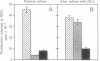
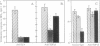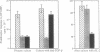Abstract
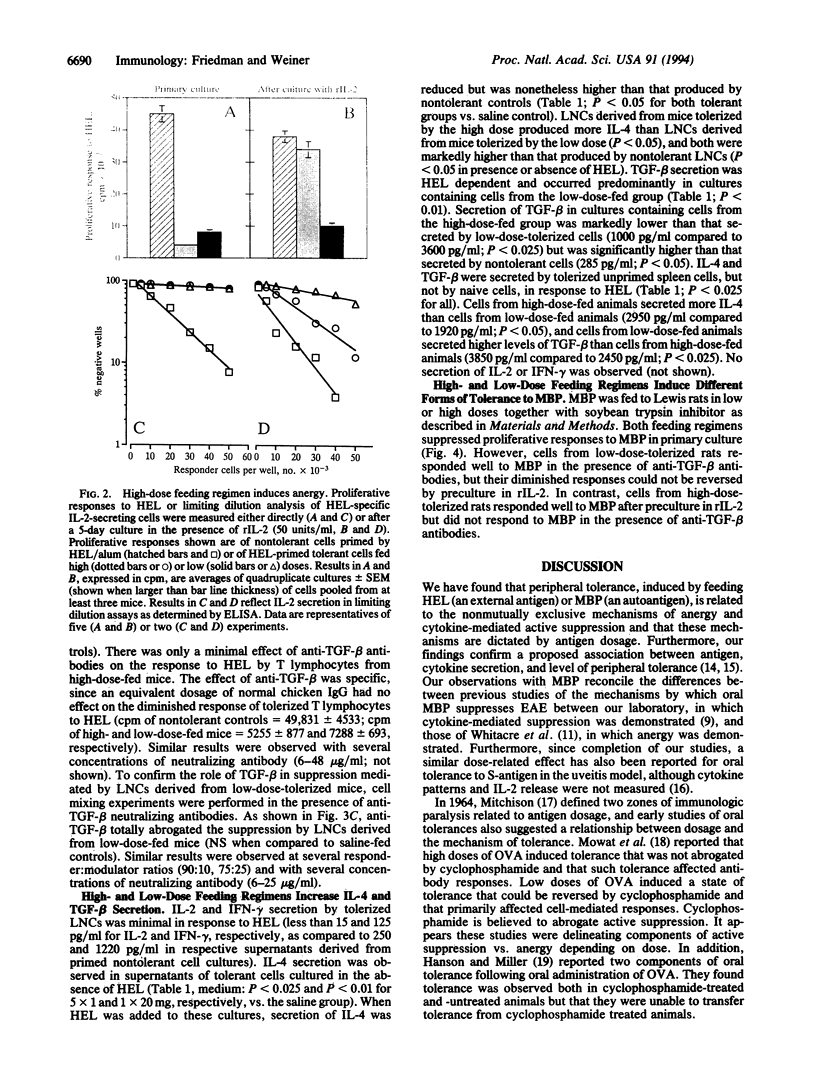
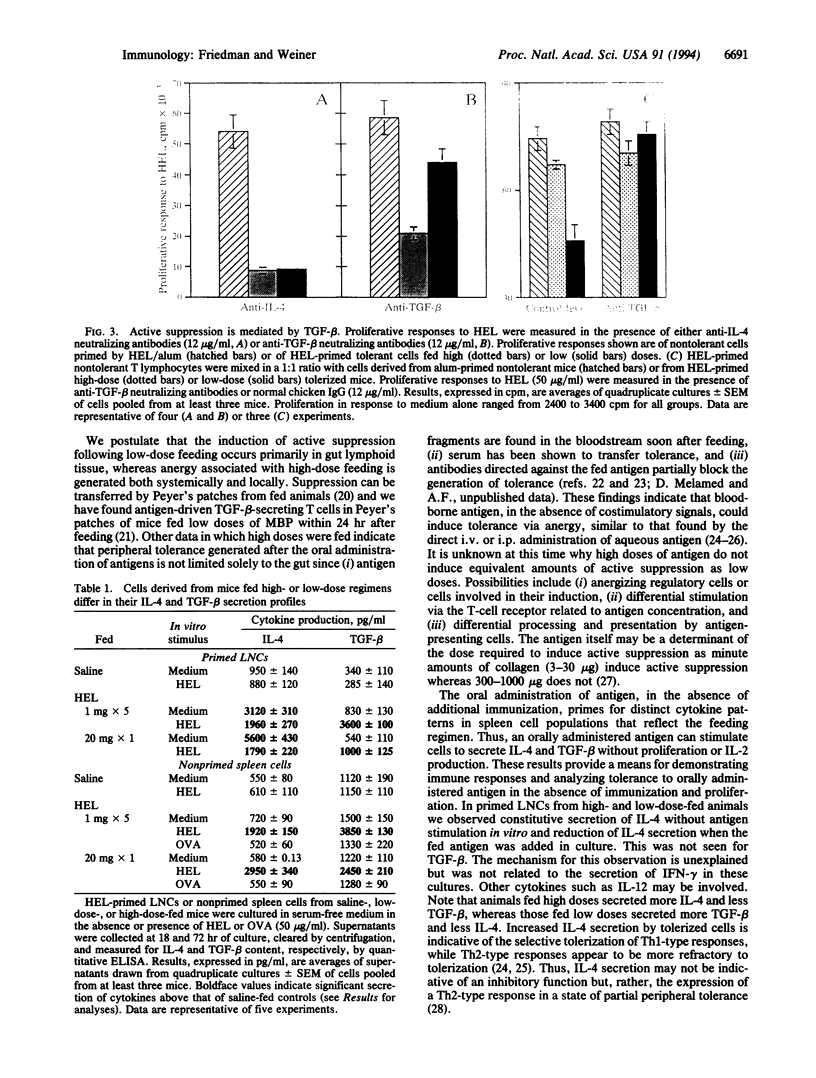
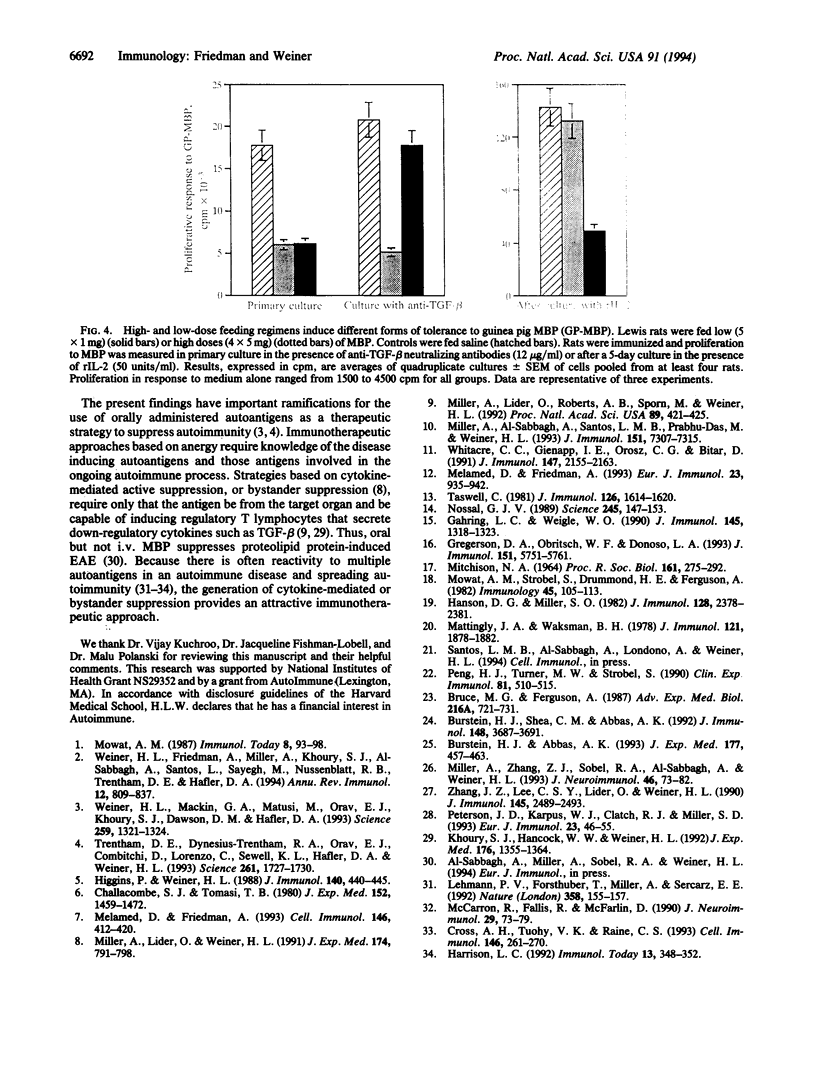
Oral tolerance was generated to hen egg white lysozyme in the mouse or to guinea pig myelin basic protein in the rat by a low-dose (1 mg) or a high-dose (5-20 mg) feeding regimen. High doses of antigen induced tolerance characterized by anergy with little or no active suppression and increased secretion of interleukin 4 (IL-4). Anergy was shown by an increase in frequency of IL-2-secreting cells following culture in recombinant IL-2. Low doses of antigen induced tolerance characterized by antigen-driven active suppression with increased secretion of transforming growth factor beta (TGF-beta) and IL-4 and minimal anergy. Without further immunization, spleen cells from animals orally tolerized by both regimens secreted increased levels of IL-4 and TGF-beta in an antigen-specific manner. Animals fed high doses secreted more IL-4 and less TGF-beta, whereas those fed low doses secreted more TGF-beta and less IL-4. These results demonstrate that the two feeding regimens induced cell populations that differed in their cytokine secretion profile and their capacity to actively suppress in vitro and to induce anergy. Our results provide a basis for distinguishing different forms of antigen-driven peripheral tolerance and have important implications for orally induced antigen-specific modulation of human autoimmune diseases.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce M. G., Ferguson A. Oral tolerance induced by gut-processed antigen. Adv Exp Med Biol. 1987;216A:721–731. doi: 10.1007/978-1-4684-5344-7_84. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H. J., Shea C. M., Abbas A. K. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma-producing (Th1) cells. J Immunol. 1992 Jun 15;148(12):3687–3691. [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. H., Tuohy V. K., Raine C. S. Development of reactivity to new myelin antigens during chronic relapsing autoimmune demyelination. Cell Immunol. 1993 Feb;146(2):261–269. doi: 10.1006/cimm.1993.1025. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Weigle W. O. The regulatory effects of cytokines on the induction of a peripheral immunologic tolerance in mice. J Immunol. 1990 Sep 1;145(5):1318–1323. [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Donoso L. A. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993 Nov 15;151(10):5751–5761. [PubMed] [Google Scholar]
- Hanson D. G., Miller S. D. Inhibition of specific immune responses by feeding protein antigens. V. Induction of the tolerant state in the absence of specific suppressor T cells. J Immunol. 1982 May;128(5):2378–2381. [PubMed] [Google Scholar]
- Harrison L. C. Islet cell antigens in insulin-dependent diabetes: Pandora's box revisited. Immunol Today. 1992 Sep;13(9):348–352. doi: 10.1016/0167-5699(92)90170-C. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol. 1988 Jan 15;140(2):440–445. [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- MITCHISON N. A. INDUCTION OF IMMUNOLOGICAL PARALYSIS IN TWO ZONES OF DOSAGE. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- McCarron R. M., Fallis R. J., McFarlin D. E. Alterations in T cell antigen specificity and class II restriction during the course of chronic relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1990 Sep-Oct;29(1-3):73–79. doi: 10.1016/0165-5728(90)90149-h. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Modification of the immune response by oral tolerance: antigen requirements and interaction with immunogenic stimuli. Cell Immunol. 1993 Feb;146(2):412–420. doi: 10.1006/cimm.1993.1037. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Weiner H. L. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991 Oct 1;174(4):791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Zhang Z. J., Sobel R. A., al-Sabbagh A., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993 Jul;46(1-2):73–82. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- Miller A., al-Sabbagh A., Santos L. M., Das M. P., Weiner H. L. Epitopes of myelin basic protein that trigger TGF-beta release after oral tolerization are distinct from encephalitogenic epitopes and mediate epitope-driven bystander suppression. J Immunol. 1993 Dec 15;151(12):7307–7315. [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Immunologic tolerance: collaboration between antigen and lymphokines. Science. 1989 Jul 14;245(4914):147–153. doi: 10.1126/science.2526369. [DOI] [PubMed] [Google Scholar]
- Peng H. J., Turner M. W., Strobel S. The generation of a 'tolerogen' after the ingestion of ovalbumin is time-dependent and unrelated to serum levels of immunoreactive antigen. Clin Exp Immunol. 1990 Sep;81(3):510–515. doi: 10.1111/j.1365-2249.1990.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Karpus W. J., Clatch R. J., Miller S. D. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur J Immunol. 1993 Jan;23(1):46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Trentham D. E., Dynesius-Trentham R. A., Orav E. J., Combitchi D., Lorenzo C., Sewell K. L., Hafler D. A., Weiner H. L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993 Sep 24;261(5129):1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Mackin G. A., Matsui M., Orav E. J., Khoury S. J., Dawson D. M., Hafler D. A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993 Feb 26;259(5099):1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- Whitacre C. C., Gienapp I. E., Orosz C. G., Bitar D. M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991 Oct 1;147(7):2155–2163. [PubMed] [Google Scholar]
- Zhang Z. Y., Lee C. S., Lider O., Weiner H. L. Suppression of adjuvant arthritis in Lewis rats by oral administration of type II collagen. J Immunol. 1990 Oct 15;145(8):2489–2493. [PubMed] [Google Scholar]