Abstract
19F MRI oximetry and 1H blood oxygen level dependent (BOLD) MRI were used to investigate tumor oxygenation in rat breast 13762NF carcinomas and correlations between the techniques were examined. A range of tissue pO2 values was found in the nine tumors while the anesthetized rats breathed air with individual tumor pO2 ranging from a mean of 1 to 36 torr and hypoxic fraction HF10 (<10 torr) ranging from 0 to 75% indicating a large intra- and inter-tumor heterogeneity. Breathing oxygen produced significant increase in tumor pO2 (mean ∆pO2 =50 torr) and decrease in HF10 (p<0.01). 1H BOLD MRI observed using a spin echo planar imaging (EPI) sequence revealed a heterogeneous response and significant increase in mean tumor signal intensity (∆SI =7%, p<0.01). R2* measured by multi-gradient echo (MGRE) MRI decreased significantly in response to oxygen (mean ∆R2* = −4 s−1; p<0.05). A significant correlation was found between changes in mean tumor pO2 and mean EPI BOLD ∆SI accompanying oxygen breathing (r2 >0.7, p<0.001). Our results suggest that BOLD MRI provides information about tumor oxygenation and may be useful to predict pO2 changes accompanying interventions. Significantly, the magnitude of the BOLD response appears to be predictive for residual tumor hypoxic fractions.
Keywords: tumor oxygenation, BOLD, 19F MRI, transverse relaxation rate R2*, oxygen, hexafluorobenzene
Introduction
Tumor oxygenation has been widely recognized as a potent factor influencing tumor response to various therapies, especially radiotherapy and hypoxia appears to promote tumor malignant progression and metastasis (1). Given the importance of tumor oxygenation, many measurement techniques have been developed (1,2). While each method has specific attributes, many are highly invasive or cannot be applied to longitudinal studies of oxygen dynamics.
BOLD (blood oxygen level dependent) MRI, extensively used in studying brain function, is increasingly being applied to assess blood oxygenation and vascular function in tumors non-invasively (3–12). The underlying rationale is that the paramagnetic deoxyhemoglobin creates microscopic field gradients, which enhance the transverse relaxation rate, R2*, of water protons in blood and in the tissue adjacent to blood vessels. Decrease in deoxyhemoglobin concentration leads to a decreased R2*, and thus, to an increased signal intensity in T2*-weighted MRI (13,14). Gradient-recalled echo (GRE) or spin echo planar imaging (EPI) is sensitive to changes in R2*. However, BOLD contrast is also influenced by other factors such as blood flow, blood volume and vascular architecture (3,15). Several recent studies have attempted to correlate BOLD MRI with tissue pO2 measured by various techniques, notably, oxygen electrodes, oxygen sensitive fiber optic probes, ESR and 19F MRI (4,11,16,17). Some of these studies have indicated a strong quantitative correlation with tissue pO2 (11), some found a qualitative relationship (16,17), while others suggested a lack of direct correlation, yet consistent temporal trends (4).
We have developed a method for measuring tumor oxygenation and dynamics based on 19F NMR EPI following direct intratumoral injection of the reporter molecule hexafluorobenzene (HFB): FREDOM (Fluorocarbon Relaxometry using Echo planar imaging for Dynamic Oxygen Mapping) (2). This technique provides quantitative pO2 measurements at multiple specific locations simultaneously within a tumor, and reveals acute dynamic changes at individual locations with respect to interventions, such as hyperoxic gas breathing and vascular modifiers. The aim of this study was to compare 19F oximetry (FREDOM) with 1H BOLD MRI in evaluating tumor oxygenation in response to hyperoxic gas (100% oxygen) challenge.
Materials and Methods
Tumor Model
Rat mammary carcinoma 13762NF (originally obtained from the Division of Cancer Treatment, NCI) was implanted syngeneically in a skin pedicle surgically created on the foreback of Fisher 344 adult female rats (~150 g, n = 9, Harlan), as described in detail previously (18). Tumors were allowed to grow and were investigated when tumor volume was 0.2 to 2.1 cm3 (mean volume = 1 cm3). Investigations were approved by the Institutional Animal Care and Use Committee.
MRI experiments
MRI was performed using a 4.7 T horizontal bore magnet with a Varian Unity Inova system. Each rat was given ketamine hydrochloride (120 µl; 100 mg/ml, Aveco, Fort Dodge, IA) as a relaxant (i.p.) and maintained under general anesthesia (air and 1% isoflurane (Baxter International Inc., Deerfield, IL)). The oxygen reporter molecule hexafluorobenzene (HFB, 50 µl, Lancaster, Gainesville, FL) was injected directly into the tumor along two or three tracks in a single central plane of the tumor, coronal to the rat’s body using a Hamilton syringe (Reno, NV) with a custom-made fine sharp needle (32G), as described in detail previously (2). A tunable (1H/19F) volume RF coil was placed around the tumor-bearing pedicle. Each animal was placed on its side in the magnet with no change in position during the whole study. A thermal blanket was used to maintain body temperature. A single 2 mm slice coronal to the rat body containing the strongest fluorine signal was chosen for both 19F pO2 and 1H BOLD studies. 1H and 19F MR images were acquired using a spin-echo sequence. Overlaying the 19F MR image on the corresponding 1H image revealed the distribution of HFB.
1H BOLD
Echo-planar imaging BOLD
A spin echo planar imaging sequence with pulse burst saturation recovery (PBSR) signal preparation was applied, as described previously (8). The initial saturation was designed to minimize in-flow effects. A series of 55 images including 5 baseline with air breathing (images 1–5) and 50 with oxygen breathing (images 6–55) was acquired on the 2 mm coronal section at 5 s intervals using MR parameters: τ = 500 ms (≡ TR), TE = 53.7 ms, field of view (FOV) = 40 × 40 mm, matrix = 32 × 32.
GRE R2*
Multi-gradient echo (MGRE) images with 8 echoes were acquired on the same 2 mm slice during air breathing (baseline) and repeated immediately after the EPI BOLD sequence (~ 5 min after start of O2 breathing) for 8 of 9 tumors. Acquisition parameters were: repetition time (TR) =195 ms, initial echo time (TE) = 7 ms, echo spacing = 6 ms, flip angle = 45°, FOV = 40 × 40 mm, matrix = 128 × 128, 2 averages, acquisition time = 6 min 40 s.
19F Tumor tissue oximetry - FREDOM
Following a re-equilibration period of air breathing (> 15 min), tumor oxygenation was estimated on the basis of 19F pulse burst saturation recovery (PBSR) EPI relaxometry of the HFB, as described previously (2). This approach provided pO2 maps with 1.25 mm in plane resolution and ~3 µl voxel size (FOV = 40 × 40 mm, matrix = 32 × 32, 2 mm thick) in 6.5 minutes. The spin-lattice relaxation rate [R1 (s−1) = 1/T1] was estimated on a voxel-by-voxel basis using a three-parameter monoexponential function, and pO2 was estimated using the relationship pO2 (torr) = (R1 – 0.0835)/0.001876 (2). Seven consecutive pO2 measurements including two baseline and five oxygen breathing were acquired on the same 2 mm section as used for 1H BOLD studies.
Data Analysis
Data were processed using IDL 5.3/5.4 (Research Systems, Boulder, CO). Signal intensity (SI) in the EPI BOLD study was assessed on a voxel-by-voxel basis and averaged at every time point for the whole tumor section. The signal intensity change (ΔSI) of each tumor was normalized to the mean baseline value expressed as a percentage change using the equation:
where SIM and SIb refer to maximum mean SI and mean baseline SI, respectively.
R2* maps were generated using all eight images with variable echo time by fitting an exponential model on a voxel-by-voxel basis. Mean R2* of the whole section was determined for baseline air and oxygen intervention. ΔR2* maps were obtained by subtracting R2* oxygen map - R2* baseline map.
For the FREDOM data, typically 40–100 voxels provided an R1 fit, and potential pO2 value. Since noise may give an apparent relaxation curve (R1) fit, data were selected by applying thresholds of T1 error < 2.5 s and ratio T1error/T1 < 50%. The two criteria are used since T1 can have a very large range from 1.5 to 12 s and thus the absolute error is particularly important for long T1s and the ratio for short T1s. While these criteria appear quite lax, only those voxels, which provided consistently reliable data throughout the whole time course of seven measurements, were included for further analysis.
Statistical significance was assessed using an Analysis of Variance (ANOVA) on the basis of Fisher's Protected Least Significant Difference (PLSD; Statview, SAS Inst. Inc., Cary, NC) or paired Student's t-tests. ANOVA was applied for comparison of multiple repeat measurements and the PLSD examines the importance of individual measurements on the overall population. The assumption is that inhaled gas at various time points is the independent variable, while pO2, R2* and BOLD signal changes are dependant variables. Paired Student t-tests were used to compare individual pairs of data such as pO2 in a specific tumor during air or oxygen breathing.
Results
Overlay of 19F on the corresponding 1H MR image confirmed that HFB was distributed in both peripheral and central regions of a 2 mm thick section, located in the central plane of a representative tumor (Figure 1). The following EPI BOLD, GRE R2* and 19F oximetry measurements all interrogated this thin slice.
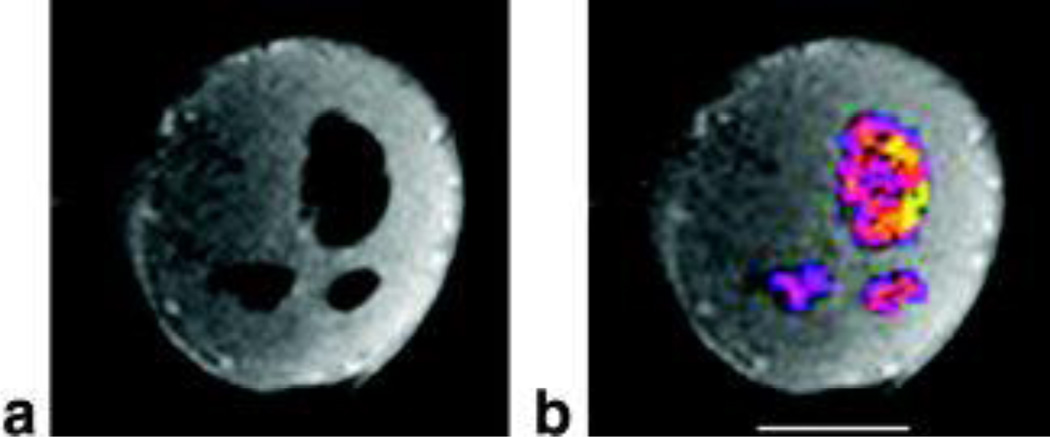
Figure 1. Distribution of oxygen reporter probe.
A. 1H T1-weighted MR image of 2 mm slice through tumor showing signal voids corresponding to presence of HFB reporter molecule.
B. Overlay of 19F signal intensity on 1H image showing distribution of oxygen reporter probe hexafluorobenzene (HFB) in both central and peripheral tumor regions (#2 in Table 1). Bar = 0.5 cm.
1H BOLD
Echo-planar imaging
Normalized SI maps at different time points after switching to oxygen breathing showed heterogeneous response (Figure 2). Significant mean signal enhancement during oxygen inhalation was observed in all nine tumors with a mean ΔSI = 6.7 ± 1.4% (range from 1% to 12%; Table 1). However, individual voxel data showed some regions with negative response (Figure 2). The percentage of voxels with negative response ranged from 12% to 38% in the nine tumors. Baseline signal was quite stable, but there was a rapid response within about 25 s of switching the inhaled gas to oxygen. Some tumors showed a continual increase, which was usually biphasic and approached a stable plateau after about 3 mins. In other cases there was a transient maximum followed by a stable lower value, just marginally above baseline.
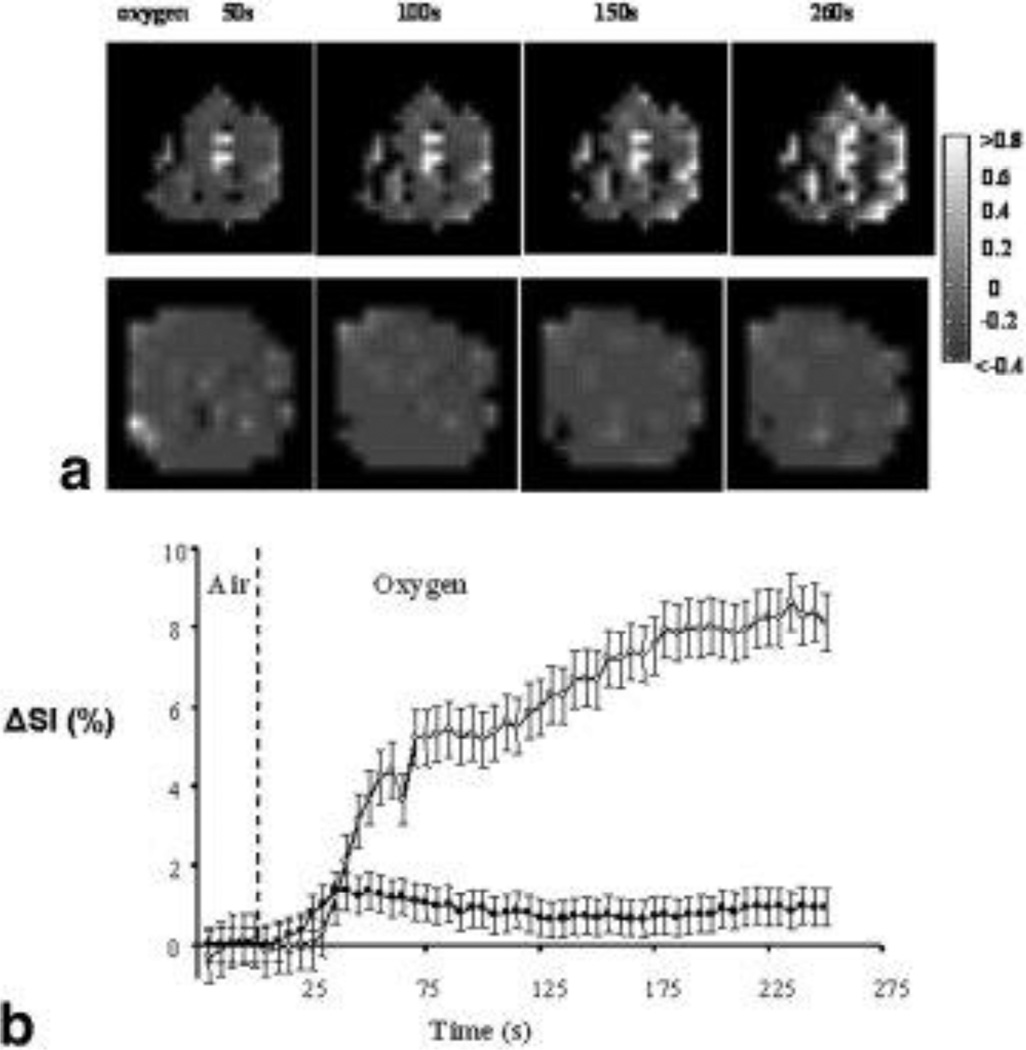
Figure 2. Variations in T2* weighted images with oxygen challenge (BOLD).
A. Normalized spin echo planar images of two tumors acquired at several time points after switching to oxygen breathing. In response to oxygen breathing the two tumors (upper, #2 0.4 cm3; lower, #6 1.4 cm3) showed heterogeneous signal enhancement. Regions of decreased signal intensity were also observed (dark regions).
B. Variations of normalized signal intensity change (ΔSI) versus time for these tumors with respect to oxygen challenge. Both tumors showed an initial rapid response. For tumor #2 (○) this became biphasic reaching a plateau with 9% increase after about 200 s of oxygen breathing. The second tumor (#6, ■) rapidly reached a peak value of ΔSI = 1.4% after 35 s, then gradually decreased to a plateau at about 0.5% increase. Data points are mean values ± standard error.
Table 1.
Comparison of pO2, BOLD and R2* in individual tumors
| Tumor | Tumor volume (cm3) |
19F oximetry | BOLD EPI (%) |
R2* (s−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | ||||||||||
| 21% O2 | 100% O2 | ΔpO2 (torr) |
21% O2 | 100% O2 | ΔR2* | |||||
| pO2 (torr)α | HF10 (%) | pO2 (torr)β | HF10 (%) | |||||||
| 1 | 0.3 | 36 ± 1 | 0 | 172 ± 7** | 0 | 136 | 12.2 ± 0.6 | 54 ± 1 | 51 ± 1* | −3 |
| 2 | 0.4 | 17 ± 2 | 29 | 76 ± 4** | 0 | 59 | 8.7 ± 0.5 | 68 ± 1 | 65 ± 1** | −3 |
| 3 | 0.4 | 13 ± 2 | 31 | 62 ± 8** | 4 | 49 | 7.6 ± 0.6 | 81 ± 1 | 77 ± 1** | −4 |
| 4 | 0.2 | 13 ± 1 | 29 | 81 ± 6** | 4 | 68 | 12.4 ± 0.4 | 116 ± 2 | 102 ± 2** | −14 |
| 5 | 2.1 | 12 ± 1 | 40 | 33 ± 4* | 0 | 21 | 2.3 ± 0.6 | 71 ± 1 | 73 ± 1 | 2 |
| 6 | 1.4 | 13 ± 1 | 38 | 26 ± 5** | 27 | 13 | 1.6 ± 0.5 | 60 ± 1 | 56 ± 1** | −4 |
| 7 | 1.0 | 24 ± 5 | 25 | 72 ± 12** | 0 | 48 | 4.6 ± 0.8 | 56 ± 1 | 51 ± 1** | −5 |
| 8 | 2.1 | 1 ± 2 | 75 | 16 ± 9* | 38 | 16 | 2.4 ± 0.6 | 90 ± 1 | 89 ± 1 | −1 |
| 9 | 0.8 | 35 ± 5 | 6 | 74 ± 9** | 0 | 39 | 8.2 ± 0.6 | NA | NA | NA |
| Mean | 1.0 ± 0.2 | 18 ± 4 | 31 ± 7 | 68 ± 15* | 8 ± 4* | 50 ± 13 | 6.7 ± 1.4 | 74 ± 7 | 70 ± 6* | −4 ± 2 |
NA: not measured;
p < 0.05,
p < 0.001 from baseline (21% O2).
Mean pO2 over all voxels in the two repeated baseline measurements.
mean highest pO2 observed in all voxels during 5 oxygen breathing measurements.
GRE R2*
Baseline maps revealed distinct heterogeneity with R2* ranging from 6 to 450 s−1 (T2*: 2 – 166 ms, Figure 3). In response to oxygen challenge, a small, but significant decrease in R2* was seen predominately in the tumor periphery (p < 0.001; Figure 3). There was no correlation between baseline R2* and ΔR2* on a voxel-by-voxel basis (r2 = 0.1; Figure 3B). For the group of eight tumors, a significant decrease in mean R2* (suggesting increased oxyhemoglobin level) was found (mean ΔR2* = −4 ± 2, p < 0.05, Table 1). One tumor (no. 5; Table 1) showed contrary behavior with increased mean R2* with oxygen breathing. A very close correlation was observed between the mean R2* values during air versus oxygen breathing (Figure 3C). A general trend was observed when the mean BOLD response for individual tumors was compared with ΔR2* (Figure 3D). In essence large ΔR2* was associated with large ΔSI, and an increase in R2* coincided with a small signal change. However, most tumors showed a ΔR2* of −4 ms and this was associated with a large range in BOLD signal change.
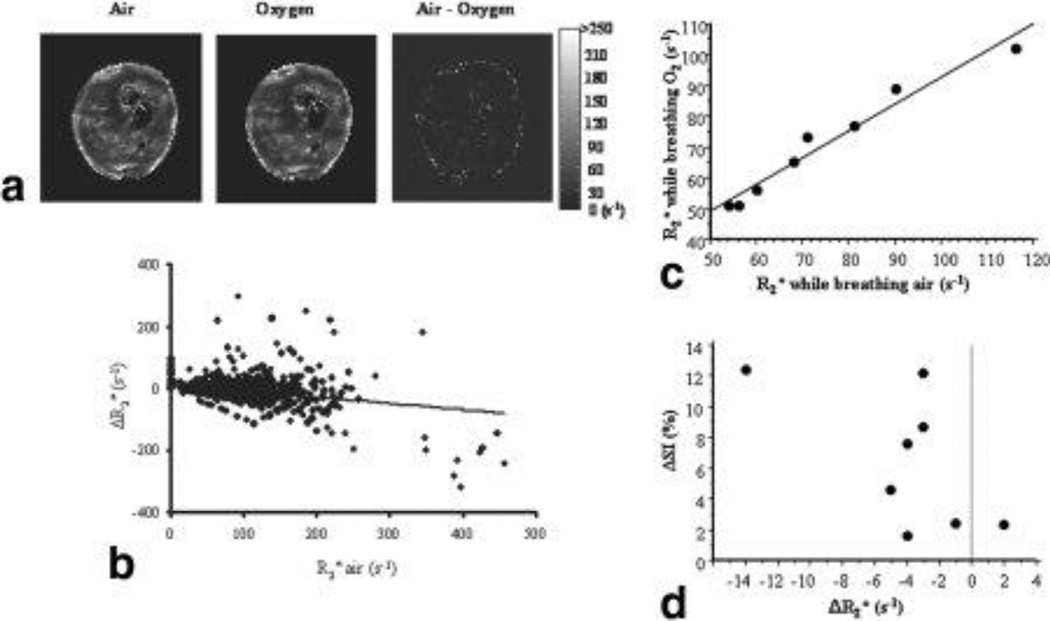
Figure 3. Response of R2* to oxygen breathing.
A. R2* maps showed heterogeneous baseline R2* values for tumor #2. Top left: R2* while breathing air; Center R2* after 5 min breathing oxygen; right difference map obtained by subtracting the oxygen map from the air map (mean ΔR2* = −2.6 s−1).
B. A voxel-voxel comparison (n = 2056) between baseline R2* and ΔR2* showed a poor correlation (r2 = 0.1).
C. Comparison of mean tumor R2* measured while rat breathed oxygen versus air showed a strong correlation (r2>0.96).
D. Comparison of mean signal change in T2*-weighted image versus change in mean R2* in tumors accompanying change in gas from air to oxygen breathing. No linear correlation was observed, but greater change in R2* was accompanied by changes in signal intensity.
19F Tumor tissue oximetry – FREDOM
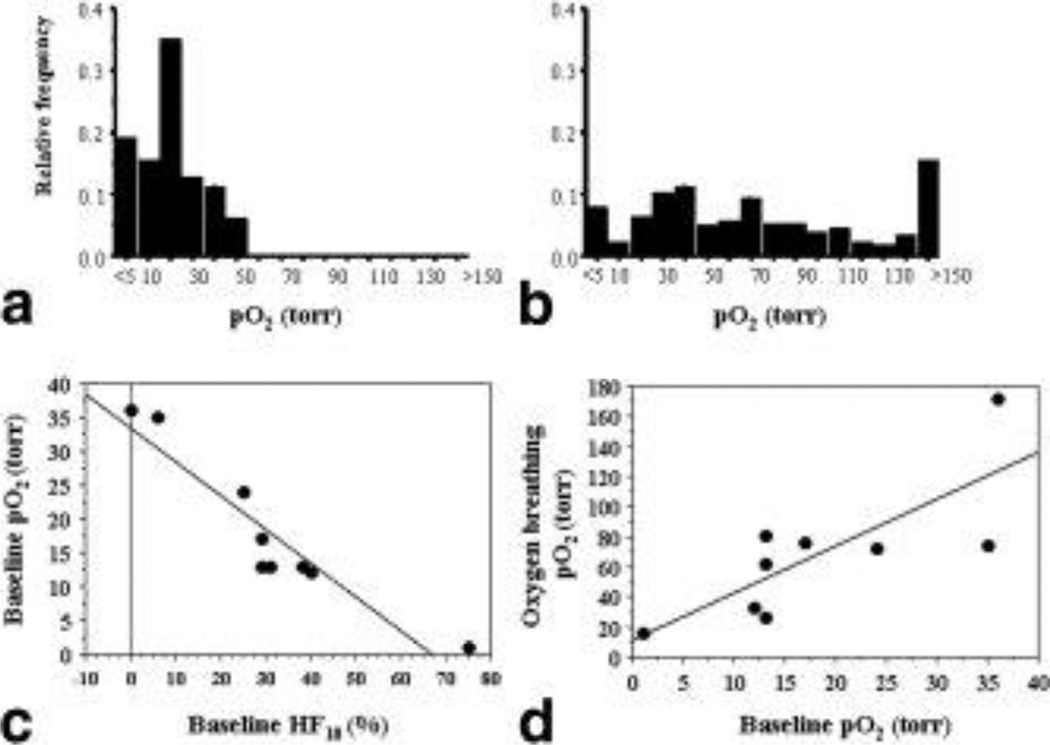
pO2 maps showed a range of baseline pO2 values and a heterogeneous response to oxygen breathing (Figure 4, Table 1). Oxygenation appeared clustered with higher pO2 regions appearing close to the periphery when overlaid on the anatomical 1H MR images of the corresponding tumor slices. Time course of pO2 dynamics showed differential response in both rate and magnitude (Figure 4B). For the group of 9 tumors, baseline pO2 varied from essentially hypoxic (0.3 torr) to well oxygenated (36 torr; Table 1). With respect to oxygen challenge, mean pO2 increased significantly in all the tumors (ΔpO2 = 50 torr; Table 1) and hypoxic fractions (< 10 torr) decreased significantly, from a mean tumor baseline 31% to 8% (p<0.05). In most tumors (7 of 9) the HF10 was essentially eliminated (<5% residual), but in two tumors a substantial hypoxic fraction remained albeit considerably diminished compared with baseline. Histograms for the pooled individual voxels (n= 265) from the nine tumors in response to oxygen challenge revealed significant increase in pO2 to a mean (75 ± 4 torr) and median (63 torr) (p < 0.001) (Figure 5A, B). Hypoxic fractions HF5 (<5 torr) and HF10 (<10 torr) decreased significantly from baseline values of 18% and 34% to 8% and 10%, respectively (p < 0.01). In common with previous observations in this tumor line baseline pO2 tended to decrease with tumor volume (r2>0.52) and as a corollary HF10 increased with tumor volume (r2>0.49) (19,20). A strong inverse correlation was found between tumor mean pO2 and HF10 (Figure 5C). The change in pO2 (ΔpO2) tended to increase for tumors with higher baseline pO2 (r2>0.4), but the relationship was much stronger when comparing pO2 during oxygen breathing versus baseline pO2 (r2>0.6; Figure 5D).
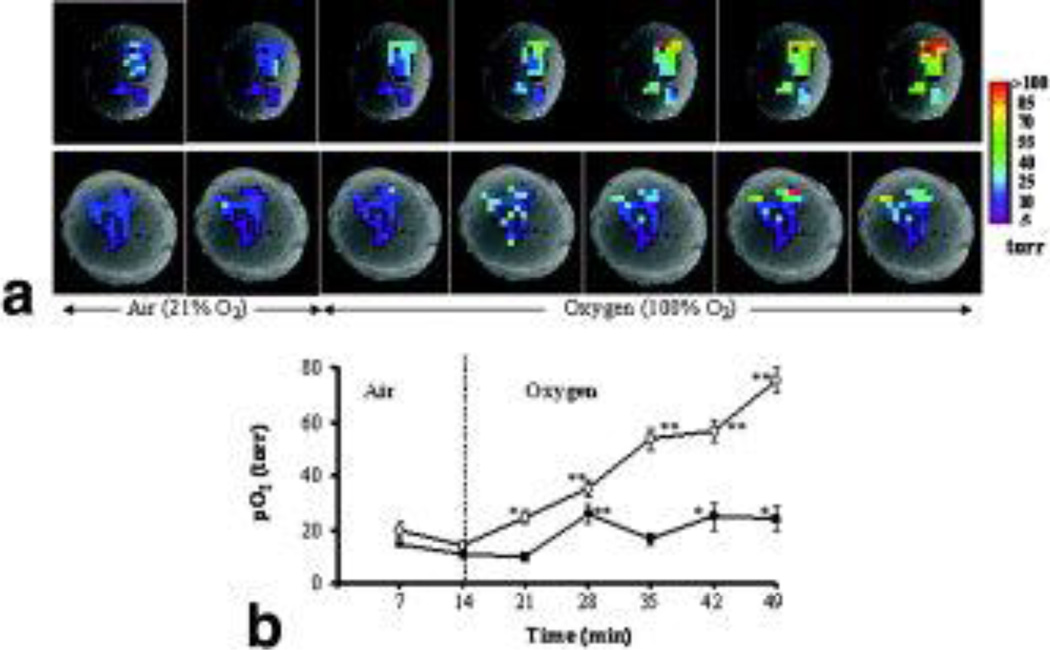
Figure 4. Variation of pO2 with oxygen challenge.
A. pO2 maps obtained at successive times overlaid on T1-weighted 1H images of two tumors (#2 and #6). A range of pO2 values was observed in both tumors under baseline conditions. In response to breathing oxygen, all the individual locations (34 voxels) in tumor # 2 (upper row) responded significantly and became well oxygenated. By contrast, some of initially hypoxic regions in tumor #6 (lower row) remained hypoxic, while others became well oxygenated.
B. Variation in mean pO2 of each tumor. Tumor #2 (○; mean baseline = 17 torr) showed significantly increased pO2 within 7 min of oxygen breathing, and continued to increase reaching 76 torr during the final measurement (49 min). Tumor #6 (■, mean baseline pO2 = 13 torr) reached a peak value (26 torr) after 14 min, but the settled back to a lower level. * p < 0.05; ** p < 0.001.
Figure 5. Tumor oxygen tension distribution.
A. Pooled pO2 values for individual regions (265) from the nine tumors showed a range of baseline pO2 values from hypoxia (<5 torr) to 55 torr, with a mean (x) = 15 ± 1 torr and median (m) = 13.1 torr, while rats breathed air. Binning is based on ranges, e.g., 10 refers to 5 ≤ pO2 <10 torr.
B. Oxygen breathing produced a significant increase in pO2 with mean (75 ± 4 torr) and median (63 torr) (p < 0.001). Hypoxic fractions HF5 (<5 torr) and HF10 (<10 torr) decreased significantly from baseline values of 18% and 34% to 8% and 10%, respectively (p < 0.01).
C. Correlation between mean baseline pO2 and hypoxic fraction showed inverse relationship (r2 > 0.87).
D. Dependence of pO2 achieved with oxygen breathing on baseline pO2 (r2 > 0.6)
Correlation of pO2 with 1H BOLD
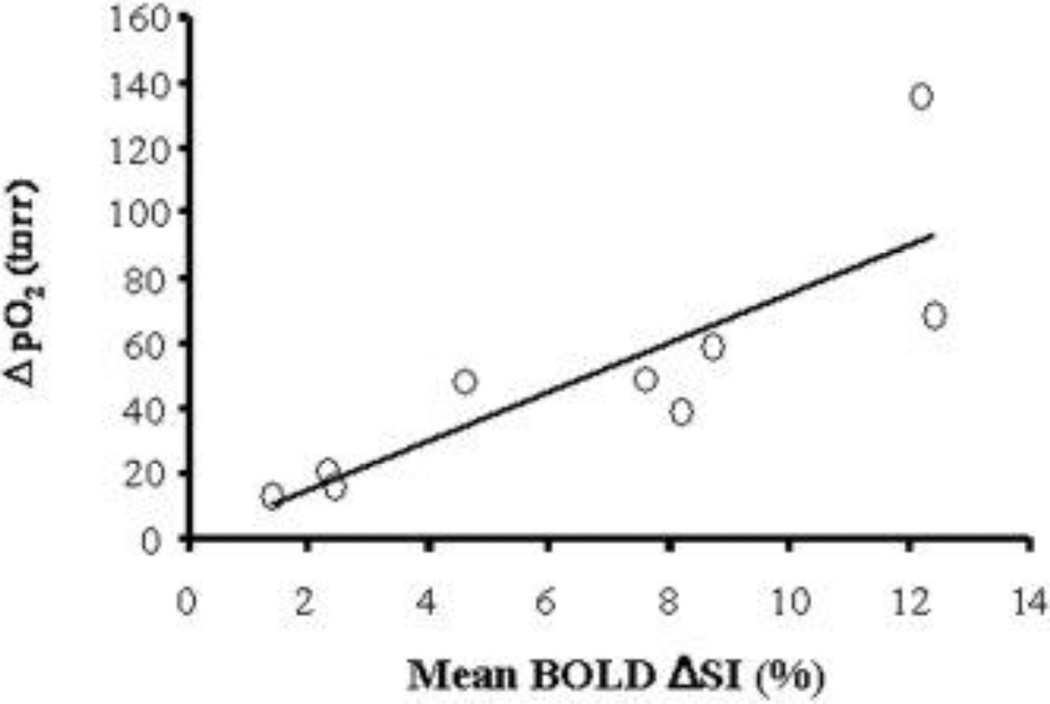
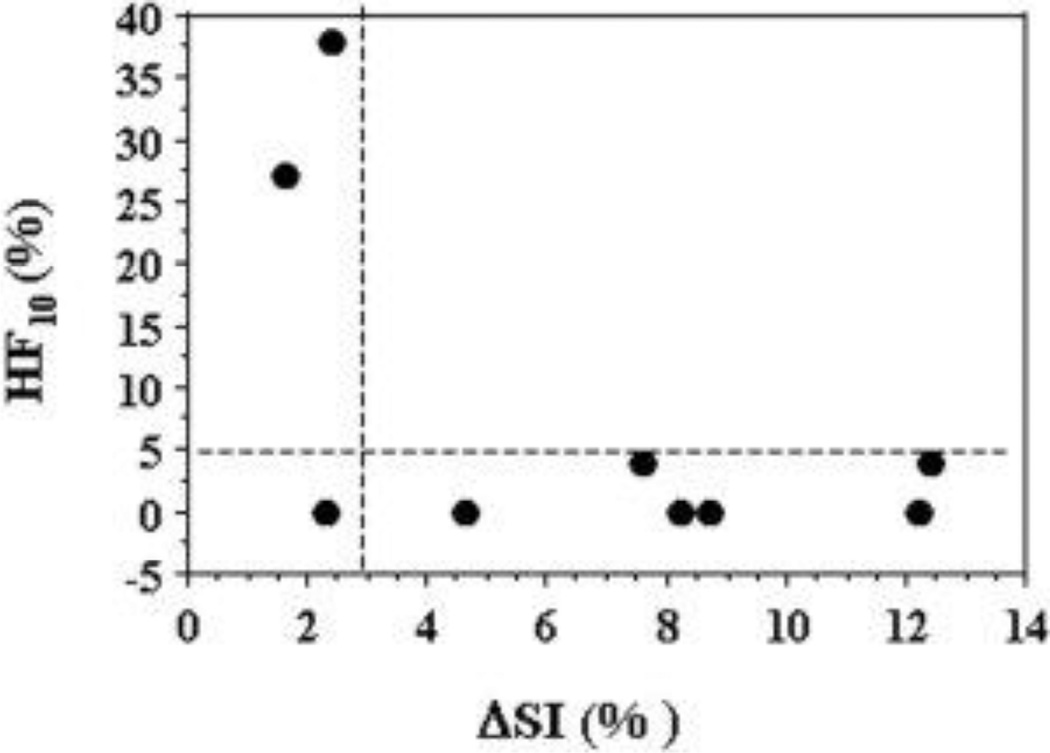
For the group of nine tumors, a significant linear correlation was found between mean ΔpO2 and ΔSI, detected by 19F oximetry and 1H EPI BOLD (r2 > 0.7, p < 0.001; Figure 6). However, there was a weak correlation between baseline pO2 and ΔSI (r2 = 0.3, data not shown). Likewise comparison of ΔR2* with baseline pO2, maximum pO2 during oxygen breathing or ΔpO2 indicated no correlations (r2<0.01). Every tumor exhibiting a large BOLD response (ΔSI>3%) had a negligible residual hypoxic fraction (HF10<5%) during oxygen breathing. In two of three tumors showing a small BOLD response a large HF10 remained (Figure 7).
Figure 6. Correlation of pO2 and BOLD responses to oxygen challenge.
A significant linear correlation was found between mean increase in tissue pO2 (ΔpO2) and mean spin echo planar BOLD signal increase in the nine tumors with respect to oxygen intervention (r2 > 0.7; p < 0.001).
Figure 7. Assessment of residual hypoxic fraction.
Comparison of the final hypoxic fraction (HF10) with oxygen breathing as a function of BOLD signal response (ΔSI) suggests strong predictive value. For most tumors (6 of 9) a large BOLD response coincided with low residual HF10. A small BOLD response indicated a large residual hypoxic fraction in 2 of 3 tumors.
Discussion
Oxygenation in 13762NF rat breast tumors was found to cover a considerable range with both intra- and inter-tumor heterogeneity, but a tendency towards lower pO2 and greater hypoxia in larger tumors, as also reported previously by us (19,20). Likewise tumor response to modulation by hyperoxic gas breathing, which resulted in increased pO2 and reduced residual hypoxic fraction was in line with previous observations (19–21). Here, we have undertaken consecutive 1H MRI BOLD and 19F MRI oximetry investigations to examine potential correlations. A strong correlation was found between mean BOLD signal response (ΔSI) and change in mean pO2 (Figure 6) supporting previous observations in various tumor types reported by others (4,11,17). More significantly, a small BOLD response usually indicates a substantial residual hypoxic fraction, while a large BOLD response to breathing oxygen indicates that the tumor is well oxygenated or becomes well oxygenated (Figure 7).
Quantitative oximetry (absolute pO2 values) has been shown to predict for local recurrence and disease free survival in several human cancers (notably, cervical and head and neck (22,23)). To date, electrodes have provided the only quantitative clinical pO2 measurements in tumors, and electrodes are not only invasive, but the Eppendorf Histograph cannot easily show changes in pO2 with respect to interventions. Furthermore, the Histograph is no longer commercially available. 19F MRI based on perfluorocarbon reporters has been used to measure pO2 in the human eye (24), but it remains generally restricted to pre-clinical animal studies (2) for two fundamental reasons: lack of access to clinical 19F MRI capabilities and lack of FDA approval for human use of PFCs. ESR can also measure pO2 distributions in rodent tumors (16,25,26), but while it has been used to measure oxygenation in humans based on India ink tattoos, it is also handicapped by lack of clinical instrumentation.
BOLD contrast MRI is an attractive surrogate for clinical pO2 measurements, since endogenous hemoglobin itself serves as the reporter molecule. A few studies of human tumor BOLD have been presented (5,27–29), but further validation relative to other techniques is of the utmost importance. Fundamental reports from Thulborn (30) and Wright (31) together with applications of fMRI in the brain lay a strong foundation for vascular oxygen measurements. Several groups (3,4,6,8,10–14,16,17,26,32) have explored BOLD responses in diverse tumor types with respect to varying oxygen concentrations and carbogen. There is considerable evidence that signal changes in T2*-weighted images reflect changes in pO2. However, some investigations have shown a lack of direct correlation, e.g., Baudelet and Gallez (4) reported that a 10% change in signal could correspond to a small (< 25 torr) or large (approaching 100 torr) change in pO2 in syngeneic FSA mouse tumors. Importantly, both represent large changes in pO2 by radiobiological standards. Elas et al. (16) showed correlation between EPRI based on vascular trityl spin probe and sequential BOLD measurements in FSA tumors in mice. As with our study their two measurements were sequential rather than concomitant and they had the added complexity of coregistering images from separate modalities.
Fan et al (17) previously compared 19F MR oximetry with BOLD response on a voxel-by-voxel basis in R3230AC rat breast tumors. In common with many experiments they infused perfluorocarbon emulsion, which progressively sequestered in the tumor tissue, while clearing from the vasculature. They reported that 19F and 1H measurement agreed in 65% of pixels, viz. when 19F MR showed increased pO2, then 1H MR linewidth decreased reflecting less deoxyhemoglobin, as assessed using HiSS (High Spatial/Spectral resolution T2* sensitive) measurements. Correlations were even stronger for subsets of pixels selected as showing no pO2 change or a BOLD change. Overall they concluded that regions identified as hypoxic tended to show a small BOLD response to carbogen inhalation in the R3230AC rat breast tumors and 1H MRI gave very few “false positives” (17). Our results are in accord with these previous observations. Tumors exhibiting a large BOLD response also showed a greater mean pO2 response (Figure 6). Most significantly, tumors exhibiting a large BOLD response (here, defined as > 4%) showed essentially no residual hypoxic fraction (HF10< 5%; Figure 7). We found no false positives and only one false negative. While breathing air 7 of 9 tumors had HF10 > 20% (Table 1). In all but two cases this fell below 5% with oxygen breathing, essentiality eliminating the hypoxic fraction. These remaining two tumors showed a particularly small BOLD effect (ΔSI <2.5%). Meanwhile six of seven responsive tumors exhibited a large BOLD effect (>4%). We do note that the 13762NF tumors show relatively low hypoxic fractions (HF10) compared with many reports for tumors implanted in rodents. However, the values are closely in line with measurements reported using the Eppendorf Histograph in breast tumors in patients (33)
We should note differences between Karczmar’s team’s approach and ours. The use of systemically delivered PFC emulsions as reporter molecules tends to provide signal from well perfused tumor regions only. Indeed. Fan et al. (17) predominantly detected 19F signal from the tumor periphery, as also noted by others (34). While our current approach allowed interrogation of central tumor regions, as well as periphery, it required direct injection of HFB into the tumor, which is invasive and samples limited regions only. Thus, it is particularly reassuring that the two approaches provide commensurate results.
BOLD changes reflect vascular oxygenation, whereas FREDOM measures tissue pO2. As expected, the BOLD changes occurred much more rapidly (seconds, Figure 2) than the pO2 changes (minutes, Figure 4). In the future it will be interesting to examine 1H T1-weighted tissue water response or so-called TOLD (Tissue Oxygen Level Dependant) response, as reported by Matsumoto et al. (35), since then both vascular and tissue changes can be assessed by 1H MRI. Matsumoto et al. (35) examined response to hyperbaric oxygen breathing, but others have reported T1 changes associated with hyperoxic gas in normal tissues (36) and tumors (3,10). The kinetic response of the mean BOLD signal was consistent with previous global near infrared observations in this tumor type (20,37). Changes in deoxyhemoglobin concentration generally followed a biphasic time course as also seen in the BOLD response (Figure 2B). This probably represents rapid arteriolar oxygenation followed by more sluggish response in the distant parts of the vascular tree. Meanwhile pO2 response was more sluggish with continued increase over 30 mins (Figure 4B) for a responsive tumor. These observations are in line with previous observations based on oxygen sensitive fiber optic probes and polarographic electrodes in this tumor type (38).
BOLD MRI or susceptibility-weighted R2* measurement based on the intrinsic paramagnetic properties of deoxyhemoglobin have been increasingly applied to assess tumor vasculature (7,9). An increase in BOLD SI or a decrease in R2* may be related to decreased blood deoxyhemoglobin. However, BOLD SI change is also related to several other factors, e.g., changes in tumor blood flow, volume, hematocrit and the ability of red blood cells to traverse tumor capillaries (3). Here, we applied a presaturation sequence across the whole tumor to minimize flow effects. It has been shown that BOLD MRI is probably less sensitive to changes in tumor oxygenation in regions containing very sparse vasculature, and hence, little deoxyhemoglobin (9). There may be concern that poorly vascularized tumors cannot show a measurable BOLD effect, due to lack of deoxyhemoglobin (9,14). As a corollary, we propose that poorly vascularized tumors will also be hypoxic. Thus, a small or absent BOLD effect will be indicative of hypoxia. Indeed, Rodrigues et al. (12) showed that well vascularized GH3 prolactinomas tended to have a much higher R2* than sparsely vascularized Radiation-induced fibrosarcomas (RIF-1). GH3 tumors showed a large ΔR2* in response to carbogen breathing, whereas the RIF-1 tumors showed essentially no change. Breathing carbogen enhanced the response of GH3 tumors to a single high dose of radiation (15 Gy), whereas there was no effect on RIF-1 tumors. Our range of R2* values (54 – 116 s−1) is commensurate with previous reports for animal tumors at 4.7 T (12). In terms of potential clinical applications the ability to stratify patients based on oxic or oxygenatable tumors versus hypoxic (and resistant to modulation) could be significant.
BOLD contrast is related to changes in local deoxyhemoglobin concentration. However, baseline R2* reflects not only blood deoxyhemoglobin level, vascular blood flow and volume, but also local tissue architecture, i.e., cell density, edema, necrosis. Thus, the baseline R2* and change in R2* (ΔR2*) likely depends on tumor type. Recently, Robinson et al. (9) showed heterogeneous inter-tumoral R2* among a variety of tumors, in which the GH3 prolactinoma had the highest mean R2* = 89 s−1 and RIF-1 had the lowest value of 58 s−1. In response to carbogen breathing, a significant decrease in R2* (−23 s−1) was found in the GH3 prolactinoma, whereas the RIF-1 fibrosarcomas showed a little increase in R2* (1 s−1). In the current study, seven out of eight tumors had a modest decrease in R2* (mean = −4 s−1), while R2* increased in one tumor (# 5) in response to oxygen breathing. As expected, the largest ΔSI in response to oxygen breathing coincided with a large decrease in R2* and the one tumor with increased R2* showed small ΔSI. However, most tumors had similar ΔR2* (about 4 s−1) yet a large range of ΔSIs (Figure 3D). Generally, there was no significant correlation between baseline ΔR2* values and R2* (Figure 3B, Table. 1). There was a strong correlation between mean R2* measured during air or oxygen breathing (Figure 3C).
In the past, we had used a thick section (essentially projection) for 19F MR oximetry (FREDOM) studies (2). The 2 mm thick slice used here allowed more satisfactory spatial correlation between 19F oximetry and 1H BOLD contrast. The success of FREDOM depends largely on the signal-to-noise ratio (SNR) of the acquired images. No obvious decrease in SNR due to reduced slice thickness was seen in the current study, which we attribute to greater emphasis on injecting the HFB in a narrow plane and the ability to image oblique angles in the upgraded Varian Inova system. On average, 29 voxels (range 10 to 43) provided reliable pO2 readings which were traceable throughout the oxygen challenge in each tumor.
We had hoped to correlate individual voxels in both 19F and 1H EPI (FREDOM vs. BOLD). However, the directly corresponding voxels did not allow meaningful correlation. Signal voids were observed, which had only 10 to 25% as much 1H signal as surrounding tumor (Figure 1A). Overlaying 19F on 1H images showed that the low 1H signal intensity regions corresponded with 19F signal (Figure 1B). In addition the R2* values were typically an order of magnitude smaller (14 to 37 s−1, as opposed to 100 s−1) with smaller and sometimes opposite changes in R2* compared with surrounding voxels in response to breathing oxygen. We have previously observed such signal voids in 1H images of tumors with HFB (19) and the new alternative 1H MRI pO2 reporter under development hexamethyldisiloxane (39). While 1H and 19F voxels did not allow direct correlation, we believe that the judicious placement of reporter molecule in central and peripheral locations can provide a representation of the whole tumor, and thus, correlation with non-labeled regions is reasonable. Importantly, pO2 values and dynamics observed using FREDOM are highly consistent with other oximetry methods in rat breast and prostate tumors, such as electrodes (38,40), fiber optic probes (37,41) and immuno histochemistry (42).
Oxygen breathing produced a significant increase in 19F measured pO2, EPI BOLD SI and decrease in GRE R2* on the same section of tumor. Data indicated a strong correlation between %ΔSI and ΔpO2 (Figure 6). Although a significant increase in global mean SI was found in all tumors by BOLD in the current study, voxel-by-voxel data analysis showed oxygen breathing induced signal loss in many voxels for each tumor, averaged as 20% of the total voxels. Similar findings have been observed in other tumor types by others and us (6,8,10,14). Indeed, Fan et al. (17) previously remarked that correlates between mean BOLD and pO2 were stronger when the whole tumor was considered, as opposed to individual regions. In a slightly different context Baudelet et al (43) reported a better correlation between mean tumor BOLD signal response and vascular kep than for individual voxels.
The ability to identify hypoxia could have far-reaching implications for radiotherapy. We have previously shown correlations between tumor pO2 measured by 19F MRI (FREDOM) and response to single high dose irradiation in Dunning prostate R3327-HI and AT1 tumors (44,45). Furthermore, we could correctly assess the ability to alter pO2 and modulate response to irradiation. Karczmar’s team also demonstrated the ability of BOLD (viz. HiSS) to predict the relative efficacy of tumor oxygenating agents (46). 1H MRI BOLD assessment would be far more practical in the clinic and we believe our results together with the previous report from Fan et al. (17) provide strong impetus for translation.
Acknowledgement
This work was supported by DOD Breast Cancer Initiative Awards IDEA DAMD 17-03-1-0363 (DZ) and pre doctoral fellowship DAMD 17-02-1-0592 (LJ), in conjunction with NCI RO1 CA79515/EB002762 and the Southwestern Small Animal Imaging research program (SW-SAIRP supported by U24 CA12660801 and P20 Pre-ICMIC CA86354). MRI experiments were performed in the Advanced Imaging Research Center (formerly, Mary Nell & Ralph B. Rogers MR Center) an NIH BTRP # P41-RR02584 facility. We are grateful to Ammar Adams and Dr. Matthew Merritt for technical and collegial support.
References
- 1.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82(10):699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D, Jiang L, Mason RP. Measuring Changes in Tumor Oxygenation. Methods Enzymol. 2004;386:378–418. doi: 10.1016/S0076-6879(04)86018-X. [DOI] [PubMed] [Google Scholar]
- 3.Howe FA, Robinson SP, Rodrigues LM, Griffiths JR. Flow and oxygenation dependent (FLOOD) contrast MR imaging to monitor the response of rat tumors to carbogen breathing. Magn Reson Imaging. 1999;17:1307–1318. doi: 10.1016/s0730-725x(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 4.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48:980–986. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 5.Padhani A, Krohn K, Lewis J, Alber M. Imaging oxygenation of human tumours. Europ Radiol. 2007;17(4):861–872. doi: 10.1007/s00330-006-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas CD, Chenu E, Walczak C, Plessis MJ, Perin F, Volk A. Morphological and carbogen-based functional MRI of a chemically induced liver tumor model in mice. Magn Reson Med. 2003;50:522–530. doi: 10.1002/mrm.10555. [DOI] [PubMed] [Google Scholar]
- 7.Neeman M, Dafni H, Bukhari O, Braun RD, Dewhirst MW. In vivo BOLD contrast MRI mapping of subcutaneous vascular function and maturation: validation by intravital microscopy. Magn Reson Med. 2001;45:887–898. doi: 10.1002/mrm.1118. [DOI] [PubMed] [Google Scholar]
- 8.Jiang L, Zhao D, Constantinescu A, Mason RP. Comparison of BOLD contrast and Gd-DTPA Dynamic Contrast Enhanced imaging in rat prostate tumor. Magn Reson Med. 2004;51:953–960. doi: 10.1002/mrm.20069. [DOI] [PubMed] [Google Scholar]
- 9.Robinson SP, Rijken PF, Howe FA, McSheehy PM, van der Sanden BP, Heerschap A, Stubbs M, Van Der Kogel AJ, Griffiths JR. Tumor vascular architecture and function evaluated by non-invasive susceptibility MRI methods and immunohistochemistry. J Magn Reson Imaging. 2003;17:445–454. doi: 10.1002/jmri.10274. [DOI] [PubMed] [Google Scholar]
- 10.Peller M, Weissfloch L, Stehling MK, Weber J, Bruening R, Senekowitsch-Schmidtke R, Molls M, Reiser M. Oxygen-induced MR signal changes in murine tumors. Magn Reson Imaging. 1998;16:799–809. doi: 10.1016/s0730-725x(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hallaq HA, River JN, Zamora M, Oikawa H, Karczmar GS. Correlation of magnetic resonance and oxygen microelectrode measurements of carbogen-induced changes in tumor oxygenation. Int J Radiat Oncol Biol Phys. 1998;41(1):151–159. doi: 10.1016/s0360-3016(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues LM, Howe FA, Griffiths JR, Robinson SP. Tumor R-2 * is a prognostic indicator of acute radiotherapeutic response in rodent tumors. Journal of Magnetic Resonance Imaging. 2004;19(4):482–488. doi: 10.1002/jmri.20024. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SP, Howe FA, Rodrigues LM, Stubbs M, Griffiths JR. Magnetic resonance imaging techniques for monitoring changes in tumor oxygenation and blood flow. Semin Radiat Oncol. 1998;8(3):198–207. doi: 10.1016/s1053-4296(98)80045-3. [DOI] [PubMed] [Google Scholar]
- 14.Baudelet C, Gallez B. Current issues in the utility of blood oxygen level dependent MRI for the assessment of modulations in tumor oxygenation. Curr Med Imaging Rev. 2005;1:229–243. [Google Scholar]
- 15.Duyn JH, Moonen CT, van Yperen GH, de Boer RW, Luyten PR. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5 T. NMR Biomed. 1994;7(1–2):83–88. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- 16.Elas M, Williams BB, Parasca A, Mailer C, Pelizzari CA, Lewis MA, River JN, Karczmar GS, Barth ED, Halpern HJ. Quantitative tumor oxymetric images from 4D electron paramagnetic resonance imaging (EPRI): Methodology and comparison with blood oxygen level-dependent (BOLD) MRI. Magn Reson Med. 2003;49:682–691. doi: 10.1002/mrm.10408. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, River JN, Zamora M, Al-Hallaq HA, Karczmar GS. Effect of carbogen on tumor oxygenation: combined fluorine-19 and proton MRI measurements. Int J Radiat Oncol Biol Phys. 2002;54:1202–1209. doi: 10.1016/s0360-3016(02)03035-3. [DOI] [PubMed] [Google Scholar]
- 18.Hahn EW, Peschke P, Mason RP, Babcock EE, Antich PP. Isolated tumor growth in a surgically formed skin pedicle in the rat: A new tumor model for NMR studies. Magn Reson Imaging. 1993;11:1007–1017. doi: 10.1016/0730-725x(93)90219-4. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Constantinescu A, Mason RP. Dynamic Breast tumor oximetry: the development of Prognostic Radiology. Technol Cancer Res Treat. 2002;1(6):471–478. doi: 10.1177/153303460200100607. [DOI] [PubMed] [Google Scholar]
- 20.Xia M, Kodibagkar V, Liu H, Mason RP. Tumour oxygen dynamics measured simultaneously by near infrared spectroscopy and 19F magnetic resonance imaging in rats. Phys Med Biol. 2006;51:45–60. doi: 10.1088/0031-9155/51/1/004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Jiang L, Hahn EW, Mason RP. Tumor physiological response to combretastatin A4 phosphate assessed by MRI. Int J Radiat Oncol Biol Phys. 2005;62:872–880. doi: 10.1016/j.ijrobp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, Hill RP. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20(3):680–687. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 23.Brizel DM, Sibly GS, Prosmitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C, Berkowitz B, McCuen B, Charles C. Measurement of preretinal pO2 in the vitrectomized human eye using 19F NMR. Arch Ophthalmol. 1992;110:1098–1100. doi: 10.1001/archopht.1992.01080200078028. [DOI] [PubMed] [Google Scholar]
- 25.Bratasz A, Pandian RP, Deng Y, Petryakov S, Grecula JC, Gupta N, Kuppusamy P. In vivo imaging of changes in tumor oxygenation during growth and after treatment. Magn Reson Med. 2007;57(5):950–959. doi: 10.1002/mrm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn JF, O'Hara JA, Zaim-Wadghiri Y, Lei H, Meyerand ME, Grinberg OY, Hou H, Hoopes PJ, Demidenko E, Swartz HM. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. JMRI. 2002;16:511–521. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- 27.Taylor NJ, Baddeley H, Goodchild KA, Powell ME, Thoumine M, Culver LA, Stirling JJ, Saunders MI, Hoskin PJ, Phillips H, Padhani AR, Griffiths JR. BOLD MRI of human tumor oxygenation during carbogen breathing. JMRI. 2001;14:156–163. doi: 10.1002/jmri.1166. [DOI] [PubMed] [Google Scholar]
- 28.Rijpkema M, Kaanders JH, Joosten FB, van der Kogel AJ, Heerschap A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002;53:1185–1191. doi: 10.1016/s0360-3016(02)02825-0. [DOI] [PubMed] [Google Scholar]
- 29.Jiang L, McColl R, Weatherall P, Tripathy D, Mason RP. ISMRM 13th Scientific Meeting. Miami Beach, Fl: 2005. BOLD and Gd-DTPA Contrast Enhanced MRI for Early Assessment of Breast Cancer Chemotherapy; p. 408. [Google Scholar]
- 30.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- 31.Wright GA, Hu BS, Macovski A. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. JMRI. 1991;1:275–283. doi: 10.1002/jmri.1880010303. [DOI] [PubMed] [Google Scholar]
- 32.Robinson SP, Collingridge DR, Howe FA, Rodrigues LM, Chaplin DJ, Griffiths JR. Tumor response to hypercapnia and hyperoxia monitored by FLOOD magnetic resonance imaging. NMR Biomed. 1999;12:98–106. doi: 10.1002/(sici)1099-1492(199904)12:2<98::aid-nbm556>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO(2) histography. Antioxid Redox Signal. 2007;9(8):1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 34.Mason RP, Antich PP, Babcock EE, Constantinescu A, Peschke P, Hahn EW. Non-invasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys. 1994;29:95–103. doi: 10.1016/0360-3016(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto K, Bernardo M, Subramanian S, Choyke P, Mitchell JB, Krishna MC, Lizak MJ. MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn Reson Med. 2006;56(2):240–246. doi: 10.1002/mrm.20961. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor JPB, Jackson A, Buonaccorsi GA, Buckley DL, Roberts C, Watson Y, Cheung S, McGrath DM, Naish JH, Rose CJ, Dark PM, Jayson GC, Parker GJM. Organ-specific effects of oxygen and carbogen gas inhalation on tissue longitudinal relaxation times. Magn Reson Med. 2007;58(3):490–496. doi: 10.1002/mrm.21357. [DOI] [PubMed] [Google Scholar]
- 37.Gu Y, Bourke V, Kim JG, Constantinescu A, Mason RP, Liu H. Dynamic Response of Breast Tumor Oxygenation to Hyperoxic Respiratory Challenge Monitored with Three Oxygen-Sensitive Parameters. Applied Optics. 2003;42:1–8. doi: 10.1364/ao.42.002960. [DOI] [PubMed] [Google Scholar]
- 38.Kim JG, Zhao D, Constantinescu A, Mason RP, Liu H. Interplay of Tumor Vascular Oxygenation and Tumor pO2 Observed Using NIRS, Oxygen Needle Electrode, and 19F MR pO2 Mapping. J Biomed Optics. 2003;8:53–62. doi: 10.1117/1.1527049. [DOI] [PubMed] [Google Scholar]
- 39.Kodibagkar VD, Wang X, Pacheco-Torres J, Gulaka P, Mason RP. Proton Imaging of Silanes to map Tissue Oxygenation Levels (PISTOL): a tool for quantitative tissue oximetry. NMR Biomed. 2008;21:899–907. doi: 10.1002/nbm.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason RP, Hunjan S, Constantinescu A, Song Y, Zhao D, Hahn EW, Antich PP, Peschke P. Tumor oximetry: Comparison of 19F MR EPI and electrodes. In: Dunn JF, Swartz HM, editors. Oxygen Transport to Tissue XXIV. Volume 530, Advances in Experimental Medicine and Biology. New York: Kluwer; 2003. pp. 19–28. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, Constantinescu A, Hahn EW, Mason RP. Tumor oxygen dynamics with respect to growth and respiratory challenge: investigation of the Dunning prostate R3327-HI tumor. Radiat Res. 2001;156:510–520. doi: 10.1667/0033-7587(2001)156[0510:todwrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Zhao D, Ran S, Constantinescu A, Hahn EW, Mason RP. Tumor oxygen dynamics: correlation of in vivo MRI with histological findings. Neoplasia. 2003;5(4):308–318. doi: 10.1016/S1476-5586(03)80024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baudelet C, Cron GO, Gallez B. Determination of the maturity and functionality of tumor vasculature by MRI: Correlation between BOLD-MRI and DCE-MRI using P792 in experimental fibrosarcoma tumors. Magnetic Resonance in Medicine. 2006;56(5):1041–1049. doi: 10.1002/mrm.21047. [DOI] [PubMed] [Google Scholar]
- 44.Bourke VA, Zhao D, Gilio J, Chang C-H, Jiang L, Hahn EW, Mason RP. Correlation of Radiation Response with Tumor Oxygenation in the Dunning Prostate R3327-AT1 Tumor. Int J Radiat Oncol Biol Phys. 2007;67(4):1179–1186. doi: 10.1016/j.ijrobp.2006.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao D, Constantinescu A, Chang C-H, Hahn EW, Mason RP. Correlation of Tumor Oxygen Dynamics with Radiation Response of the Dunning Prostate R3327-HI Tumor. Radiat Res. 2003;159:621–631. doi: 10.1667/0033-7587(2003)159[0621:cotodw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Al-Hallaq HA, Zamora M, Fish BL, Farrell A, Moulder JE, Karczmar GS. MRI measurements correctly predict the relative effects of tumor oxygenating agents on hypoxic fraction in rodent BA1112 tumors. Int J Radiat Oncol Biol Phys. 2000;47:481–488. doi: 10.1016/s0360-3016(00)00445-4. [DOI] [PubMed] [Google Scholar]