Abstract
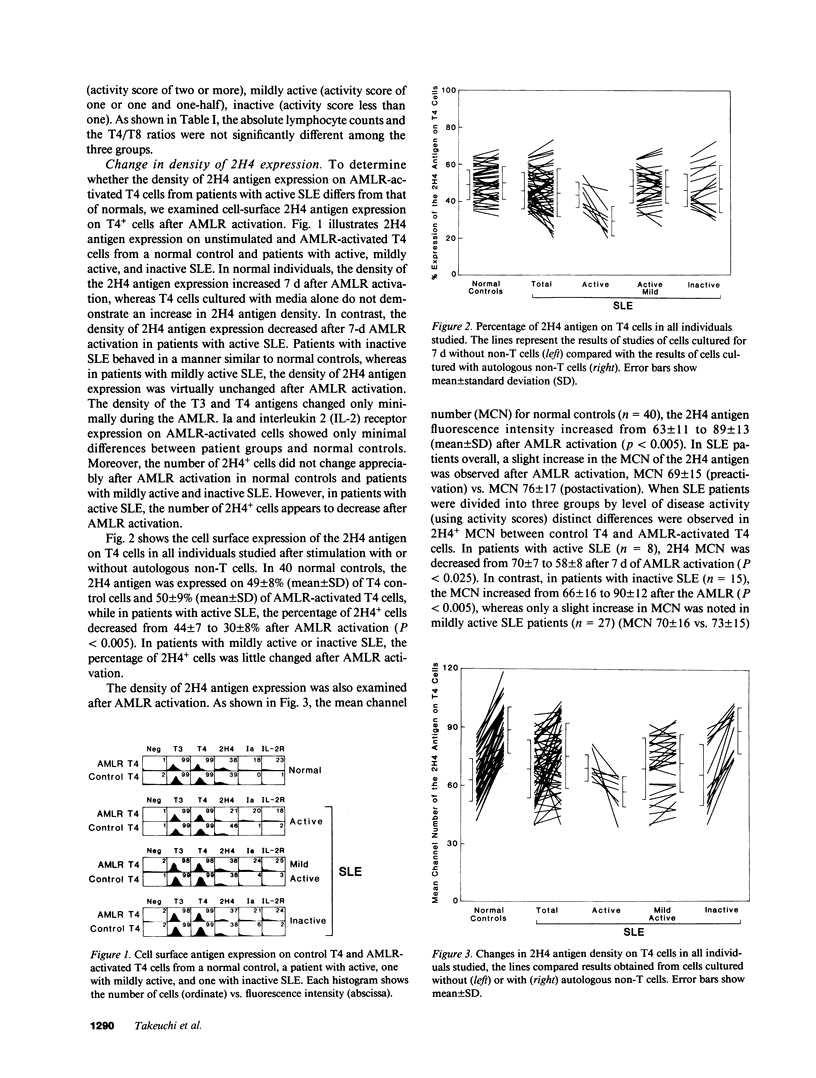
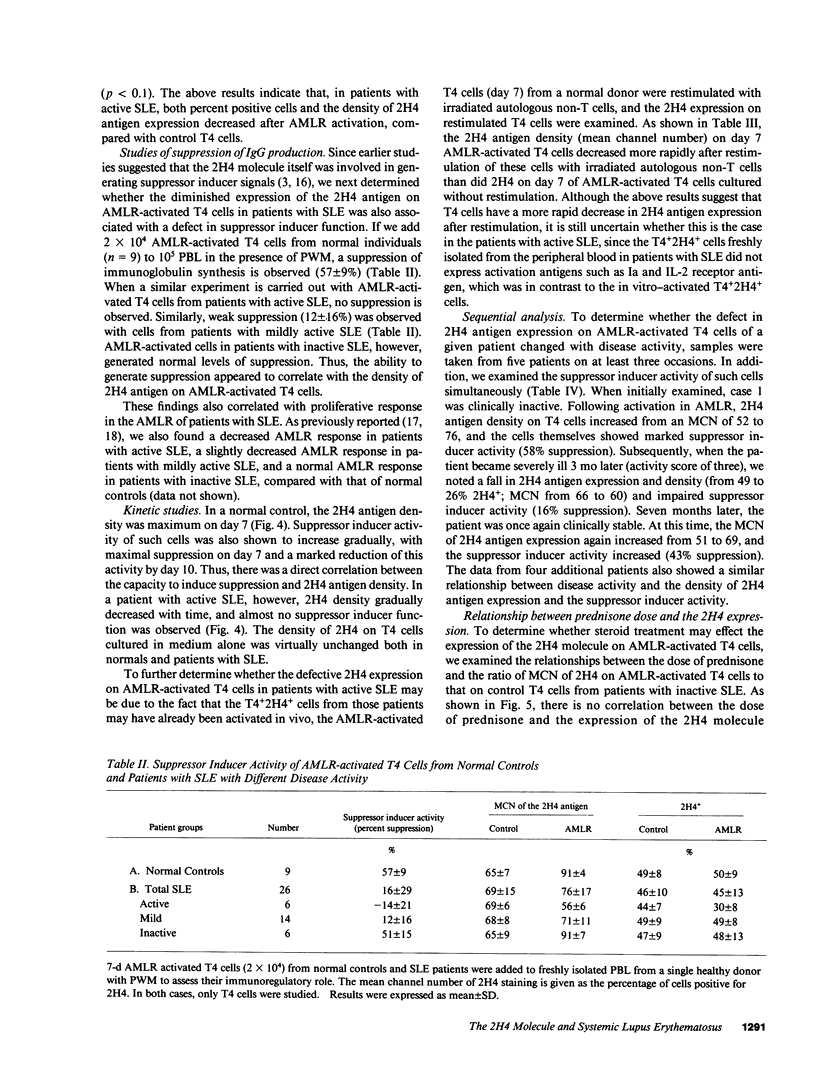
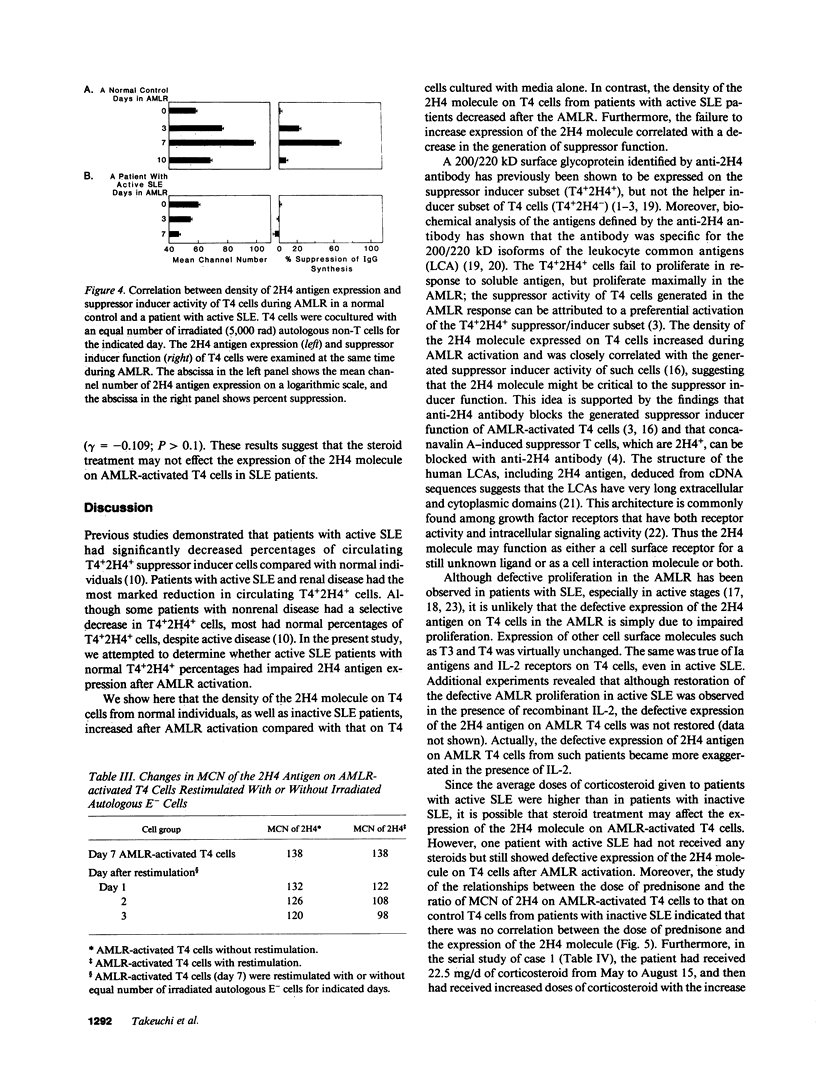
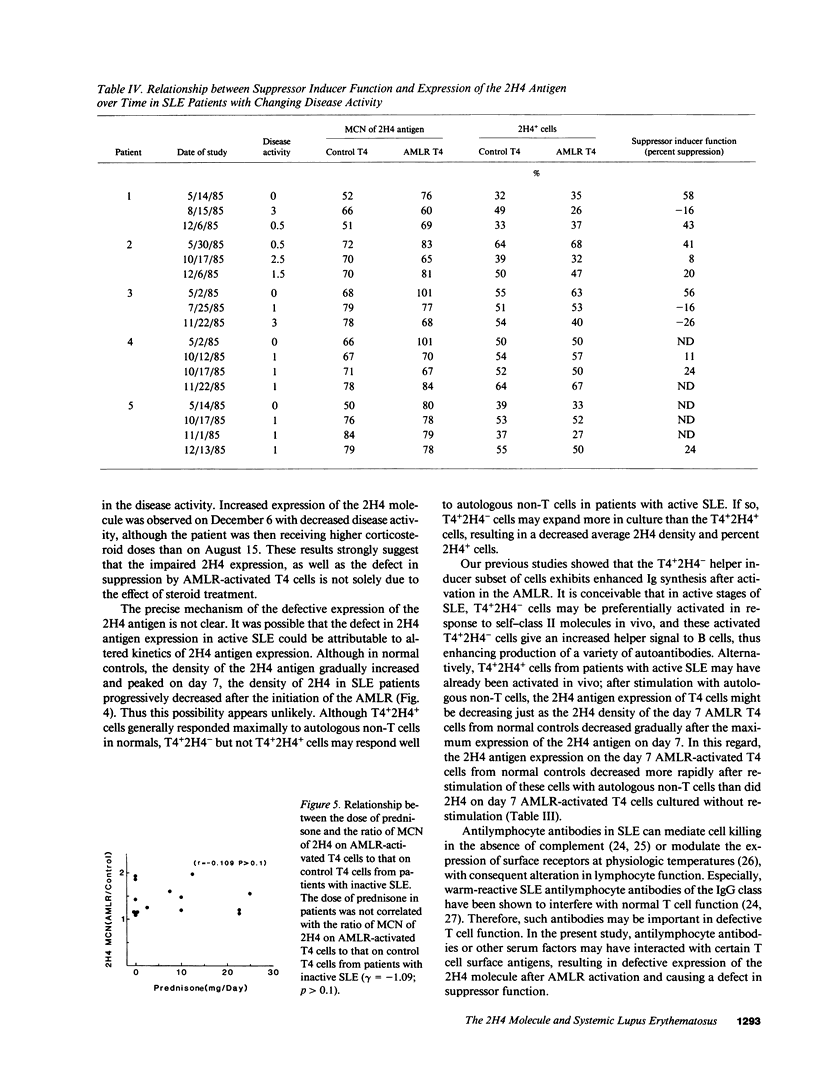
Previous studies demonstrated that patients with active systemic lupus erythematosus (SLE), especially those with active renal disease, had a marked reduction in T4+2H4+ suppressor inducer cells in their peripheral blood. However, it was puzzling to find that active SLE patients without renal diseases often had normal percentages of T4+2H4+ cells. In the present study, we attempted to determine whether active SLE patients bearing normal percentages of T4+2H4+ cells had a defect in their expression of the 2H4 molecule on T4+ cells after autologous mixed lymphocyte reaction (AMLR) activation. The peripheral blood lymphocytes (PBL) from 50 SLE patients with normal percentages of T4+2H4+ cells (greater than or equal to 7% in PBL) were studied and the results were compared with those of 40 normal individuals. The density of the 2H4 molecule on T4 cells from normal controls increased during the 7-d AMLR; in contrast T4 cells from patients with SLE, especially those with active SLE, had defective expression of the 2H4 antigen after AMLR activation. Patients with inactive SLE, like normals, showed an increase in the 2H4 molecule after AMLR activation. Moreover, a strong correlation was observed between percent suppression of pokeweed mitogen (PWM)-driven IgG synthesis and the density of the 2H4 antigen on AMLR-activated T4 cells. Serial analysis of patients with SLE showed that the density of the 2H4 antigen expression and the suppressor inducer activity of AMLR-activated T4 cells were inversely correlated with disease activity. Thus, defective expression of the 2H4 antigen may be an important mechanism for the failure of active SLE patients with normal percentages of T4+2H4+ cells to generate suppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaese R. M., Grayson J., Steinberg A. D. Increased immunoglobulin-secreting cells in the blood of patients with active systemic lupus erythematosus. Am J Med. 1980 Sep;69(3):345–350. doi: 10.1016/0002-9343(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Steinberg A. D. Fractionation of cells on a discontinuous Ficoll gradient. Study of subpopulations of human T cells using anti-T-cell antibodies from patients with systemic lupus erythematosus. J Clin Invest. 1976 Mar;57(3):604–614. doi: 10.1172/JCI108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai S., Steinberg A. D., Green I. Antibodies to T cells in patients with systemic lupus erythematosus can induce antibody-dependent cell-mediated cytotoxicity against human T cells. J Clin Invest. 1981 Mar;67(3):605–614. doi: 10.1172/JCI110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. The cellular basis of the impaired autologous mixed lymphocyte reaction in patients with systemic lupus erythematosus. J Clin Invest. 1979 Jan;63(1):151–153. doi: 10.1172/JCI109270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- Litvin D. A., Cohen P. L., Winfield J. B. Characterization of warm-reactive IgG anti-lymphocyte antibodies in systemic lupus erythematosus. Relative specificity for mitogen-activated T cells and their soluble products. J Immunol. 1983 Jan;130(1):181–186. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Brown H. M., Schlossman S. F. The cellular basis for the induction of antigen-specific T8 suppressor cells. Eur J Immunol. 1986 Feb;16(2):198–204. doi: 10.1002/eji.1830160216. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Rudd C. E., Hagan M., Takeuchi T., Schlossman S. F. The role of the 2H4 molecule in the generation of suppressor function in Con A-activated T cells. J Immunol. 1986 Nov 15;137(10):3247–3253. [PubMed] [Google Scholar]
- Morimoto C., Steinberg A. D., Letvin N. L., Hagan M., Takeuchi T., Daley J., Levine H., Schlossman S. F. A defect of immunoregulatory T cell subsets in systemic lupus erythematosus patients demonstrated with anti-2H4 antibody. J Clin Invest. 1987 Mar;79(3):762–768. doi: 10.1172/JCI112882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira K., Searles R. P., Tanimoto K., Horiuchi Y., Williams R. C., Jr T lymphocyte interaction with immunoglobulin G antibody in systemic lupus erythematosus. J Clin Invest. 1982 Apr;69(4):1026–1038. doi: 10.1172/JCI110506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Morimoto C., Wong L. L., Schlossman S. F. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987 Dec 1;166(6):1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Arnett F. C., Reinertsen J. L., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Arthritis Rheum. 1979 Jul;22(7):770–776. doi: 10.1002/art.1780220713. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Rudd C. E., Schlossman S. F., Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987 Jan;17(1):97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Schlossman S. F., Morimoto C. The 2H4 molecule but not the T3-receptor complex is involved in suppressor inducer signals in the AMLR system. Cell Immunol. 1987 Jun;107(1):107–114. doi: 10.1016/0008-8749(87)90270-x. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Meuer S. C., Romain P. L., Schlossman S. F. A monoclonal antibody that blocks class II histocompatibility-related immune interactions. Hum Immunol. 1984 May;10(1):23–40. doi: 10.1016/0198-8859(84)90083-1. [DOI] [PubMed] [Google Scholar]
- Wernet P., Kunkel H. G. Antibodies to a specific surface antigen of T cells in human sera inhibiting mixed leukocyte culture reactions. J Exp Med. 1973 Oct 1;138(4):1021–1026. doi: 10.1084/jem.138.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B. Anti-lymphocyte antibodies in systemic lupus erythematosus. Clin Rheum Dis. 1985 Dec;11(3):523–549. [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Kunkel H. G. Association of cold-reactive antilymphocyte antibodies with lymphopenia in systemic lupus erythematosus. Arthritis Rheum. 1975 Nov-Dec;18(6):587–594. doi: 10.1002/art.1780180609. [DOI] [PubMed] [Google Scholar]


