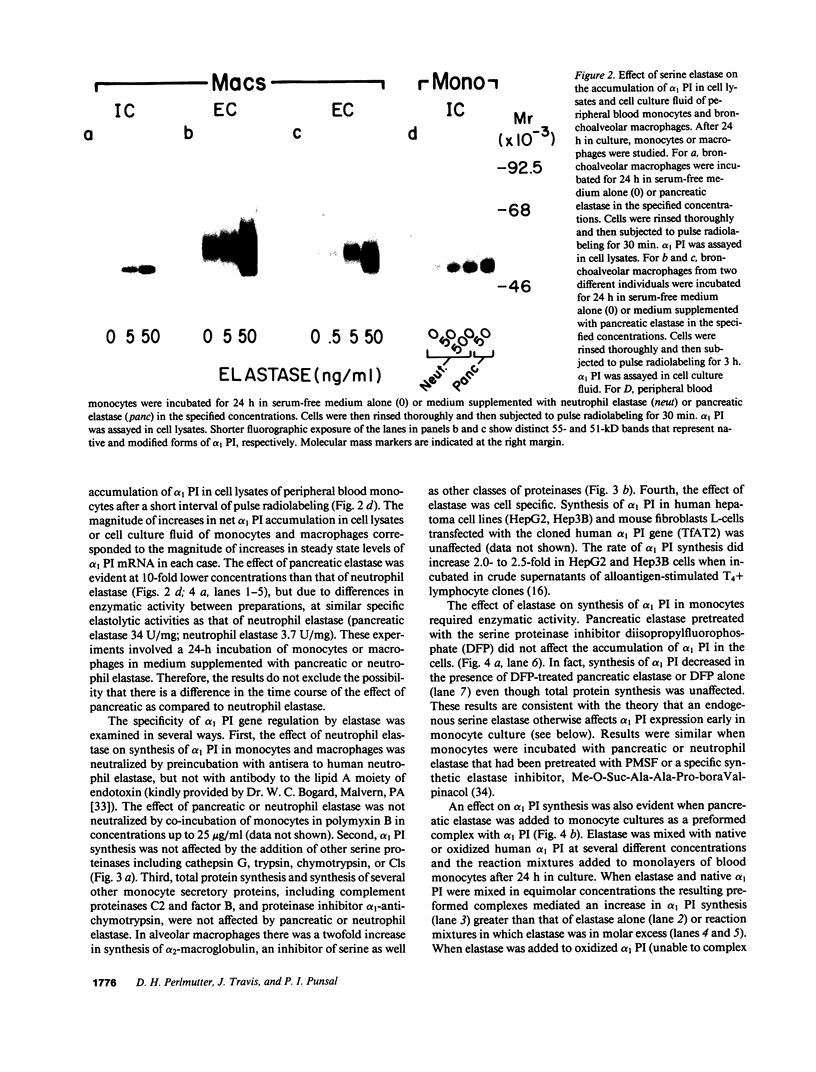
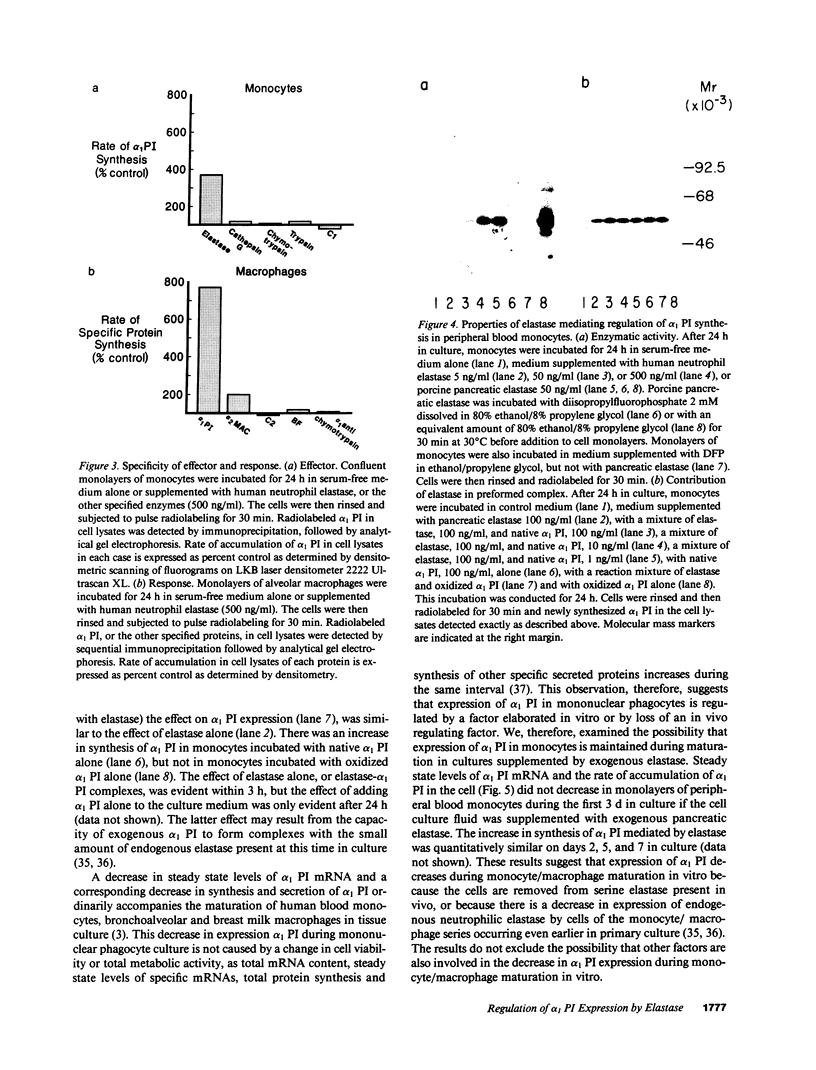
Abstract
The net balance of neutrophil elastase, an enzyme that degrades many components of the extracellular matrix, and its inhibitor, alpha-1-proteinase inhibitor (alpha 1 PI), is thought to be a critical determinant in the development of destructive lung disease, especially in individuals with homozygous alpha 1 PI deficiency. Synthesis and secretion of alpha 1 PI has been recently demonstrated in cells of mononuclear phagocyte lineage, including peripheral blood monocytes and tissue macrophages. In this study we show that alpha 1 PI gene expression in human monocytes and bronchoalveolar macrophages is affected by a novel mechanism, whereby elastase directly regulates the synthesis of its inhibitor. In nanomolar concentrations, neutrophil or pancreatic elastase mediates a dose- and time-dependent increase in steady state levels of alpha 1 PI mRNA and in the rate of synthesis of alpha 1 PI in human monocytes and bronchoalveolar macrophages. Antisera to neutrophil elastase or pretreatment of elastase with the serine proteinase inhibitor diisopropylfluorophosphate abrogates the effect of elastase on alpha 1 PI expression. Elastase also stimulates the synthesis of alpha 1 PI in monocytes from homozygous PiZZ alpha 1 PI-deficient individuals, but has no effect on the rate of secretion; hence, the enzyme mediates an effect on alpha 1 PI that increases the intracellular accumulation of inhibitor and exaggerates the intrinsic defect in secretion of alpha 1 PI that characterizes the homozygous PiZZ alpha 1 PI deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Clark E. J., Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980 Dec 1;152(6):1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey-Morel C., Pierce J. A., Campbell E. J., Perlmutter D. H. Lipopolysaccharide modulates the expression of alpha 1 proteinase inhibitor and other serine proteinase inhibitors in human monocytes and macrophages. J Exp Med. 1987 Oct 1;166(4):1041–1054. doi: 10.1084/jem.166.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Beatty K., Robertie P., Senior R. M., Travis J. Determination of oxidized alpha-1-proteinase inhibitor in serum. J Lab Clin Med. 1982 Aug;100(2):186–192. [PubMed] [Google Scholar]
- Bensa J. C., Reboul A., Colomb M. G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983 Nov 15;216(2):385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing D. H., Andrews J. M., Morris K. M., Cole E., Irish V. Purification of subcomponents Clq, Cl(-)r and Cl(-)s of the first component of complement from Cohn Fraction I by affinity chromatography. Prep Biochem. 1980;10(3):269–296. doi: 10.1080/10826068009412829. [DOI] [PubMed] [Google Scholar]
- Carrell R. W. alpha 1-Antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986 Dec;78(6):1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Cole F. S., Auerbach H. S., Goldberger G., Colten H. R. Tissue-specific pretranslational regulation of complement production in human mononuclear phagocytes. J Immunol. 1985 Apr;134(4):2610–2616. [PubMed] [Google Scholar]
- Cole F. S., Matthews W. J., Jr, Rossing T. H., Gash D. J., Lichtenberg N. A., Pennington J. E. Complement biosynthesis by human bronchoalveolar macrophages. Clin Immunol Immunopathol. 1983 May;27(2):153–159. doi: 10.1016/0090-1229(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson I., Alper C. A. Changes in serum proteinase inhibitor levels following bone surgery. Clin Chim Acta. 1974 Aug 20;54(3):381–385. doi: 10.1016/0009-8981(74)90257-5. [DOI] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R. C., Judah J. D., Colman A. Xenopus oocytes can synthesise but do not secrete the Z variant of human alpha 1-antitrypsin. FEBS Lett. 1984 Mar 12;168(1):84–88. doi: 10.1016/0014-5793(84)80211-2. [DOI] [PubMed] [Google Scholar]
- Garver R. I., Jr, Mornex J. F., Nukiwa T., Brantly M., Courtney M., LeCocq J. P., Crystal R. G. Alpha 1-antitrypsin deficiency and emphysema caused by homozygous inheritance of non-expressing alpha 1-antitrypsin genes. N Engl J Med. 1986 Mar 20;314(12):762–766. doi: 10.1056/NEJM198603203141207. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):165–169. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves C. B., Munns T. W., Carlisle T. L., Grant G. A., Strauss A. W. Induction of prothrombin synthesis by prothrombin fragments. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4772–4776. doi: 10.1073/pnas.78.8.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves C. B., Munns T. W., Willingham A. K., Strauss A. W. Rat factor X is synthesized as a single chain precursor inducible by prothrombin fragments. J Biol Chem. 1982 Nov 10;257(21):13108–13113. [PubMed] [Google Scholar]
- Hovi T., Mosher D., Vaheri A. Cultured human monocytes synthesize and secrete alpha2-macroglobulin. J Exp Med. 1977 Jun 1;145(6):1580–1589. doi: 10.1084/jem.145.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Barrett A. J., Mason R. W. Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region. J Biol Chem. 1986 Nov 5;261(31):14748–14751. [PubMed] [Google Scholar]
- Johnson D. A., Travis J. Human alpha-1-proteinase inhibitor mechanism of action: evidence for activation by limited proteolysis. Biochem Biophys Res Commun. 1976 Sep 7;72(1):33–39. doi: 10.1016/0006-291x(76)90956-6. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Kettner C. A., Shenvi A. B. Inhibition of the serine proteases leukocyte elastase, pancreatic elastase, cathepsin G, and chymotrypsin by peptide boronic acids. J Biol Chem. 1984 Dec 25;259(24):15106–15114. [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Larsson C., Dirksen H., Sundström G., Eriksson S. Lung function studies in asymptomatic individuals with moderately (Pi SZ) and severely (Pi Z) reduced levels of alpha1-antitrypsin. Scand J Respir Dis. 1976;57(6):267–280. [PubMed] [Google Scholar]
- Morii M., Odani S., Ikenaka T. Characterization of a peptide released during the reaction of human alpha 1-antitrypsin and bovine alpha-chymotrypsin. J Biochem. 1979 Oct;86(4):915–921. doi: 10.1093/oxfordjournals.jbchem.a132623. [DOI] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Owen M. C., Carrell R. W. alpha-1-Antitrypsin: sequence of the Z variant tryptic peptide. FEBS Lett. 1977 Jul 15;79(2):245–247. doi: 10.1016/0014-5793(77)80796-5. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Goldberger G., Colten H. R. Distinct primary translation products from human liver mRNA give rise to secreted and cell-associated forms of complement protein C2. J Biol Chem. 1984 Aug 25;259(16):10380–10385. [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Colten H. R., Grossberger D., Strominger J., Seidman J. G., Chaplin D. D. Expression of complement proteins C2 and factor B in transfected L cells. J Clin Invest. 1985 Oct;76(4):1449–1454. doi: 10.1172/JCI112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E. A fast-acting elastase inhibitor in human monocytes. J Exp Med. 1985 Dec 1;162(6):2142–2155. doi: 10.1084/jem.162.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. G., Levy B. A., Adams M. A., Fuller G. M. Regulation of fibrinogen synthesis by plasmin-derived fragments of fibrinogen and fibrin: an indirect feedback pathway. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1530–1534. doi: 10.1073/pnas.79.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Sveger T. Prospective study of children with alpha 1-antitrypsin deficiency: eight-year-old follow-up. J Pediatr. 1984 Jan;104(1):91–94. doi: 10.1016/s0022-3476(84)80599-5. [DOI] [PubMed] [Google Scholar]
- Takemura S., Rossing T. H., Perlmutter D. H. A lymphokine regulates expression of alpha-1-proteinase inhibitor in human monocytes and macrophages. J Clin Invest. 1986 Apr;77(4):1207–1213. doi: 10.1172/JCI112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Bowen J., Baugh R. Human alpha-1-antichymotrypsin: interaction with chymotrypsin-like proteinases. Biochemistry. 1978 Dec 26;17(26):5651–5656. doi: 10.1021/bi00619a011. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Bar-Shavit Z., Senior R. M., Teitelbaum S. L. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest. 1985 Jul;76(1):219–224. doi: 10.1172/JCI111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]