Abstract
Sexual reproduction is an obligate step in the life cycle of many parasites, including the causative agents of malaria (Plasmodium). Mixed-species infections are common in nature and consequently, interactions between heterospecific gametes occur. Given the importance of managing gene flow across parasite populations, remarkably little is understood about how reproductive isolation between species is maintained. We use the rodent malaria parasites P. berghei and P. yoelii to investigate the ecology of mixed-species mating groups, identify proteins involved in pre-zygotic barriers, and examine their evolution. Specifically, we show that (i) hybridization occurs, but at low frequency; (ii) hybridization reaches high levels when female gametes lack the surface proteins P230 or P48/45, demonstrating that these proteins are key for pre-zygotic reproductive isolation; (iii) asymmetric reproductive interference occurs, where the fertility of P. berghei gametes is reduced in the presence of P. yoelii and (iv) as expected for gamete recognition proteins, strong positive selection acts on a region of P230 and P47 (P48/45 paralogue). P230 and P48/45 are leading candidates for interventions to block malaria transmission. Our results suggest that depending on the viability of hybrids, applying such interventions to populations where mixed-species infections occur could either facilitate or hinder malaria control.
Keywords: malaria, hybridization, transmission, reproductive isolation, P230, P48/45
1. Introduction
Interactions between species lie at the core of evolution because they can facilitate, or undermine, reproductive isolation and the process of speciation [1]. Mating interactions between heterospecifics can also shape geographical distributions of species via reproductive interference, the phenomenon in which the fitness of both (symmetric) or one (asymmetric) of the interacting species is reduced [2]. Reproductive isolation requires the evolution of barriers to genetic exchange between species and can act before (pre-zygotic) or after (post-zygotic) mating [3]. Pre-zygotic barriers include spatial or temporal segregation, behavioural isolation (e.g. through mate choice), gametic incompatibility and lack of gamete transfer/activation [3,4]. Post-zygotic barriers generally occur through hybrid sterility and inviability [3,4]. For Plasmodium (and related Apicomplexan) parasites, a single round of sexual reproduction is obligatory for transmission between hosts and parasites are hermaphroditic organisms that can self-fertilize and outcross. Developmentally arrested male and female sexual stages (gametocytes) are produced throughout infections in the vertebrate host and are taken up in the vector's blood meal. Once inside a blood meal, gametocytes have 30–60 min to differentiate into gametes and achieve fertilization [5]. However, the mechanisms responsible for the origin and maintenance of reproductive isolation are unknown.
Mixed-species infections of Plasmodium are common in humans (e.g. 12–65% in Thailand; [6]) and were present in approximately 28% of wild-caught rodent Plasmodium isolates [7]. Several lines of evidence suggest that gametocytes from co-infecting species co-transmit to vectors during blood feeding: (i) multiple Plasmodium species naturally infect the same host species, including humans (P. falciparum, P. knowlesi, P. malariae, P. ovale and P. vivax) and thicket rats (P. berghei, P. chabaudi, P. vinckei and P. yoelii) [6,8]. (ii) Co-infecting species concurrently produce gametocytes [9]. (iii) Mixed-species infections are found in wild captured Anopheles mosquitoes [10], and mosquitoes in the laboratory can simultaneously acquire and transmit multiple species [10,11]. (iv) The cues that stimulate gametogenesis are conserved across Plasmodium species (e.g. temperature drop > 5°C, xanthurenic acid [12,13]). Given the drive to develop interventions that block disease transmission by preventing mating [14], determining how heterospecific gametes interact is necessary.
Fertilization generally involves gamete attachment and recognition (potentially at the same time), followed by fusion [15]. In Plasmodium, the following proteins are required for conspecific gamete interactions during fertilization. HAP2/GCS1 is expressed at the surface of male, but not female gametes, and is required for fusion, but not attachment [16,17]. The proteins P230, P47 and P48/45 belong to the 6-cys multi-domain protein family and are expressed at the surface of gametocytes/gametes [18,19]. P47 (a paralogue of P48/45) is only expressed at the surface of female gametes and its deletion in P. berghei prevents viable male gametes from attaching to females [18,20]. P230 and P48/45 are important targets of transmission-blocking immunity [21] and are expressed at the surface of both male and female gametes [18,22]. Deletion of either P230 or P48/45 in P. berghei renders males infertile but has no apparent impact on female fertility [18,22]. However, the fertility of female gametes lacking P230 or P48/45 has only been investigated in mating crosses between conspecifics [18,22].
We investigated what, if anything, prevents gene flow between different Plasmodium species using two rodent malaria species, P. berghei and P. yoelii, as model systems (see ‘Supplementary methods’ for the rationale on using these species, electronic supplementary material). We determined that pre-zygotic barriers do exist, and that heterospecific mating occurs at a high rate when P230 or P48/45 is absent from the surface of female gametes. Therefore, our approach has identified proteins important for reproductive isolation, which is of particular relevance for taxa where hybridization is suspected (e.g. Haemoproteus [23,24]). We then examined the ecology of mixed-species mating groups and show that asymmetric reproductive interference occurs. Finally, because organisms with external fertilization often have fast-evolving gamete recognition proteins [25], we examined the rate of evolution of p230, p48/45 and the related gene, p47.
2. Material and methods
We carried out two experiments taking advantage of the ability of P. yoelii or P. berghei to mate in culture. Compared with mosquito transmission experiments, our in vitro approach allowed us to more accurately standardize the conditions parasites experienced and simultaneously set up and assay a larger number of samples. The first experiment tested whether hybridization occurs between P. yoelii and P. berghei, in reciprocal crosses between males and females of each species (referred to as ‘intact’ parasites). The second experiment examined hybridization rates between P. yoelii males and P. berghei female gametes that could not produce either P230 or P48/45 (referred to as ‘knockout’ parasites). We used genetically modified reference lines of P. berghei and P. yoelii whose female gametes and ookinetes express different fluorescent proteins to distinguish between offspring resulting from conspecific and heterospecific fertilizations (see figure 1 for experimental design). The parasite lines used and their phenotypes with respect to the fertility of male/female gametes and expression of fluorescent proteins are shown in electronic supplementary material, table S1 and the numbers of infections contributing to each type of culture are shown in electronic supplementary material, table S2.
Figure 1.
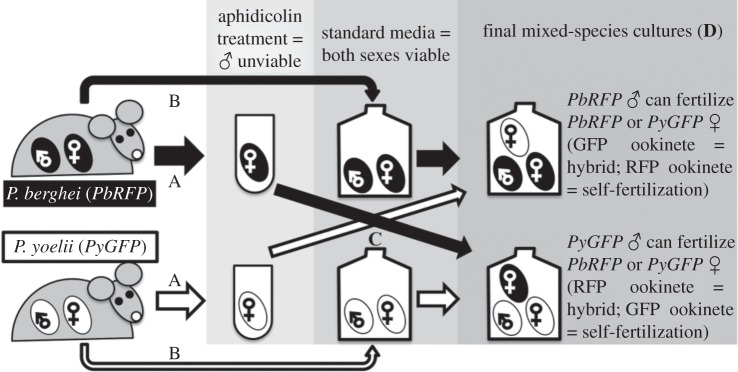
Experimental design for mixed-species mating cultures of intact parasites. Blood was taken from mice infected with P. berghei (PbRFP—black circles; RFP-expressing females/ookinetes) or P. yoelii (PyGFP—white circles; GFP-expressing females/ookinetes). Parasites were incubated in media that did (A) or did not (B) contain aphidicolin. Aphidicolin is an inhibitor of DNA polymerase-α that makes males unviable because male gametocytes, unlike females, have to replicate their DNA to produce gametes. Aphidicolin was washed off and parasites then were added to cultures containing heterospecifics (C) in vector mimicking media, which triggers gametogenesis and fertilization. Final cultures (D) contained fertile males from a single species (at a high enough density to ensure they were not limiting) and females from both species, expressing different fluorescent proteins.
(a). Hosts and parasites
We infected MF1 male mice, aged 8–12 weeks (Harlan-Olac, UK; or in-house supplier, University of Edinburgh), with either P. yoelii or P. berghei (as described in [26]). Two to four days before infection, we treated mice with phenylhydrazine (PHZ) to elevate gametocyte (gamete precursor stages) production [27]. For the first experiment, using intact parasites, we inoculated mice with either 107 red blood cells (RBCs) parasitized with P. berghei lines PbRFP or PbΔp47 (PHZ: 125 mg kg−1, day −2 post-infection (PI)) or 108 RBCs parasitized with P. yoelii lines PyGFP or Py17X (PHZ: 60 mg kg−1, day −3PI). For the second experiment, using knockout parasites, we inoculated mice with 107 RBCs parasitized with either P. berghei lines PbWT, PbΔp230, PbΔp48/45 or with P. yoelii line PyWT (60 mg kg−1 PHZ, day −4 PI). These parasite and PHZ dose combinations ensured high gametocyte densities within a few days of infection, so that the presence of transmission-blocking immune factors was minimized.
(b). Mating cultures
We performed all of the experiments using the conditions described in [26]. Briefly, we harvested gametocytes (on day 3 or 4 post-infection), from mice infected either with P. berghei or P. yoelii, and incubated them in RPMI (Roswell Park Memorial Institute) media with 10% calf serum at pH 8 and 21°C. This mimics the vector environment, immediately triggering gametogenesis, fertilization and ookinete development [26]. P. yoelii is thought to transmit at 24°C but preliminary work revealed that culturing at 21°C does not significantly affect fertilization success. We counted the number of females and exflagellating males present in each infection (as described in [26]) to determine the densities of males and females of each species in the mating cultures. Each infected mouse contributed parasites to only one mixed-species culture and to one culture of each the controls (see below). This maximizes statistical power while avoiding pseudo-replication [26].
To measure both con- and heterospecific fertilization success, we assayed ookinete numbers 18–20 h after fertilization (when ookinetes have developed). To do this, we examined 10 μl of each culture in a Neubauer haemocytometer and counted ookinetes using a fluorescence microscope. Zygotes resulting from heterospecific matings can develop into ookinetes because males make little contribution to zygote-to-ookinete development. Ookinetes develop via translationally repressed proteins, whose mRNAs are present in females before mating [28]. Indeed, male gene expression has not been observed until the ookinete-to-oocyst transition [29]. We used ookinetes to assay fertilization success because, compared with zygotes, the distinct crescent shape of ookinetes means they can be more accurately distinguished from unfertilized females. We distinguished ookinetes resulting from conspecific and heterospecific fertilizations by the fluorescent protein that they expressed (figure 1; electronic supplementary material, table S1).
(c). Hybridization between ‘intact’ Plasmodium berghei and Plasmodium yoelii
We tested whether hybridization occurs between ‘intact’ (i.e. non knockout) P. berghei and P. yoelii gametes by mixing both species together in mating cultures after making the males of one species infertile. Therefore, in one set of cultures the P. berghei males were infertile and so, hybrid ookinetes could only be produced by matings between P. berghei females and P. yoelii males and would express red fluorescent protein (RFP). Conversely, conspecific matings would give rise to ookinetes expressing green fluorescent protein (GFP). In the second set, the P. yoelii males were infertile, and so hybrid ookinetes (GFP) could only occur when P. berghei males mated P. yoelii females (conspecific ookinetes: RFP).
To make males infertile we used aphidocolin, which stops male gametogenesis but leaves females unaffected. We incubated 15 μl of PbRFP- or PyGFP-infected blood (for 12 min) in 1 ml RPMI with 5 × 10−4 M aphidicolin (Sigma-Aldrich, UK) [22]. We then washed the aphidicolin by centrifuging (12000 r.p.m., 5 s) and replacing the supernatant with new RPMI (without aphidicolin). During the time that parasites were undergoing aphidicolin treatment, we collected parasites from all other PbRFP or PyGFP infections and added 60 μl infected blood to 4 ml RPMI. We then combined the cultures of infertile males plus fertile females with cultures of the other parasite species that contained viable males and females (final culture volume: 5 ml). These steps are illustrated in figure 1. We used different volumes of blood (15 μl and 60 μl) to ensure a high ratio of viable males relative to females, minimizing the possibility of male limitation constraining fertilization of either con- or heterospecific females.
(i). Control cultures
Several types of control cultures were required to validate that (i) conspecific mating occurs within the PbRFP and PyGFP lines; (ii) aphidicolin treatment did not adversely affect PyGFP and PbRFP females; (iii) aphidicolin treatment blocked male fertility of PyGFP and PbRFP. We verified these assumptions by (i) independently culturing PbRFP- or PyGFP-infected blood and observing ookinetes; (ii) inactivating PbRFP or PyGFP males with aphidicolin and mixing them with the conspecifics P. berghei Δp47 (only males are fertile) and P. yoelii 17X wild-type (ookinetes are wild-type) and observing fluorescent ookinetes; (iii) inactivating PbRFP or PyGFP males with aphidicolin and not observing ookinetes. See electronic supplementary material, table S2 for the number of replicates and the ookinete densities produced, per control type. A full description of these results is given in electronic supplementary material, ‘Supplementary results’.
(d). Hybridization between P230 and P48/45 ‘knockouts' and Plasmodium yoelii
To test whether P230 and/or P48/45 mediate species recognition, we set up mating crosses between a wild-type line of P. yoelii (PyWT) and P. berghei lines that constitutively express GFP and lack either p230 (PbΔp230) or p48/45 (PbΔp48/45; electronic supplementary material, table S1). The deletion of P230 or P48/45 renders males unviable, so aphidocolin treatment was not required. Thus, we simply mixed 10 μl of PyWT-infected blood (males and females are viable) with 10 μl of PbΔp230 or PbΔp48/45 (only females are viable) in 1 ml cultures. We then assayed the densities of GFP ookinetes (mating between P. yoelii males and P. berghei females) or wild-type ookinetes (P. yoelii self-fertilizations).
(i). Control cultures
We set up control cultures to verify that (i) PyWT produces ookinetes but PbΔp230 and PbΔp48/45 do not and (ii) females from PbΔp230 and PbΔp48/45 can be fertilized by conspecific males. We verified these assumptions by (i) culturing each line alone and observing ookinetes in PyWT but not PbΔp230 or PbΔp48/45 cultures and (ii) mixing P. berghei wild-type with PbΔp230 or PbΔp48/45 and observing ookinetes. See electronic supplementary material, table S2 for the number of replicates and the ookinete densities produced, per control type. A full description of these results is given in electronic supplementary material, ‘Supplementary results’.
(e). Data analysis
Plasmodium berghei and P. yoelii produce different numbers of gametocytes during infections, so the numbers of heterospecific and conspecific females differed in mixed-species cultures. However, because all mixed-species cultures contain fertile males from only one species, if parasites mated randomly, the proportion of hybrid ookinetes would be equal to the proportion of females that are heterospecific. Therefore, we term the proportion of heterospecific females as ‘expected hybridization’ (under random mating) and the proportion of hybrid ookinetes as ‘observed hybridization’. In the majority of our analyses of the mixed-species cultures, we compare expected with observed hybridization because we are testing for deviations from random mating. All analyses were performed in R v. 2.14.0 (http://www.r-project.org/) and consisted of generalized-linear and linear mixed-effects models and t-tests. This depended on the distribution of the data, the need to account for random effects and sample sizes. Non-parametric Wilcoxon tests were used when the assumptions of normality could not be met by data transformation. Further details on data analysis of Results sections (§§3(b,d)) are given in electronic supplementary material, ‘Supplementary methods’.
(f). Molecular evolution
We determined the DNA sequence of p230, p48/45 and p47 using previously collected genomic DNA [7], spanning 58 genotypes from field isolates of the four rodent malaria species (P. berghei, P. chabaudi, P. vinckei and P. yoelii; see electronic supplementary material, table S3 for primers and PCR cycling conditions). We sequenced the entire p47 and p48/45 loci. However, as p230 is a large locus (approx. 8.3 kb), we examined two regions of this locus thought to be fast- or slow-evolving [18] (region I ranges from 2242 to 3466 bp and region II from 6741 to 7832 bp; reference: P. berghei at PlasmoDB, see http://plasmodb.org). We investigated the female-specific surface protein P47 because it plays a role in male recognition of female gametes and it has been suggested that it directly interacts with P230 and/or P48/45 during gamete recognition and attachment [18]. We refer to p230, p47 and p48/45 collectively as ‘mating’ loci (GenBank: KP849590–KP849808). Moreover, for some of the analysis, we included a set of 11 ‘control’, house-keeping loci (GenBank: JX904678–JX905153, JX984464–JX984513; [7]).
After aligning the sequences and testing for recombination, we computed a variety of standard population-genetic summary statistics: (i) counts of fixed differences at non-synonymous and synonymous sites (DN, DS) and polymorphisms (PN, PS); (ii) divergence ratios (substitutions per site: KA, KS, KA/KS) and nucleotide diversity (πA, πS, πA/πS) [30]; (iii) Tajima's D [31]; (iv) single-locus McDonald–Kreitman (MK) tests [32]; (v) multi-locus MK [33] and Hudson–Kreitman–Aguade tests (HKA, [34]). Moreover, we used codon evolution models to test whether the strength of selection varied along each locus [35]. Further details on each type of analysis are given in electronic supplementary material, ‘Supplementary methods’.
3. Results
(a). Hybridization can occur between Plasmodium berghei and Plasmodium yoelii
When we tested whether hybridization occurs between ‘intact’ lines of P. berghei and P. yoelii, we obtained hybrid ookinetes in 60% of the crosses between male PyGFP and female PbRFP (as identified by RFP-positive ookinetes) and in 40% of the crosses between male PbRFP and female PyGFP (GFP-positive ookinetes). However, the proportion of hybrid ookinetes (observed hybridization) was much lower than the proportion of heterospecific females (expected hybridization; figure 2). Specifically, in the cross between PyGFP males and PbRFP females, expected hybridization was on average 0.85 ± 0.02 (±s.e.) but observed hybridization was 0.15 ± 0.06 (Wilcoxon; V = 0; p < 0.0001; d.f. = 14). In the cross between PbRFP males and PyGFP females, expected hybridization was 0.16 ± 0.03 and observed hybridization was 0.03 ± 0.01 (Wilcoxon; V = 5; p < 0.0001; d.f. = 19; see electronic supplementary material, table S2 for ookinete densities). While this demonstrates that hybridization can occur between different species of malaria parasites, there is clearly strong preference for mating between conspecifics suggesting that pre-zygotic reproductive barriers operate during mating.
Figure 2.
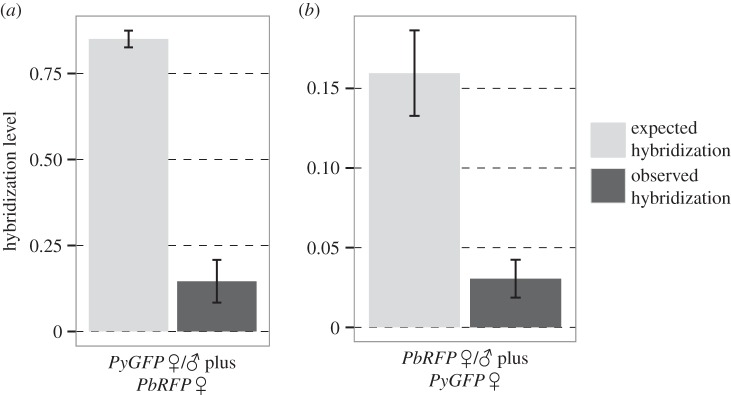
Hybridization occurs between P. berghei and P. yoelii. Expected hybridization, under random mating (i.e. proportion of heterospecific female gametes; light bars), and observed hybridization (i.e. proportion of hybrid ookinetes; dark bars). Mean ± s.e. is shown for matings between (a) P. yoelii (PyGFP) males and P. berghei (PbRFP) or PyGFP females and (b) PbRFP males and PyGFP or PbRFP females.
(b). Reproductive interference
We used the data from the experiment above to test whether reproductive interference occurs. For P. berghei, self-fertilizations (i.e. the proportion of conspecific fertilized females) are reduced in the presence of P. yoelii (likelihood ratio test, 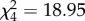 , p < 0.001, single species cultures: 0.31 ± 0.03; mixed species: 0.20 ± 0.03). By contrast, the presence of P. berghei had no significant effect on P. yoelii self-fertilizations (LRT
, p < 0.001, single species cultures: 0.31 ± 0.03; mixed species: 0.20 ± 0.03). By contrast, the presence of P. berghei had no significant effect on P. yoelii self-fertilizations (LRT 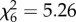 ; p = 0.51, single species: 0.28 ± 0.05). Therefore, asymmetric reproductive interference can occur and in particular, it reduces conspecific mating rates for P. berghei by approximately 30% (figure 3).
; p = 0.51, single species: 0.28 ± 0.05). Therefore, asymmetric reproductive interference can occur and in particular, it reduces conspecific mating rates for P. berghei by approximately 30% (figure 3).
Figure 3.
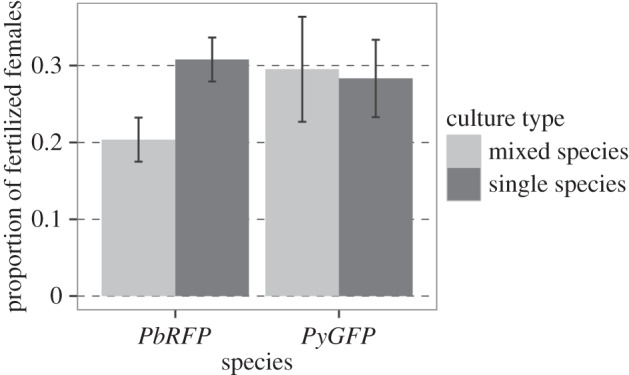
Asymmetric reproductive interference between P. berghei and P. yoelii. The proportion of conspecific females (mean±s.e.) that were fertilized in single- (dark bars) and mixed-species (light bars) cultures for P. berghei (PbRFP; left) and P. yoelii (PyGFP; right). Fertilization success is significantly reduced for P. berghei, but not P. yoelii, in mixed-species mating groups.
(c). P230 and P48/45 are involved in pre-zygotic reproductive barriers
Next, we tested whether P230 and P48/45 proteins influence the levels of observed hybridization. We obtained hybrid ookinetes in crosses between male PyWT and females from PbΔp230 (approx. 80% of the cultures) and PbΔp48/45 (approx. 90% of the cultures). In contrast to the crosses between intact parasites, observed hybridization approached the levels of expected hybridization (cf. figures 2 and 4). Specifically, while the mean difference between expected and observed hybridization was 0.70 (95% CI: 0.58–0.83; assuming a normal distribution) for crosses between ‘intact’ P. yoelii males and P. berghei females, this difference was approximately five times smaller for crosses between P. yoelii males and PbΔp230 females (0.14; 95% CI: 0.016–0.28) or PbΔp48/45 females (0.16; 95% CI: −0.04–0.36; see electronic supplementary material, table S2 for ookinete densities). Moreover, while there was a significant difference between expected (0.71 ± 0.07) and observed hybridization (0.56 ± 0.09) for the cross between PyWT males and PbΔp230 females (t = −2.49, d.f. = 10, p = 0.032), this was not the case for the cross between PyWT males and PbΔp48/45 females (t = −1.75, d.f. = 13, p = 0.104; observed hybridization: 0.39 ± 0.10; figure 4), suggesting that PyWT males randomly mate with conspecific and PbΔp48/45 females, but not PbΔp230 females (we address this issue in the next section). These results indicate that P230 and P48/45, at the surface of female gametes, are key for pre-zygotic reproductive isolation.
Figure 4.
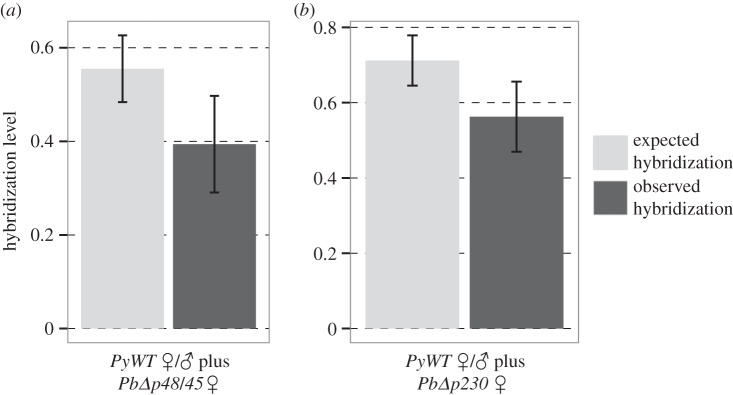
Hybridization between P. yoelii males and P. berghei females occurs at higher rates when P. berghei females lack P48/45 or P230. Expected hybridization, under random mating (i.e. proportion of heterospecific female gametes; light bars), and observed hybridization (i.e. proportion of hybrid ookinetes; dark bars). Mean ± s.e. is shown for matings between (a) P. yoelii (PyWT) males and PbΔp48/45 or PyWT females; and (b) PyWT males and PbΔp230 or PyWT females.
(d). Mating is non-random in the absence of P230 and P48/45
The number of con- and heterospecific female gametes was variable in cultures of P. yoelii males plus PbΔp230 or PbΔp48/45 females. This enabled us to examine whether P. yoelii males randomly mate with conspecific and PbΔp48/45 or PbΔp230 females. Our analysis showed that P. yoelii males do not have a significant preference for PbΔp48/45 or PbΔp230 females (F1,20 = 0.813; p = 0.378). However, observed hybridization was significantly affected by the interaction between the densities of con- and heterospecific females available (F1,21 = 10.199, p = 0.004). To visualize this interaction, we generated a matrix of values for the densities of con- and heterospecific females (within the observed ranges). We then inputted this matrix into the minimal generalized-linear model (see electronic supplementary material, ‘Supplementary methods’) to predict how observed hybridization correlates with the density of con- and heterospecific females (figure 5). Unsurprisingly, observed hybridization rises as the density of heterospecific females increases and decreases when the density of conspecific females increases. However, these patterns are nonlinear; when the density of heterospecific females is low, the level of observed hybridization is dominated by the density of conspecific females (figure 5, colours change vertically), but at high densities of heterospecific females (greater than 120 × 106 ml), observed hybridization becomes independent of the density of conspecific females (figure 5, colours change horizontally). The key point is that random mating is only predicted at very low densities of both con- and heterospecific females or at very high densities of heterospecific females.
Figure 5.
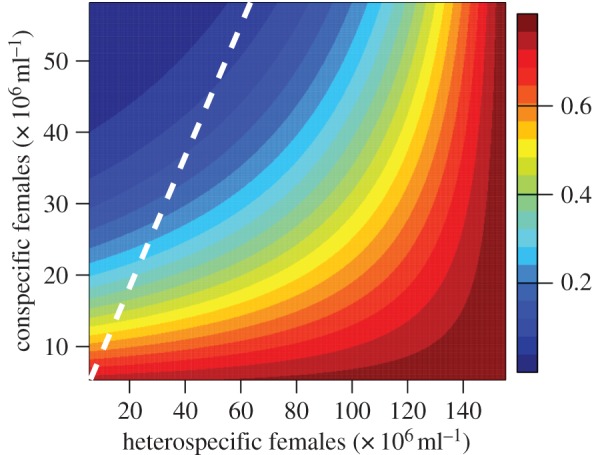
Hybridization rates correlate with the interaction between the densities of con- and heterospecific females. The proportion of matings that produce hybrids (red, high; blue, low) follows a nonlinear pattern with respect to the densities of con- (y-axis) and heterospecific females (x-axis) available in culture. When the densities of con- and heterospecific females are equal (white line), random mating would result in 50% of matings producing hybrids. The yellow band (50% hybrids) mostly sits to the right of the white line. This suggests that fewer hybrids are observed than expected under random mating for most combinations of con- and heterospecific female densities and so P230 and P48/45 are not the only pre-zygotic barriers.
(e). P230, P47 and P48/45 are under positive selection
Since the surface proteins P230 and P48/45 play a role in conspecific gamete recognition [18,22] and mediate mating between heterospecific gametes (this study), we investigated the evolution of p230, p48/45 and p47 (‘mating’ loci) and identified domains under selection. In our analyses, single gene MK tests were significant only for region I of p230, with α varying between 0.7 and 0.85 (α is the estimated proportion of non-synonymous substitutions owing to positive selection [36]; electronic supplementary material, table S4). However, the greater statistical power of the multi-locus MK tests suggests that the ‘mating’ loci experience a higher rate of adaptive change (i.e. non-synonymous divergence) than the ‘control’ loci (table 1; see electronic supplementary material, tables S5 and S6, for the full multi-locus MK results). This is probably driven by region I of p230 and p47, which are the only loci that better fit a model where α varies between ‘control’ and ‘mating’ loci (when each locus is separately tested; table 1 and tables S5 and S6). Moreover, for p230 (region I) and p47, α is substantially higher than for the remaining loci (α varies between 0.7–0.86 and 0.52–0.82 for region I of p230 and p47, respectively; electronic supplementary material, tables S5 and S6). On the other hand, the HKA test did not detect significant differences in polymorphism between mating and control loci (data not shown), for any of the comparisons tested. This suggests an absence of either long-term balancing selection or recent selective sweeps and is in agreement with the lack of significance of single-locus Tajima's D ([37];electronic supplementary material, table S4).
Table 1.
The ‘mating’ loci are under positive selection. α is shown for the ‘mating’ loci individually or as a group (All) and for a group of 11 ‘control’ loci [7]. α was estimated using a multi-locus MK test [33]. α values in bold indicate loci for which the best-fitting model allowed α to vary between ‘mating’ and ‘control’ loci, which indicated that the ‘mating’ loci are adaptively evolving faster than the ‘control’. Data in this table were obtained using polymorphism counts for P. chabaudi chabaudi clones, but qualitatively similar results are obtained for the subspecies P. y. yoelii or P. vinckei petteri and at the species level (electronic supplementary material, tables S5 and S6).
| locus tested |
|||||
|---|---|---|---|---|---|
| All | p230 region I | p230 region II | p47 | p48/45 | |
| αmating | 0.75 | 0.86 | 0.67 | 0.84 | 0.45 |
| αcontrol | 0.37 | 0.47 | 0.47 | 0.39 | 0.47 |
Finally, using codon evolution models ([35]; see electronic supplementary material, ‘Supplementary methods’), we detected positive selection across all mating loci, with estimated KA/KS always above 2.3 for the most strongly selected class of codons (see electronic supplementary material, table S7 for details on statistical significance, KA/KS values and % of selected codons). Moreover, we identified 65 candidate codons as being positively selected (across all loci) and therefore greatly expand on the 15 codons previously identified in [18] (electronic supplementary material, table S8). Taken together, these analyses suggest that the ‘mating loci’ (particularly p47 and region I of p230) are evolving under positive selection, but there is no evidence for balancing selection or recent selective sweeps.
4. Discussion
We combined in vitro fertilization experiments with molecular evolution tools to examine the mating biology of Plasmodium species. We show that P. berghei and P. yoelii gametes can hybridize and that asymmetric reproductive interference occurs in mixed-species mating groups. Hybridization rates significantly increase when either of the proteins P230 or P48/45 is absent from the surface of P. berghei female gametes, suggesting an unexpected role for these proteins in species recognition during fertilization. However, the absence of these proteins does not lead to random mating between con- and heterospecifics, indicating that other, yet unidentified, factors also contribute to pre-zygotic barriers. Finally, we reveal strong positive selection on a region of P230 and on the female surface protein P47 and suggest specific codons in these genes that experience strong selection.
(a). Hybridization and introgression
Recent research shows that hybridization occurs more frequently than previously thought [38], and that introgression can have important evolutionary consequences for diverse organisms, including the parasites Schistosoma and Leishmania (hybridization extends the vector-species range [39,40]). We observed low levels of hybridization between wild-type P. yoelii and P. berghei gametes (figure 2). To our knowledge, this is the first time that hybridization has been demonstrated for species of the Plasmodium genus (although hybridization has been reported in the related Haemoproteus genus [23]). Whether hybrids are able to complete the life cycle is not clear. We investigated this by carrying out a small number of mosquito feeds on mice co-infected with P. yoelii wild-type and GFP-labelled P. berghei lines lacking P230 or P48/45. We observed hybrid oocysts (GFP) of abnormally small size (without sporozoites) in mixed, but not in single-species infections (data not shown). This suggests that hybrids may often fail to proceed further than the early oocyst stage and fits with the failure of earlier attempts to produce crosses of these species in vivo [8]. However, experimentally assessing the viability and evolutionary impact of hybrids is very difficult because the number and genetic diversity of the parasites circulating in natural environments is much higher than what can be experimentally tested. Furthermore, introgression can be important at the population level even when the probability of individual hybrids completing the life cycle is extremely low. In this case, examining individual parasites will only very rarely reveal a fit hybrid. Thus, evidence for hybrid viability is better obtained from genome sequence data, as has been the case for schistosomes [39].
Interestingly, we also observe that, in the absence of P230 or P48/45, the frequency of hybridization changes nonlinearly with the interaction between the densities of con- and heterospecific females. Moreover, the frequency of hybridization approaches random mating very rarely. This is the case at very low densities of both con- and heterospecific females. Low gametocyte densities are the norm in natural infections [41], suggesting that hybridization may be more common than we observed. In this case, parasites could reduce chances of hybridization (assuming it is costly for fitness) by increasing the number of circulating gametocytes.
(b). Reproductive interference
We demonstrate that asymmetric reproductive interference occurs in Plasmodium, in which the self-fertilization success of P. berghei, but not P. yoelii, is reduced in the presence of heterospecifics (figure 3). Similar results have been obtained by Paul et al. [42] and Valkiunas et al. [24] for avian Plasmodium and Haemoproteus, respectively. There are several mechanisms that could underpin this phenomenon, including (i) host immune factors produced in P. yoelii infections that act in the blood meal (e.g. [21,26]) could have more severe effects on P. berghei than on P. yoelii; (ii) if male gametes use chemotaxis to locate females, P. yoelii males may be better able to distinguish between con- and heterospecific signals than P. berghei males. (iii) Direct, chemically mediated, antagonistic interactions between gametes of different species (allelopathy) could also occur. Paul et al. [42] suggest that allelopathy occurs based on the observation that asymmetric reproductive interference among avian malaria parasites is independent of immunity. Whatever the reproductive interference mechanism, understanding whether reproductive interference occurs in the wild is important because it can be a determinant of epidemiological dynamics and geographical distributions of the interacting species [2].
(c). The role of P230 and P48/45 in pre-zygotic isolation
We show that the absence of proteins P230 and P48/45 from the surface of female P. berghei gametes markedly increases hybridization with P. yoelii males (figures 2 and 4), suggesting that these proteins are involved in species recognition. An important role for these proteins in females was unexpected because all previous work has focused on their essential role for male fertility. While P230 and P48/45 are key for the maintenance of pre-zygotic barriers, it is unclear if P230 and P48/45 directly mediate species recognition, or whether P230 and P48/45 underpin the functionality of recognition proteins (e.g. P230 and P48/45 may ensure that recognition proteins are correctly localized). Importantly, our statistical model predicts that mating is generally non-random, despite the absence of P230 or P48/45, suggesting that other factors are also involved in species recognition/attachment (figure 5, §3d). Potential candidates include other male/female surface proteins, such as P47 or LAP/CCp-family members, which are known to interact with P230 and P48/45 [18,43]. It is also possible that species-specific chemotactic signals influence encounters between con- and heterospecific gametes.
While further work is required to determine if P230 or P48/45 or both are involved in mediating gamete recognition, there are several reasons to suspect that P230 plays a dominant role in pre-zygotic isolation, by mediating gamete recognition. First, P230 and P48/45 are expressed at the surface of male and female gametes [44,45] and form a complex, anchored to the gamete surface by P48/45 [46,47]. In P. falciparum, deletion of P48/45 prevents P230 expression at the gamete surface [47], but not vice-versa. Second, mating can occur in the absence of P230 and/or P48/45 from the surface of female gametes (§3 and [18]), suggesting that female recognition is not essential for fertilization to occur. Similar observations have been made for organisms as divergent as humans and hamsters [48]. Third, positive selection is commonly found in gamete recognition proteins across taxa and we find that region I of p230, but not p48/45, is fast evolving relative to the control loci. Fourth, the protein structure of P. falciparum P230 indicates that domain IV (defined in [19]) is an external domain available for molecular interactions—a domain where we identify eight fast-evolving codons and for which several non-synonymous polymorphisms have been identified in P. falciparum [19].
(d). Molecular evolution of p230, p48/45 and p47
Our results provide evidence of adaptive evolution for the proteins involved in fertilization in Plasmodium, particularly for region I of p230 and for p47 (table 1). These results are in agreement with work on other taxa showing that genes involved in gamete recognition are fast-evolving [25] and provide further support for their role in gamete recognition. However, more work is needed to identify the ecological factors driving this fast evolution. In metazoans, sexual conflict/selection (e.g. polyspermy, assortative mating) or reinforcement are often thought to be the key forces driving this fast evolution [25]. While sexual conflict has not been studied in Plasmodium, natural transmission-blocking immunity targets P230 and P48/45, leading to reduced transmission success (e.g. [21]). Thus, natural antibodies could provide a selective pressure for driving the evolution of these proteins [49]. If P230 and/or P48/45 both contribute to reproductive isolation and are under divergent selection owing to immunity, this selective pressure could contribute towards non-random mating [50]. Such a pleiotropic effect cannot be broken down by recombination and so, could facilitate speciation in the presence of gene flow [51]. If this is the case, immunity against P230 or P48/45 could accelerate the rate at which Plasmodium lineages diverge. By contrast, no adaptive immune responses have been detected against P47 [20]. However, it may be coevolving with the mosquito immune response [52].
(e). Conclusions and implications
Our results illustrate that considering the molecular and organismal interactions within an ecological context provides a broader understanding of the mating biology of parasites. While care should be taken in extrapolating from model systems to human parasites, the implications may have medical relevance. Hybridization may have complex consequences for the success of transmission-blocking interventions directed against P230 and P48/45. Both natural and vaccine-induced antibodies can greatly reduce transmission by complement-dependent or independent processes [21,53–55]. However, while complement-dependent processes lead to gamete lysis, complement-independent processes may only mask/inactivate P230 [53–55]. Thus, antibodies that induce complement-independent processes may simply interfere with gamete recognition/attachment, allowing fertilization to proceed. Because such antibodies will be species-specific, females of the target species could be fertilized by heterospecific males in blood meals from hosts with mixed-species infections. In this case, enhancing the production of antibodies to P230 (e.g. by vaccination) could facilitate hybridization. If hybrids are viable, introgression could facilitate the spread of medically unfavourable alleles (e.g. virulence determinants, drug resistance). However, if hybrids are not viable, facilitating hybridization may bring unexpected benefits by reducing transmission of both the target and the non-target species. Furthermore, given that specific regions of the ‘mating’ loci are fast-evolving, transmission-blocking vaccines should avoid targeting these epitopes in order to delay the emergence of vaccine-escape mutants, especially if selection pressures resulting from mate recognition and immunity target similar protein regions. Finally, understanding the molecular interactions responsible for reproductive interference could provide novel targets for transmission-blocking interventions.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Aidan O'Donnell, Will Chadwick and Claire Webster for assistance in the laboratory, and Andy Waters for discussion.
Ethics Statement
Protocols involving mice passed an ethical review process and were approved by the UK Home Office (Project License 60/4121). All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986.
Data accessibility
Data for figures 2–5 is available as electronic supplementary material and DNA sequences are available at GenBank (KP849590–KP849808).
Funding statement
This research was funded by the Fundação para a Ciência e Tecnologia (R.S.R.: SFRH/BD/39960/2007), the Wellcome Trust (S.E.R.: WT082234MA and D.J.O.: 085064/Z/08/Z), the Royal Society, NERC, the Centre for Infection, Immunity and Evolution, the EU Seventh Framework Program (FP7/2007–2013; 242095; C.J.J.) and the European Commission (FP7, EVIMalaR Network of Excellence; C.J.J. and S.M.K.).
Author Contributions
R.S.R. and S.E.R. designed and analysed the in vitro experiments. R.S.R. and D.J.O. designed and analysed the molecular evolution work. R.S.R. performed all experiments. S.M.K., B.F.-F. and C.J.J. provided materials and helped interpret data. All authors contributed towards writing the manuscript.
Conflict of interest
We have no competing interests.
References
- 1.Wolf JBW, Lindell J, Backström N. 2010. Speciation genetics: current status and evolving approaches. Phil. Trans. R. Soc. B 365, 1717–1733. ( 10.1098/rstb.2010.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groning J, Hochkirch A. 2008. Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282. ( 10.1086/590510) [DOI] [PubMed] [Google Scholar]
- 3.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 4.Barton NH, Briggs DEG, Eisen JA, Goldstein DB, Patel NH. 2007. Evolution. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 5.Alano P, Carter R. 1990. Sexual differentiation in malaria parasites. Annu. Rev. Microbiol. 44, 429–449. ( 10.1146/annurev.mi.44.100190.002241) [DOI] [PubMed] [Google Scholar]
- 6.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. 2004. Mixed-species malaria infections in humans. Trends Parasitol. 20, 233–240. ( 10.1016/J.Pt.2004.03.006) [DOI] [PubMed] [Google Scholar]
- 7.Ramiro R, Reece S, Obbard D. 2012. Molecular evolution and phylogenetics of rodent malaria parasites. BMC Evol. Biol. 12, 219 ( 10.1186/1471-2148-12-219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killick-Kendrick R, Peters W. 1978. Rodent malaria. New York, NY: Academic Press. [Google Scholar]
- 9.McKenzie FE, Bossert WH. 1997. Mixed-species Plasmodium infections of humans. J. Parasitol. 83, 593–600. ( 10.2307/3284229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie F, Bossert W. 1997. Mixed-species Plasmodium infections of Anopheles (Diptera: Culicidae). J. Med. Entomol. 34, 417–425. ( 10.1093/jmedent/34.4.417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imwong M, Nakeesathit S, Day NPJ, White NJ. 2011. A review of mixed malaria species infections in anopheline mosquitoes. Malaria J. 10, 253 ( 10.1186/1475-2875-10-253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. 1998. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392, 289–292. ( 10.1038/32667) [DOI] [PubMed] [Google Scholar]
- 13.Billker O, Shaw MK, Margos G, Sinden RE. 1997. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 115, 1–7. ( 10.1017/S0031182097008895) [DOI] [PubMed] [Google Scholar]
- 14.Carter R. 2001. Transmission blocking malaria vaccines. Vaccine 19, 2309–2314. ( 10.1016/S0264-410X(00)00521-1) [DOI] [PubMed] [Google Scholar]
- 15.Vieira A, Miller DJ. 2006. Gamete interaction: is it species-specific? Mol. Reprod. Dev. 73, 1422–1429. ( 10.1002/mrd.20542) [DOI] [PubMed] [Google Scholar]
- 16.Liu YJ, et al. 2008. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Gene Dev. 22, 1051–1068. ( 10.1101/Gad.1656508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai M, Arai M, Mori T, Miyagishima S, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H. 2008. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr. Biol. 18, 607–613. ( 10.1016/J.Cub.2008.03.045) [DOI] [PubMed] [Google Scholar]
- 18.van Dijk MR, et al. 2010. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 6, e1000853 ( 10.1371/journal.ppat.1000853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerloff DL, Creasey A, Maslau S, Carter R. 2005. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc. Natl Acad. Sci. USA 102, 13 598–13 603. ( 10.1073/pnas.0502378102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Schaijk BCL, et al. 2006. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specitic surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 149, 216–222. ( 10.1016/j.molbiopara.2006.05.015) [DOI] [PubMed] [Google Scholar]
- 21.Bousema T, et al. 2010. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PLoS ONE 5, e14114 ( 10.1371/journal.pone.0014114.t003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dijk MR, et al. 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164. ( 10.1016/S0092-8674(01)00199-4) [DOI] [PubMed] [Google Scholar]
- 23.Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Bensch S. 2008. In vitro hybridization of Haemoproteus spp.: an experimental approach for direct investigation of reproductive isolation of parasites. J. Parasitol. 94, 1385–1394. ( 10.1645/ge-1569.1) [DOI] [PubMed] [Google Scholar]
- 24.Valkiunas G, Palinauskas V, Krizanauskiene A, Bernotiene R, Kazlauskiene R, Iezhova TA. 2013. Further observations on in vitro hybridization of hemosporidian parasites: patterns of ookinete development in Haemoproteus spp. J. Parasitol. 99, 124–136. ( 10.1645/GE-3226.1) [DOI] [PubMed] [Google Scholar]
- 25.Palumbi SR. 2009. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity 102, 66–76. ( 10.1038/Hdy.2008.104) [DOI] [PubMed] [Google Scholar]
- 26.Ramiro RS, Alpedrinha J, Carter L, Gardner A, Reece SE. 2011. Sex and death: the effects of innate immune factors on the sexual reproduction of malaria parasites. PLoS Pathog. 7, e1001309 ( 10.1371/journal.ppat.1001309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautret P, Coquelin F, Chabaud AG, Landau I. 1997. The production of gametocytes by rodent Plasmodium species in mice during phenylhydrazine induced reticulocytosis. Acta Parasitol. 42, 65–67. [Google Scholar]
- 28.Mair GR, et al. 2010. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 6, e1000767 ( 10.1371/journal.ppat.1000767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bushell ESC, Ecker A, Schlegelmilch T, Goulding D, Dougan G, Sinden RE, Christophides GK, Kafatos FC, Vlachou D. 2009. Paternal effect of the nuclear formin-like protein MISFIT on Plasmodium development in the mosquito vector. PLoS Pathog. 5, e1000539 ( 10.1371/journal.ppat.1000539.g005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nei M, Gojobori T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3, 418–426. [DOI] [PubMed] [Google Scholar]
- 31.Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald JH, Kreitman M. 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351, 652–654. ( 10.1038/351652a0) [DOI] [PubMed] [Google Scholar]
- 33.Welch JJ. 2006. Estimating the genomewide rate of adaptive protein evolution in Drosophila. Genetics 173, 821–837. ( 10.1534/genetics.106.056911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright SI, Charlesworth B. 2004. The HKA test revisited: a maximum-likelihood-ratio test of the standard neutral model. Genetics 168, 1071–1076. ( 10.1534/genetics.104.026500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang ZZ. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591. ( 10.1093/molbev/msm088) [DOI] [PubMed] [Google Scholar]
- 36.Eyre-walker A. 2006. The genomic rate of adaptive evolution. Trends Ecol. Evol. 21, 569–575. ( 10.1016/j.tree.2006.06.015) [DOI] [PubMed] [Google Scholar]
- 37.Kreitman M. 2000. Methods to detect selection in populations with applications to the human. Annu. Rev. Genom. Hum. Genet. 1, 539–559. ( 10.1146/annurev.genom.1.1.539) [DOI] [PubMed] [Google Scholar]
- 38.Mallet J. 2005. Hybridization as an invasion of the genome. Trends Ecol. Evol. 20, 229–237. ( 10.1016/j.tree.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 39.Huyse T, Webster BL, Geldof S, Stothard JR, Diaw OT, Polman K, Rollinson D. 2009. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 5, e1000571 ( 10.1371/journal.ppat.1000571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volf P, Benkova I, Myskova J, Sadlova J, Campino L, Ravel C. 2007. Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int. J. Parasitol. 37, 589–593. ( 10.1016/j.ijpara.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, Basáñez M-G. 2013. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2, e00626 ( 10.7554/eLife.00626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul REL, Nu VT, Krettli AU, Brey PT. 2002. Interspecific competition during transmission of two sympatric malaria parasite species to the mosquito vector. Proc. R. Soc. Lond. B 269, 2551–2557. ( 10.1098/rspb.2002.2171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholz S, Dude M, Templeton T, Pradel G. 2008. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int. J. Parasitol. 38, 327–340. ( 10.1016/j.ijpara.2007.08.009) [DOI] [PubMed] [Google Scholar]
- 44.Kaushal DC, Carter R, Rener J, Grotendorst CA, Miller LH, Howard RJ. 1983. Monoclonal-antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitos. J. Immunol. 131, 2557–2562. [PubMed] [Google Scholar]
- 45.Vermeulen AN, Vandeursen J, Brakenhoff RH, Lensen THW, Ponnudurai T, Meuwissen J. 1986. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronized gametocyte cultures. Mol. Biochem. Parasitol. 20, 155–163. ( 10.1016/0166-6851(86)90027-7) [DOI] [PubMed] [Google Scholar]
- 46.Kumar N. 1987. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9, 321–335. ( 10.1111/j.1365-3024.1987.tb00511.x) [DOI] [PubMed] [Google Scholar]
- 47.Eksi S, Czesny B, Van Gemert G-J, Sauerwein RW, Eling W, Williamson KC. 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 61, 991–998. ( 10.1111/mmi.2006.61.issue-4) [DOI] [PubMed] [Google Scholar]
- 48.Wassarman PM, Jovine L, Litscher ES. 2001. A profile of fertilization in mammals. Nat. Cell Biol. 3, E59–E64. ( 10.1038/35055178) [DOI] [PubMed] [Google Scholar]
- 49.Reece SE, Khan SM, Waters AP, Janse CJ, Kaczanowski S. 2012. Why are male malaria parasites in such a rush? Sex-specific evolution and host–parasite interactions. Evol. Med. Public Health 2013, 3–13. ( 10.1093/emph/eos003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung H, Loehlin DW, Dufour HØD, Vaccarro K, Millar JG, Carroll SB. 2014. A single gene affects both ecological divergence and mate choice in Drosophila. Science 343, 1148–1151. ( 10.1126/science.1249998) [DOI] [PubMed] [Google Scholar]
- 51.Servedio MR, Doorn GSV, Kopp M, Frame AM, Nosil P. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26, 389–397. ( 10.1016/j.tree.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 52.Molina-Cruz A, et al. 2013. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984–987. ( 10.1126/science.1235264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter R, Graves P, Keister D, Quakyi I. 1990. Properties of epitopes of Pfs48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. 12, 587–603. ( 10.1111/j.1365-3024.1990.tb00990.x) [DOI] [PubMed] [Google Scholar]
- 54.Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. 1997. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect. Immun. 65, 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tachibana M, Sato C, Otsuki H, Sattabongkot J, Kaneko O, Torii M, Tsuboi T. 2012. Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate. Vaccine 30, 1807–1812. ( 10.1016/j.vaccine.2012.01.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for figures 2–5 is available as electronic supplementary material and DNA sequences are available at GenBank (KP849590–KP849808).


