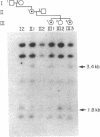
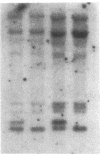
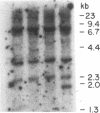
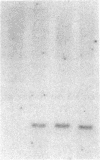
Abstract
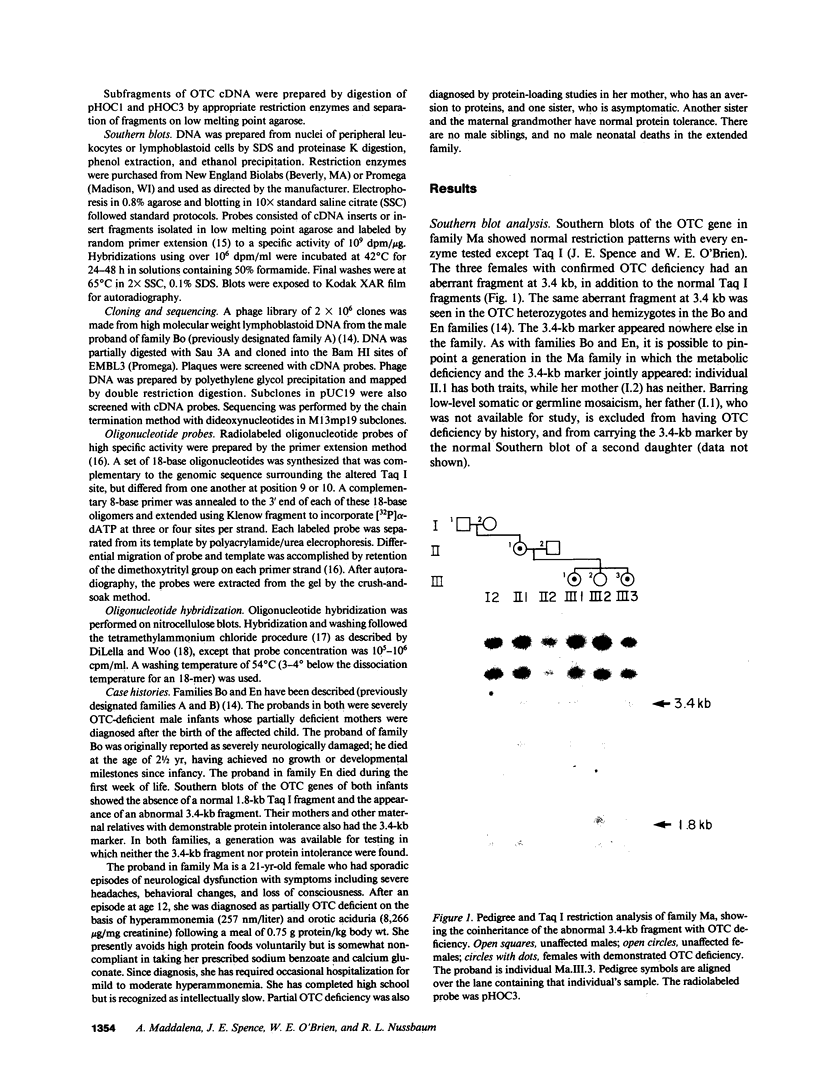
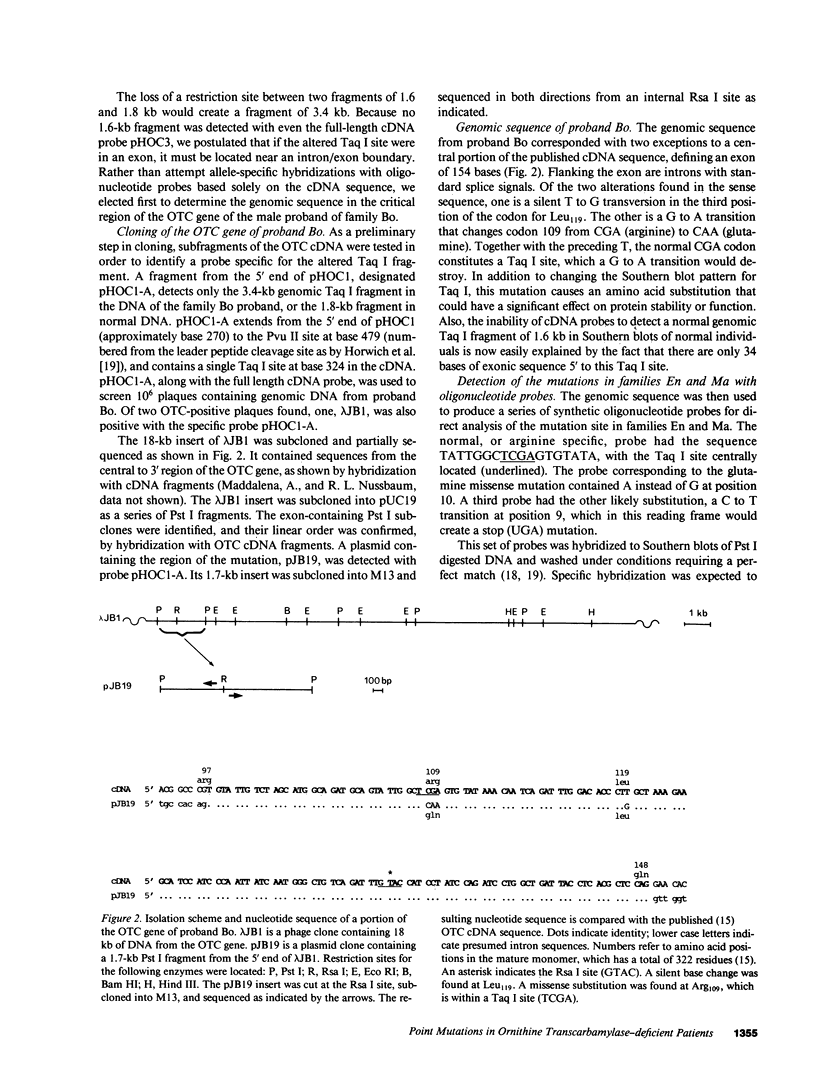
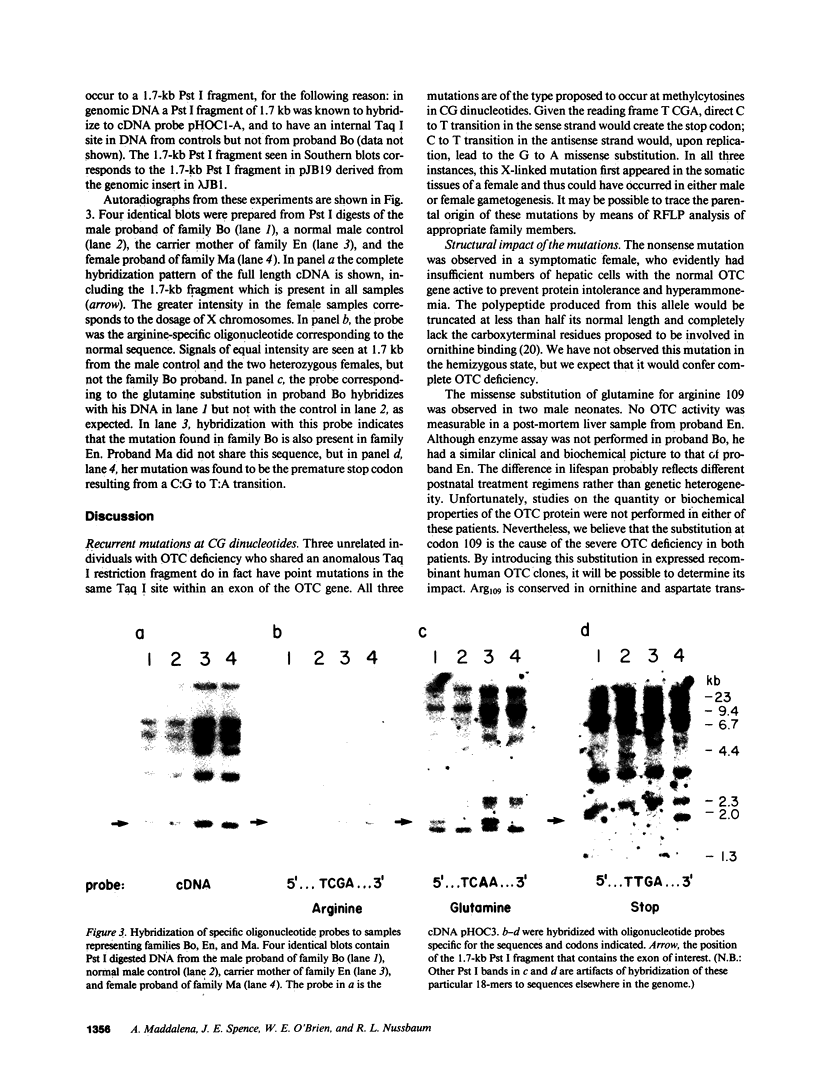
Point mutations in the X-linked ornithine transcarbamylase (OTC) gene have been detected at the same Taq I restriction site in 3 of 24 unrelated probands with OTC deficiency. A de novo mutation could be traced in all three families to an individual in a prior generation, confirming independent recurrence. The DNA sequence in the region of the altered Taq I site was determined in the three probands. In two unrelated male probands with neonatal onset of severe OTC deficiency, a guanine (G) to adenine (A) mutation on the sense strand (antisense cytosine [C] to thymine [T]) was found, resulting in glutamine for arginine at amino acid 109 of the mature polypeptide. In the third case, where the proband was a symptomatic female, C to T (sense strand) transition converted residue 109 to a premature stop. These results support the observation that Taq I restriction sites, which contain an internal CG, are particularly susceptible to C to T transition mutation due to deamination of a methylated C in either the sense or antisense strand. The OTC gene seems especially sensitive to C to T transition mutation at arginine codon 109 because either a nonsense mutation or an extremely deleterious missense mutation will result.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Briand P., Francois B., Rabier D., Cathelineau L. Ornithine transcarbamylase deficiencies in human males. Kinetic and immunochemical classification. Biochim Biophys Acta. 1982 May 21;704(1):100–106. doi: 10.1016/0167-4838(82)90136-4. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Hybridization of genomic DNA to oligonucleotide probes in the presence of tetramethylammonium chloride. Methods Enzymol. 1987;152:447–451. doi: 10.1016/0076-6879(87)52052-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Hack A. M., Fenton W. A., Rosenberg L. E. Identification and application of additional restriction fragment length polymorphisms at the human ornithine transcarbamylase locus. Am J Hum Genet. 1986 Jun;38(6):841–847. [PMC free article] [PubMed] [Google Scholar]
- Fox J., Hack A. M., Fenton W. A., Golbus M. S., Winter S., Kalousek F., Rozen R., Brusilow S. W., Rosenberg L. E. Prenatal diagnosis of ornithine transcarbamylase deficiency with use of DNA polymorphisms. N Engl J Med. 1986 Nov 6;315(19):1205–1208. doi: 10.1056/NEJM198611063151907. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Shuman M. A., Lawn R. M. Identification of a missense mutation in the factor VIII gene of a mild hemophiliac. Science. 1986 Jun 13;232(4756):1415–1416. doi: 10.1126/science.3012775. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Tuddenham E. G., Shuman M. A., Goralka T. M., Chen E. Y., Lawn R. M. Detection and sequence of mutations in the factor VIII gene of haemophiliacs. 1985 May 30-Jun 5Nature. 315(6018):427–430. doi: 10.1038/315427a0. [DOI] [PubMed] [Google Scholar]
- Hata A., Tsuzuki T., Shimada K., Takiguchi M., Mori M., Matsuda I. Isolation and characterization of the human ornithine transcarbamylase gene: structure of the 5'-end region. J Biochem. 1986 Sep;100(3):717–725. doi: 10.1093/oxfordjournals.jbchem.a121764. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Huygen R., Crabeel M., Glansdorff N. Nucleotide sequence of the ARG3 gene of the yeast Saccharomyces cerevisiae encoding ornithine carbamoyltransferase. Comparison with other carbamoyltransferases. Eur J Biochem. 1987 Jul 15;166(2):371–377. doi: 10.1111/j.1432-1033.1987.tb13525.x. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Evidence for an exceptionally reactive arginyl residue at the binding site for carbamyl phosphate in bovine ornithine transcarbamylase. J Biol Chem. 1980 Aug 10;255(15):7301–7305. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- McClead R. E., Jr, Rozen R., Fox J., Rosenberg L., Menke J., Bickers R., Morrow G., 3rd Clinical application of DNA analysis in a family with OTC deficiency. Am J Med Genet. 1986 Nov;25(3):513–518. doi: 10.1002/ajmg.1320250313. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Boggs B. A., Beaudet A. L., Doyle S., Potter J. L., O'Brien W. E. New mutation and prenatal diagnosis in ornithine transcarbamylase deficiency. Am J Hum Genet. 1986 Feb;38(2):149–158. [PMC free article] [PubMed] [Google Scholar]
- Old J. M., Briand P. L., Purvis-Smith S., Howard N. J., Wilcken B., Hammond J., Pearson P., Cathelineau L., Williamson R., Davies K. E. Prenatal exclusion of ornithine transcarbamylase deficiency by direct gene analysis. Lancet. 1985 Jan 12;1(8420):73–75. doi: 10.1016/s0140-6736(85)91966-x. [DOI] [PubMed] [Google Scholar]
- Pembrey M. E., Old J. M., Leonard J. V., Rodeck C. H., Warren R., Davies K. E. Prenatal diagnosis of ornithine carbamoyl transferase deficiency using a gene specific probe. J Med Genet. 1985 Dec;22(6):462–465. doi: 10.1136/jmg.22.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. E., Kalousek F., Orsulak M. D. Biogenesis of ornithine transcarbamylase in spfash mutant mice: two cytoplasmic precursors, one mitochondrial enzyme. Science. 1983 Oct 28;222(4622):426–428. doi: 10.1126/science.6623083. [DOI] [PubMed] [Google Scholar]
- Rozen R., Fox J. E., Hack A. M., Fenton W. A., Horwich A. L., Rosenberg L. E. DNA analysis for ornithine transcarbamylase deficiency. J Inherit Metab Dis. 1986;9 (Suppl 1):49–57. doi: 10.1007/BF01800858. [DOI] [PubMed] [Google Scholar]
- Rozen R., Fox J., Fenton W. A., Horwich A. L., Rosenberg L. E. Gene deletion and restriction fragment length polymorphisms at the human ornithine transcarbamylase locus. 1985 Feb 28-Mar 6Nature. 313(6005):815–817. doi: 10.1038/313815a0. [DOI] [PubMed] [Google Scholar]
- Saheki T., Imamura Y., Inoue I., Miura S., Mori M., Ohtake A., Tatibana M., Katsumata N., Ohno T. Molecular basis of ornithine transcarbamylase deficiency lacking enzyme protein. J Inherit Metab Dis. 1984;7(1):2–8. doi: 10.1007/BF01805609. [DOI] [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Takiguchi M., Murakami T., Miura S., Mori M. Structure of the rat ornithine carbamoyltransferase gene, a large, X chromosome-linked gene with an atypical promoter. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6136–6140. doi: 10.1073/pnas.84.17.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Kazazian H. H., Jr, Phillips D. G., Aronis S., Tsiftis G., Brown V. A., Antonarakis S. E. Recurrent mutations in haemophilia A give evidence for CpG mutation hotspots. 1986 Nov 27-Dec 3Nature. 324(6095):380–382. doi: 10.1038/324380a0. [DOI] [PubMed] [Google Scholar]