Abstract
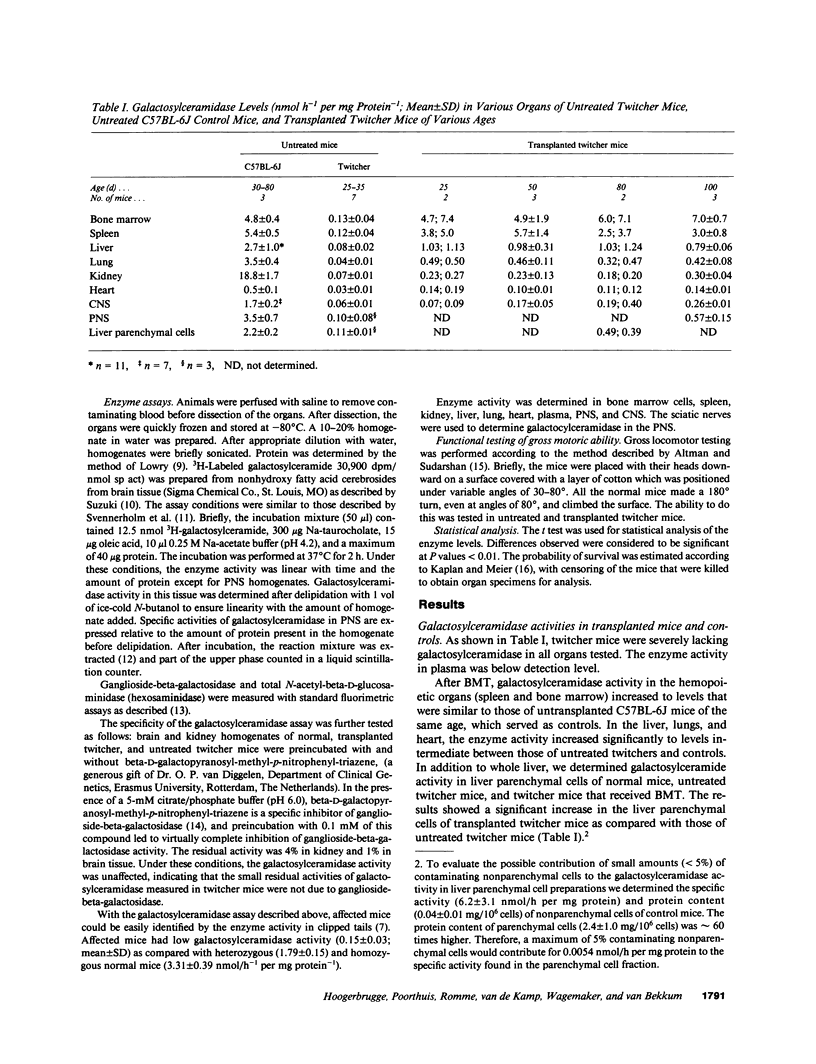
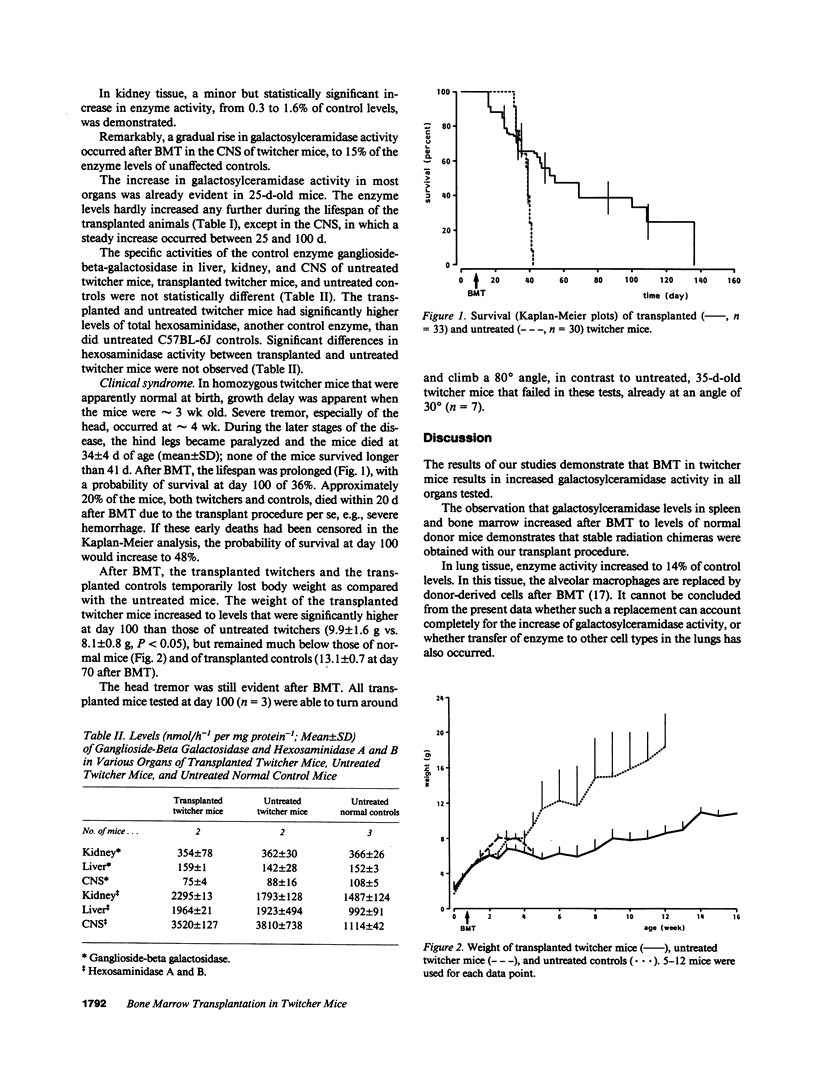
The effect of allogeneic bone marrow transplantation (BMT) was investigated in the neurologically affected twitcher mouse, a model for human Krabbe's disease. Twitcher mice have a hereditary deficiency of the lysosomal enzyme galactosylceramidase, which causes growth delay, tremor, and paralysis of the hind legs. Death occurs at 30-40 d of age. After BMT galactosylceramidase activity increased to donor levels in hemopoietic organs. In lung, heart, and liver, galactosylceramidase activity rose to levels intermediate between those of twitcher and normal mice. Increased galactosylceramidase activity in liver parenchymal cells indicated uptake of the donor enzyme by recipient cells of nonhemopoietic origin. Enzyme activity also increased in kidney tissue. BMT resulted in a gradual increase in galactosylceramidase activity in the central nervous system to 15% of normal donor levels. A 5-6-fold increase in galactosylceramidase activity was found in the peripheral nervous system. This increase in enzyme activity was accompanied by a partial alleviation of neurological symptoms. In particular, paralysis of the hind legs was prevented by BMT. BMT led to a modest restoration of growth and prolonged survival. In several cases, the mice survived for more than 100 d, but eventually all animals died with severe neurological disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers C. E., Beckstead J. H. Monocyte/macrophage derived cells in normal and transplanted human kidneys. Clin Immunol Immunopathol. 1985 Aug;36(2):129–140. doi: 10.1016/0090-1229(85)90114-x. [DOI] [PubMed] [Google Scholar]
- Altman J., Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975 Nov;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F. Pluripotential hemopoietic stem cells in adult mouse brain. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2722–2725. doi: 10.1073/pnas.79.8.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayever E., Ladisch S., Philippart M., Brill N., Nuwer M., Sparkes R. S., Feig S. A. Bone-marrow transplantation for metachromatic leucodystrophy. Lancet. 1985 Aug 31;2(8453):471–473. doi: 10.1016/s0140-6736(85)90402-7. [DOI] [PubMed] [Google Scholar]
- Costantino-Ceccarini E., Morell P. Biosynthesis of brain sphingolipids and myelin accumulation in the mouse. Lipids. 1972 Oct;7(10):656–659. doi: 10.1007/BF02533072. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Eicher E. M., Jacobs J. M., Scaravilli F., Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980 Sep;103(3):695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Fujita S., Kitamura T. Origin of brain macrophages and the nature of the so-called microglia. Acta Neuropathol Suppl. 1975;Suppl 6:291–296. doi: 10.1007/978-3-662-08456-4_51. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R., Hugh-Jones K., Barrett A. J., Byrom N., Chambers D., Henry K., James D. C., Lucas C. F., Rogers T. R., Benson P. F. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet. 1981 Oct 3;2(8249):709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Poorthuis B. J., Mulder A. H., Wagemaker G., Dooren L. J., Vossen J. M., van Bekkum D. W. Correction of lysosomal enzyme deficiency in various organs of beta-glucuronidase-deficient mice by allogeneic bone marrow transplantation. Transplantation. 1987 May;43(5):609–614. doi: 10.1097/00007890-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Wagemaker G., van Bekkum D. W. Failure to demonstrate pluripotential hemopoietic stem cells in mouse brains. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4268–4269. doi: 10.1073/pnas.82.12.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igisu H., Takahashi H., Suzuki K., Suzuki K. Abnormal accumulation of galactosylceramide in the kidney of twitcher mouse. Biochem Biophys Res Commun. 1983 Feb 10;110(3):940–944. doi: 10.1016/0006-291x(83)91053-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nagara H., Suzuki K., Suzuki K. The twitcher mouse: determination of genetic status by galactosylceramidase assays on clipped tail. Biochem Med. 1982 Feb;27(1):8–14. doi: 10.1016/0006-2944(82)90003-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Yamanaka T., Jacobs J. M., Teixeira F., Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease). Brain Res. 1980 Dec 8;202(2):479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- Krivit W., Pierpont M. E., Ayaz K., Tsai M., Ramsay N. K., Kersey J. H., Weisdorf S., Sibley R., Snover D., McGovern M. M. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI). Biochemical and clinical status 24 months after transplantation. N Engl J Med. 1984 Dec 20;311(25):1606–1611. doi: 10.1056/NEJM198412203112504. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipton M., Lockman L. A., Ramsay N. K., Kersey J. H., Jacobson R. I., Krivit W. Bone marrow transplantation in metachromatic leukodystrophy. Birth Defects Orig Artic Ser. 1986;22(1):57–67. [PubMed] [Google Scholar]
- Marshall R. J., MacIver A. G. The monocyte/macrophage population of the normal human kidney. J Pathol. 1984 Aug;143(4):275–280. doi: 10.1002/path.1711430407. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Barranger J. A., Brady R. O., Pagel M., Furbish F. S., Quirk J. M., Mook G. E., Frenkel E. Delivery of hexosaminidase A to the cerebrum after osmotic modification of the blood--brain barrier. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5838–5841. doi: 10.1073/pnas.78.9.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R. M., Hastings N. E., Selcer R. R., Jones J. B., Smith J. R., Cullen W. C., Constantopoulos G. Bone marrow transplantation in canine mucopolysaccharidosis I. Effects within the central nervous system. J Clin Invest. 1987 Feb;79(2):435–443. doi: 10.1172/JCI112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin S., Yatziv S. Correction of enzyme deficiency in mice by allogeneic bone marrow transplantation with total lymphoid irradiation. Science. 1980 Dec 5;210(4474):1150–1152. doi: 10.1126/science.7003711. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Vanier M. T., Häkansson G., Mänsson J. E. Use of leukocytes in diagnosis of Krabbe disease and detection of carriers. Clin Chim Acta. 1981 May;112(3):333–342. doi: 10.1016/0009-8981(81)90456-3. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Igisu H., Suzuki K., Suzuki K. Murine globoid cell leukodystrophy: the twitcher mouse. An ultrastructural study of the kidney. Lab Invest. 1984 Jan;50(1):42–50. [PubMed] [Google Scholar]
- Thomas E. D., Ramberg R. E., Sale G. E., Sparkes R. S., Golde D. W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976 Jun 4;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Toyoshima E., Yeager A. M., Brennan S., Santos G. W., Moser H. W., Mayer R. F. Nerve conduction studies in the Twitcher mouse (murine globoid cell leukodystrophy). J Neurol Sci. 1986 Jul;74(2-3):307–318. doi: 10.1016/0022-510x(86)90116-4. [DOI] [PubMed] [Google Scholar]
- Van Bezooijen C. F., Grell T., Knook D. L. The effect of age on protein synthesis by isolated liver parenchymal cells. Mech Ageing Dev. 1977 Jul-Aug;6(4):293–304. doi: 10.1016/0047-6374(77)90031-8. [DOI] [PubMed] [Google Scholar]
- Van Diggelen O. P., Galjaard H., Sinnott M. L., Smith P. J. Specific inactivation of lysosomal glycosidases in living fibroblasts by the corresponding glycosylmethyl-p-nitrophenyltriazenes. Biochem J. 1980 May 15;188(2):337–343. doi: 10.1042/bj1880337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin P. I., Dixon M. S., Jr, Schafer I., Strandjord S. E., Coccia P. F. Bone marrow transplantation in Hunter syndrome: a preliminary report. Birth Defects Orig Artic Ser. 1986;22(1):31–39. [PubMed] [Google Scholar]
- Wenger D. A., Gasper P. W., Thrall M. A., Dial S. M., LeCouteur R. A., Hoover E. A. Bone marrow transplantation in the feline model of arylsulfatase B deficiency. Birth Defects Orig Artic Ser. 1986;22(1):177–186. [PubMed] [Google Scholar]
- Wenger D. A., Satter M., Markey J. P. Deficiency of monogalactosyl diglycerid beta-B-galactosidase activity in krabbe's disease. Biochem Biophys Res Commun. 1973 Jul 17;53(2):680–685. doi: 10.1016/0006-291x(73)90715-8. [DOI] [PubMed] [Google Scholar]
- Whitley C. B., Ramsay N. K., Kersey J. H., Krivit W. Bone marrow transplantation for Hurler syndrome: assessment of metabolic correction. Birth Defects Orig Artic Ser. 1986;22(1):7–24. [PubMed] [Google Scholar]
- Yeager A. M., Brennan S., Tiffany C., Moser H. W., Santos G. W. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science. 1984 Sep 7;225(4666):1052–1054. doi: 10.1126/science.6382609. [DOI] [PubMed] [Google Scholar]


