Abstract
Pathogen transmission by mosquitoes is tightly linked to blood feeding which, in turn, is required for egg development. Studies of these processes would greatly benefit from genetic methods, such as the binary Gal4/UAS system. The latter has been well established for model organisms, but its availability is limited for mosquitoes. The objective of this study was to develop the blood-meal-activated, gut-specific Gal4/UAS system for the yellow-fever mosquito Aedes aegypti and utilize it to investigate the regulation of gut-specific gene expression. A 1.1-kb, 5' upstream region of the carboxypeptidase A (CP) gene was used to genetically engineer the CP-Gal4 driver mosquito line. The CP-Gal4 specifically activated the Enhanced Green Fluorescent Protein (EGFP) reporter only after blood feeding in the gut of the CP-Gal4>UAS-EGFP female Ae. aegypti. We used this system to study the regulation of CP gene expression. In vitro treatments with either amino acids (AAs) or insulin stimulated expression of the CP-Gal4>UAS-EGFP transgene; no effect was observed with 20-hydroxyecdysone (20E) treatments. The transgene activation by AAs and insulin was blocked by rapamycin, the inhibitor of the Target-of-Rapamycin kinase (TOR). RNA interference (RNAi) silence of the insulin receptor (IR) reduced the expression of the CP-Gal4>UAS-EGFP transgene. Thus, in vitro and in vivo experiments have revealed that insulin and TOR pathways control expression of the digestive enzyme CP. In contrast, 20E, the major regulator of post-blood-meal vitellogenic events in female mosquitoes, has no role in regulating the expression of this gene. This novel CP-Gal4/UAS system permits functional testing of midgut-specific genes that are involved in blood digestion and interaction with pathogens in Ae. aegypti mosquitoes.
Keywords: Genetic transformation, Carboxypeptidase, Insulin, TOR, Mosquito, Aedes aegypti
1. Introduction
Female mosquitoes require vertebrate blood for egg development, and their cyclic feeding results in transmitting pathogens of numerous devastating human diseases. The yellow fever mosquito Aedes aegypti has become the predominant vector for the virus that causes Dengue fever, a life-threatening and debilitating disease, throughout many parts of the world. Understanding functions linked to blood feeding is essential for elucidating mechanisms of acquisition and transmission of disease pathogens. It could pave the way toward developing novel approaches for pathogen and vector control.
Significant progress has been made through genomic and post-genomic studies in mosquitoes. Genomes of three mosquito species—Anopheles gambiae, Ae. aegypti and Culex quinquefasciatus—have been sequenced, permitting identification of genes involved in mosquito-specific functions (Holt et al., 2002; Nene et al., 2007; Arensburger et al., 2010). However, hypotheses arising from genetic and genomic data need to be tested in vivo to elucidate the function of individual genes. Germ-line transformation allows introduction of such genes of interest into mosquitoes for identification of their functions. These transgenic studies in mosquitoes have been limited to direct overexpression of the gene of interest under the control of a selected promoter (Kokoza et al., 2000; Moreira et al., 2000; Adelman et al., 2008; Papathanos et al., 2009; Cho et al., 2006; Catteruccia et al., 2005; Nolan et al., 2011). Establishment of the Gal4/UAS system in model organisms has initiated a new era in elucidating gene functions (Brand and Perrimon, 1993; Ornitz et al., 1991; Scheer et al., 1999; Hartley et al., 2002; Imamura et al., 2003; Schinko et al., 2010). Essential genes, which when modified can have potentially harmful effects on development, behavior and fertility, can be investigated using the Gal4/UAS system. However, development of the Gal4/UAS system in mosquitoes has been slow, due to the difficulties in genetic transformation of these organisms and scarcity of available promoters with tissue/cell-, sex- and stage-specific expression. Kokoza and Raikhel established the first binary Gal4/UAS system for the female-, blood-meal-induced- and fat-body-specific expression in the mosquito Ae. aegypti (Kokoza and Raikhel, 2011). Lynd and Lycett have reported the midgut-specific Gal4/UAS system in the malaria vector An. gambiae (Lynd and Lycett, 2012). However, further refinement and development of the midgut-specific Gal4/UAS system is essential for characterization of regulatory mechanisms, governing expression of genes involved in blood digestion and pathogen interaction in mosquitoes. The goal of this work is to establish the midgut-specific Gal4/UAS system in Ae. aegypti.
In this study, we used the 5' upstream region of the CP gene to establish the Ae. aegypti CP-Gal4 driver line. We show that the CP-Gal4 driver activates a UAS-EGFP responder in midguts of CP-Gal4>UAS-EGFP females in a blood meal dependent manner. Using this CP-Gal4/UAS system, we have investigated the regulation of transgene expression and shown it to be regulated by insulin and amino acid/TOR pathways. The development of the female midgut-specific CP-Gal4/UAS system for Ae. aegypti will enhance our ability to investigate genes involved in blood digestion. It will also be instrumental for studies of midgut factors that play a role in interactions with invading pathogens.
2. Materials and methods
2.1. Mosquito rearing
The Ae. aegypti UGAL/Rockefeller strain and transgenic lines were reared under identical laboratory conditions (27°C and 80% humidity) and kept in cages with unlimited access to 10% sugar solution and water until blood feeding. Three- to four-day-old female mosquitoes were blood-fed on White Leghorn chickens.
2.2. Plasmid construction
For construction of the pAehsp-pBac helper plasmid (Fig. S1), we used the 0.66-kb 5' regulatory region of the gene encoding the heat shock protein 70 (Aehsp70) (Isoe et al., 2007). PCR was used to amplify the Aehsp70 fragment with genomic DNA from the Ae. aegypti UGAL/Rockefeller strain (wt) as template and Aehsp70 gene-promoter-specific primers (Table S3). This fragment was incorporated into the SacI-blunted site of the pBΔSac (Handler et al., 1998) to create pAehsp-pBac, placing the Aehsp70 promoter upstream of the piggyBac transposase gene. The cloning strategy for the CP-Gal4 driver construct was as follows. Plasmid pBluescript-AeCPA promoter (Franz et al., 2006) was excised using SacI and BamHI, and the resulting 1.1-kb fragment was subcloned into the SmaI-BamHI site of the pSLfa1180fa shuttle vector (Horn and Wimmer, 2000) to generate pSL-CP. Then, a 0.8-kb BamHI-XbaI fragment of the chimeric Gal4 activator, excised from the pBac[3×P3-EGFP afm, Vg-Gal4] (Kokoza and Raikhel, 2011), was assembled into pSL-CP to form pSL-CP-Gal4. Adding a 0.25-kb NotI-AflII SV40 terminator fragment into the pSL-CP-Gal4 produced the complete CP-Gal4 driver cassette. This resulting CP-Gal4 driver cassette was subcloned into the pBac [3×P3-EGFP afm] transformation vector (Horn and Wimmer, 2000) at the AscI site to produce the CP-Gal4 driver construct.
2.3. Germ-line transformation of Ae. aegypti
Plasmid DNA used in injections was purified using the EndoFree Plasmid Maxi Kit (QIAGEN, Valencia, CA). CP-Gal4 driver (0.35mg/ml) and pAehsp-pBac helper (0.25mg/ml) plasmids were re-suspended in 0.1mM phosphate buffer (pH 6.8, containing 5mM KCl) and injected into the pre-blastoderm-stage eggs. The development of CP-Gal4 driver line was performed following a previously described process (Kokoza and Raikhel, 2011). G1 progeny were selected by monitoring the EGFP fluorescent eye marker under a Nikon SMZ800 fluorescence microscope fitted with GFP-B filter. The CP-Gal4>UAS-EGFP line was established as described previously (Kokoza and Raikhel, 2011), and the hybrid mosquitoes were selected by the presence of two eye-specific selectable markers, DsRed and EGFP. A Nikon SMZ800 fluorescence microscope fitted with DsRed and GFP-B filter sets was used for this selection.
2.4. Molecular analysis
Genomic DNA was extracted from adult mosquitoes using DNeasy tissue kit (QIAGEN, Valencia, CA). In genomic PCR reactions, 200 ng genomic DNA was used under conditions following the manufacturer’s protocol for the Platinum PCR SuperMix (Invitrogen, Carlsbad, CA). Inverse PCR was performed on the M16-1 and F4-4 driver lines to investigate the insertion location in the genome. A total of 2 µg genomic DNA was digested with MboI and HpaII (NEB, Ipswich, MA), and 1 µg of digested product was self-ligated in 400-µl total volume overnight at 16°C using T4 DNA Ligase (NEB, Ipswich, MA). The ligation product was purified and dissolved in 30 µl ddH2O. PCR was carried out to amplify DNA flanking the insertion locations using specific primers (Table S3), and the products were sequenced and verified against the VectorBase sequences, using Basic Local Alignment Search Tool (BLAST).
For each experimental treatment, total RNA was extracted from 10 midguts using Trizol (Invitrogen, Carlsbad, CA). cDNAs were synthesized from 2 µg of total RNA after DNaseI (Invitrogen, Carlsbad, CA) treatment, using Omniscript Reverse transcriptase kit (QIAGEN, Valencia, CA) in 20 µl of the reaction mixture. RT-PCR and qRT-PCR for selected transcripts were done and analyzed as described previously (Kokoza and Raikhel, 2011; Roy et al., 2007).
2.5. Immunofluorescence
10 midguts from each treatment were fixed at room temperature for 20 min in 3.7% (w/v) formaldehyde in phosphate buffered saline (PBS). Fixed midguts were washed (three times, 5 min each) in PBS-T, which contained 0.3% Triton X-100 (v/v) in PBS, before being blocked for 1 h in 3% (w/v) bovine serum albumin in PBS-T at room temperature. After rinsing in PBS-T, midguts were incubated at 4°C overnight with anti-GFP antibodies diluted 1:200 in PBS-T (polyclonal, developed in rabbit, Sigma-Aldrich, St. Louis, MO). Midguts were washed and incubated with Fluorescein-conjugated Anti-Rabbit IgG (Vector, Burlingame, CA) diluted 1:400 in PBS-T for 2 h at room temperature, then mounted using VECTASHIELD Mounting Medium (Vector, Burlingame, CA). Images were captured and analyzed under a Leica M165 FC fluorescent stereo microscope and using LAS V4.0 software. Photographs are representative of ten mosquitoes for each treatment.
2.6. In vitro midgut culture
The in vitro organ culture was performed as previously described for the fat body (Roy et al., 2007). Tissue was incubated either in culture medium lacking AAs (AA−; containing equimolar amounts of mannitol in place of AAs) or with medium containing all the AAs (AA+) for 8 h at 27°C (1 midgut/60 µl/dish). In addition, the following reagents were used: bovine insulin at a final concentration of 17 µM (Sigma-Aldrich, St. Louis, MO, USA), 20 pmol rapamycin (LC Laboratory; dissolved in ethanol) and 20E at the final concentration of 10 µM (Sigma-Aldrich, St. Louis, MO, USA; dissolved in ethanol). Midguts were dissected from 3-day-old, non-blood-fed females.
2.7. RNA interference
Double-stranded RNA (dsRNA) was produced using the MEGAscript T7 kit (Ambion, Austin, TX, USA), and a total of 2 µg in 0.3–0.5 µl distilled water was injected into 1-day-old female mosquitoes. These mosquitoes were maintained on sucrose solution for 4 days, then given a blood meal, after which their midguts were isolated (at 24 h PBM). Transcript abundance was analyzed using qRT-PCR analysis as described above. RNAi depletion of luciferase (dsLuc) served as a control.
3. Results
3.1. Establishment of CP-Gal4 transgenic lines
piggyBac transposable element was utilized to create the transgenic Ae. aegypti CP-Gal4 driver plasmid (Fig. 1A). This CP-Gal4 driver plasmid contained the 1.1-kb 5' upstream region of the CP gene that was linked to a modified yeast Gal4 protein coding sequence (Kokoza and Raikhel, 2011; Kulkarni and Arnosti, 2003), followed by a SV40 polyadenylation signal. The CP-Gal4 driver cassette contained a selectable marker, consisting of the EGFP gene driven by the eye-specific 3×P3 gene promoter. To construct the piggyBac helper plasmid, we employed the 0.66-kb 5' upstream promoter region of the Ae. aegypti heat shock protein70 gene (Aehsp70) (Isoe et al., 2007) instead of the previously used Drosophila melanogaster hsp70 gene (Handler and Harrell II, 1999) (Fig. S1).
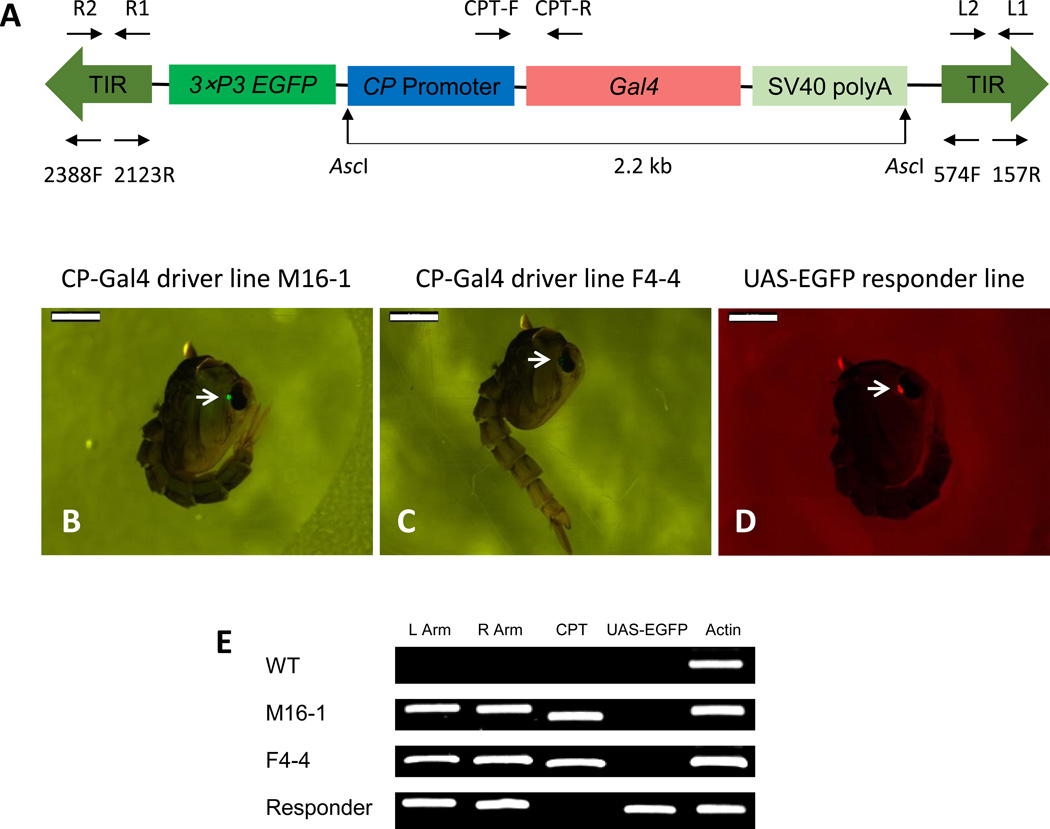
Figure 1.
Germline transformation and molecular characterization of Ae. aegypti CP-Gal4 lines. (A) Schematic representation of the CP-Gal4 cassette. The CP-Gal4 cassette consists of the piggyBac vector plasmid containing the 5' regulatory region of the CP gene placed upstream of the modified Gal4 and the EGFP marker gene under the control of the eye-specific 3×P3 gene promoter. Arrows above and below the A diagram represent the transgene-specific primers used for genomic PCR, RT-PCR and inverse PCR. (B–D) Eye-specific expression of the selectable markers, EGFP and DsRed, in Ae. aegypti transgenic mosquito lines. Transgenic mosquitoes were selected based on the presence of EGFP marker in CP-Gal4 lines and DsRed marker in the UAS-EGFP line at the pupal stage (arrows). Screening was carried out using a Nikon SMZ800 fluorescence microscope with GFP-B and DsRed filters, respectively. Scale bar: 1mm. (E) Genomic PCR analysis demonstrating integration of CP-Gal4 and UAS-EGFP constructs into the Ae. aegypti genome. Wild type (WT), CP-Gal4 driver line M16-1 (M16-1), CP-Gal4 driver line F4-4 (F4-4) and UAS-EGFP responder line (Responder), were checked. Primers were specific to the left and right arms of the piggyBac vector, left arm (L Arm), right arm (R Arm), the CP-Gal4 transgene (CPT) and the UAS-EGFP transgene (UAS-EGFP). Primers for actin were used as control (Actin).
The pre-blastoderm embryos of the UGAL/Rockefeller Ae. aegypti strain (wt) were injected with a mixture of the CP-Gal4 driver and helper constructs. Of 1084 injected embryos, 90 survived to adulthood. These G0 mosquitoes were separated by sex with 43 males and 47 females. Each G0 male was out-crossed individually with 5 wt females giving rise to 43 male families (M1-M43). 47 females were separated into 6 groups. In each of these six G0 group, females were out-crossed with an equal number of wt males, forming 6 female families (F1–F6). G1 progeny (larvae and pupae) were examined for the presence of EGFP expression in their eyes. Based on this criterion, two individuals were selected, M16-1 from M16 male family and F4-4 from F4 female family. Their progeny was established as homozygous based on the selection for 100% eye-specific EGFP expression of mosquitoes in each line. Pupae of the M16-1 line showed much stronger eye-specific marker gene expression than those of the F4-4 line (Fig. 1B–C). Results indicated that the transformation efficiency was 2.2% (Table S1).
Genome integration of the CP-Gal4 construct in the M16-1 and F4-4 driver lines was confirmed by means of genomic polymerase chain reaction (PCR) using specific primers for the piggyBac transposon left and right arms, as well as the CP-Gal4 sequence (Fig. 1E). The UAS-EGFP sequence was used in these PCR analyses as a control. While piggyBac transposon sequences (left arm and right arm) were detected in the driver and responder transgenic lines, the CP-Gal4 sequence was only present in the genomic DNA isolated from the CP-Gal4 driver lines, M16-1 and F4-4. DNA from the UAS-EGFP responder line contained UAS-EGFP responder sequence that was not present in either the driver line or wt mosquitoes (Fig. 1E).
Genome incorporation of the CP-Gal4 transgene (CPT) was determined by means of inverse PCR analysis using genomic DNA from M16-1 and F4-4 transgenic lines. Insertion of the piggyBac transposon with canonical TTAA target sites containing the CPT was found in M16-1 and F4-4 transgenic lines (Table S2). The insertion sites were located outside of predicted gene coding sequences (VectorBase AaegL 1.3) in both transgenic lines. The UAS-EGFP responder line with a DsRed selectable marker has been generated and characterized previously (Kokoza and Raikhel, 2011).
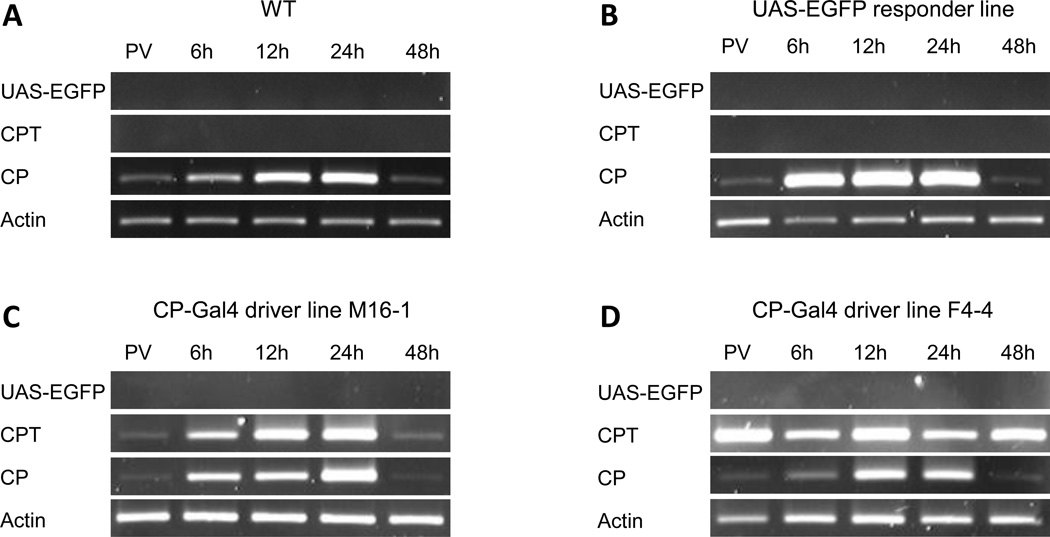
The pattern of CPT expression in M16-1 and F4-4 transgenic lines was investigated using RNA isolated from the digestive system (midgut) of female mosquitoes from the wt, UAS-EGFP responder, M16-1 and F4-4 transgenic driver lines at 72 h post-eclosion, a time point during the previtellogenic (PV) stage before blood feeding and several time points after blood feeding (Fig. 2A–D). This analysis was accomplished by means of the reverse-transcriptase polymerase chain reaction (RT-PCR), using primers specific to the endogenous CP, transgenic CPT and UAS-EGFP (Table S3). There was no expression of the UAS-EGFP transgene in midguts of any of the tested mosquito lines (Fig. 2A–D). The expression pattern of the CP gene was similar in all four tested mosquito lines; the CP transcript was barely visible before blood feeding, it increased PBM, reaching the peak at 24 h PBM, and decreased thereafter (Fig. 2A–D). In the M16-1 line, CPT expression followed a temporal pattern similar to that of the endogenous CP gene (Fig. 2C). However, in the F4-4 line, the CPT was expressed during the previtellogenic stage and peaked at 12 h PBM (Fig. 2D). Because the CPT expression pattern in the F4-4 line did not match that of the endogenous CP, the M16-1 CP-Gal4 driver line was chosen for further analysis. Expression of CPT was not observed in the wt or the UAS-EGFP responder lines.
Figure 2.
RT-PCR analysis of the CP-Gal4 transgene expression in Ae. aegypti females. (A) WT, (B) UAS-EGFP responder line, (C) M16-1 CP-Gal4 driver line, (D) F4-4 CP-Gal4 driver line. Primers were specific to the UAS-EGFP transgene (UAS-EGFP), CP-Gal4 transgene (CPT) and endogenous CP gene (CP). Actin primers were used as control (Actin). RNA samples were extracted from isolated digestive systems of female mosquitoes at different time points: 72-h-old mosquitoes prior to blood feeding (PV), 6, 12, 24 and 48 hours PBM.
3.2. Development of CP-Gal4>UAS-EGFP hybrid mosquitoes
The CP-Gal4>UAS-EGFP hybrid mosquitoes were produced by crossing the M16-1 CP-Gal4 homozygous driver line with the UAS-EGFP homozygous responder line. These CP-Gal4>UAS-EGFP hybrid mosquitoes exhibited the presence of both EGFP and DsRed eye-specific markers (Fig. 3A–D). Genomic PCR analysis confirmed the presence of piggyBac transposon left arm, right arm, CP-Gal4 driver and UAS-EGFP responder sequences in the hybrid mosquitoes (Fig. 3E).
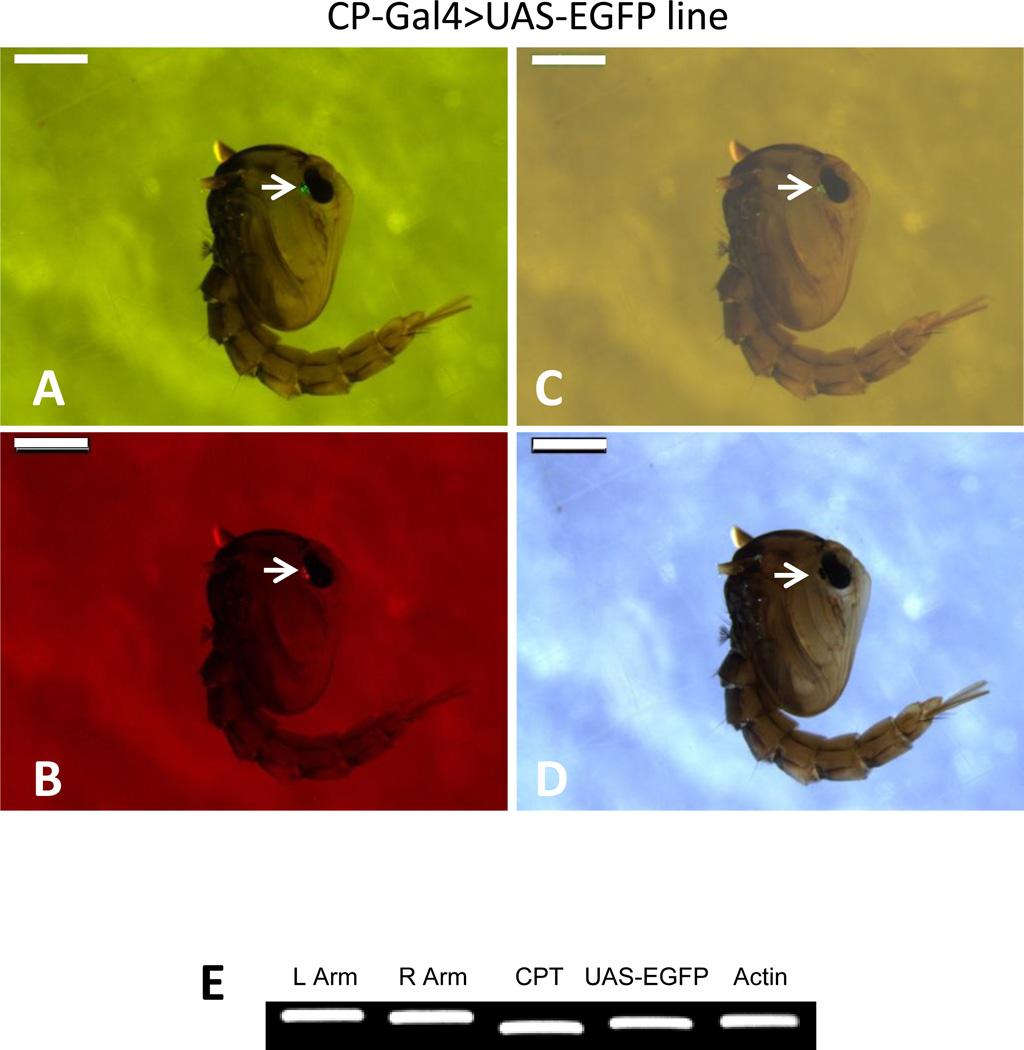
Figure 3.
Establishment of the Ae. aegypti CP-Gal4>UAS-EGFP hybrid line. (A–D). Pupae of hybrid mosquitoes were screened for the presence of eye-specific expression of the selectable markers, EGFP and DsRed. (A) GFP-B filter; (B) DsRed filter; (C) merged; (D) white light. Scale bar: 1mm. Nikon SMZ800 fluorescence microscope with GFP-B and DsRed filters was used for screening. (E) Genomic PCR analysis for verifying the integration of the CP-Gal4 driver and UAS-EGFP responder constructs in the Ae. aegypti CP-Gal4>UAS-EGFP hybrid line. Specific primers used for the analysis: L Arm and R Arm – left and right arms of the piggyBac vector; CPT - CP-Gal4 transgene; and UAS-EGFP - UAS-EGFP transgene. Actin probe was used as the loading control.
In the CP-Gal4>UAS-EGFP hybrid female mosquitoes, the EGFP transgene expression followed a pattern similar to that of the endogenous CP and CPT (Fig. 4A). Expression of the UAS-EGFP transcript was midgut-specific and was not found in the fat body (FB), ovaries (OV) or Malpighian tubules (MT) of hybrid female mosquitoes 24 h PBM (Fig. 4B). Males from this hybrid line did not express UAS-EGFP (Fig. 4B). These data indicated that, in the CP-Gal4>UAS-EGFP mosquitoes, the UAS-EGFP transgene exhibited a tissue-, sex-, and stage-specific expression pattern similar to that of the endogenous CP gene.
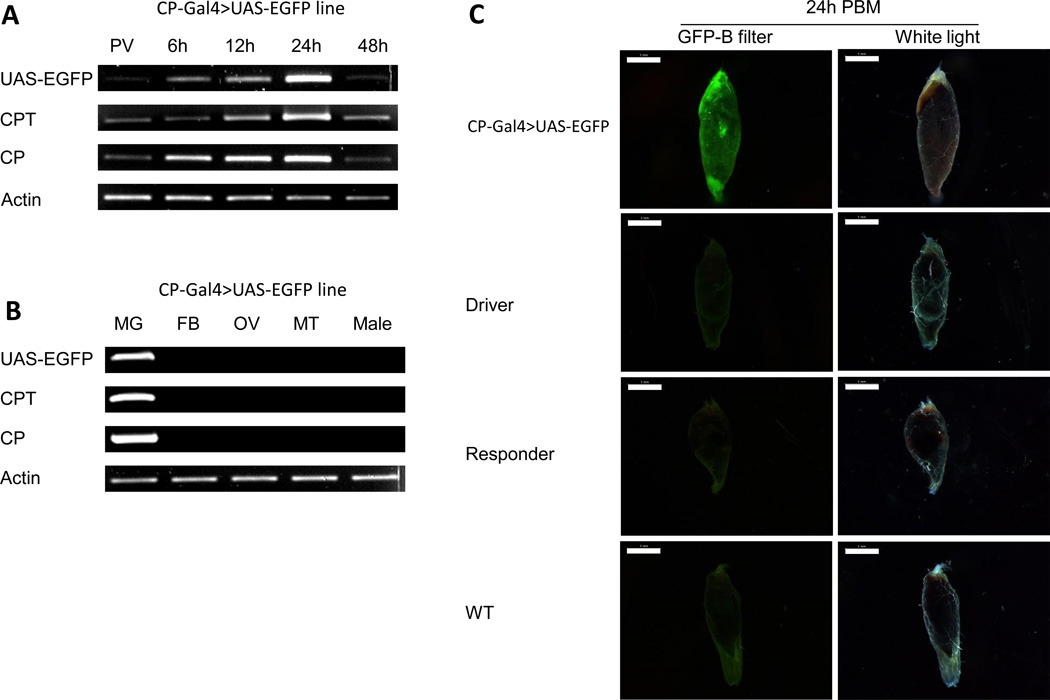
Figure 4.
Expression of the CP-Gal4>UAS-EGFP transgene in the Ae. aegypti hybrid line. (A) The time course of transcript abundance in midguts of hybrid transgenic female mosquito line using gene-specific primers. RNA samples were extracted from different time points: PV, 6, 12, 24 and 48 h PBM. (B) Tissue- and sex-specific expression of the CP-Gal4>UAS-EGFP transgene in the CP-Gal4>UAS-EGFP hybrid line mosquitoes. RNA samples were extracted from midgut (MG), fat bodies (FB), ovaries (OV), Malpighian tubules (MT) of females at 24 h PBM and adult hybrid males (M). Analyses in (A) and (B) were performed by means of RT-PCR using UAS-EGFP, CPT and CP probes. Actin was used as a loading control in (A) and (B). (C) EGFP expression in midguts from females of the CP-Gal4>UAS-EGFP line, CP-Gal4 driver line M16-1 (driver), UAS-EGFP responder line (responder), and wild type (WT). Midguts were dissected at 24 h PBM. Images were obtained using Leica M165FC fluorescent stereomicroscope using GFP-B filter or transparent filter (White light) and LAS V4.0 software. Scale bar: 1mm.
To assess the EGFP protein level in the CP-Gal4>UAS-EGFP mosquitoes, we dissected and examined midgut with a fluorescence microscope. This analysis showed a strong signal in midguts of CP-Gal4>UAS-EGFP female mosquitoes at 24 h PBM. In contrast, only background signal was observed in midguts from the CP-Gal4 driver, UAS-EGFP responder lines and wt mosquitoes examined at the same time (Fig. 4C). This confirmed that the EGFP protein was produced in the CP-Gal4>UAS-EGFP female mosquitoes only after blood-meal activation.
3.3. Insulin and amino acid/TOR signaling pathways are involved in activation of CP-Gal4>UAS-EGFP transgene expression
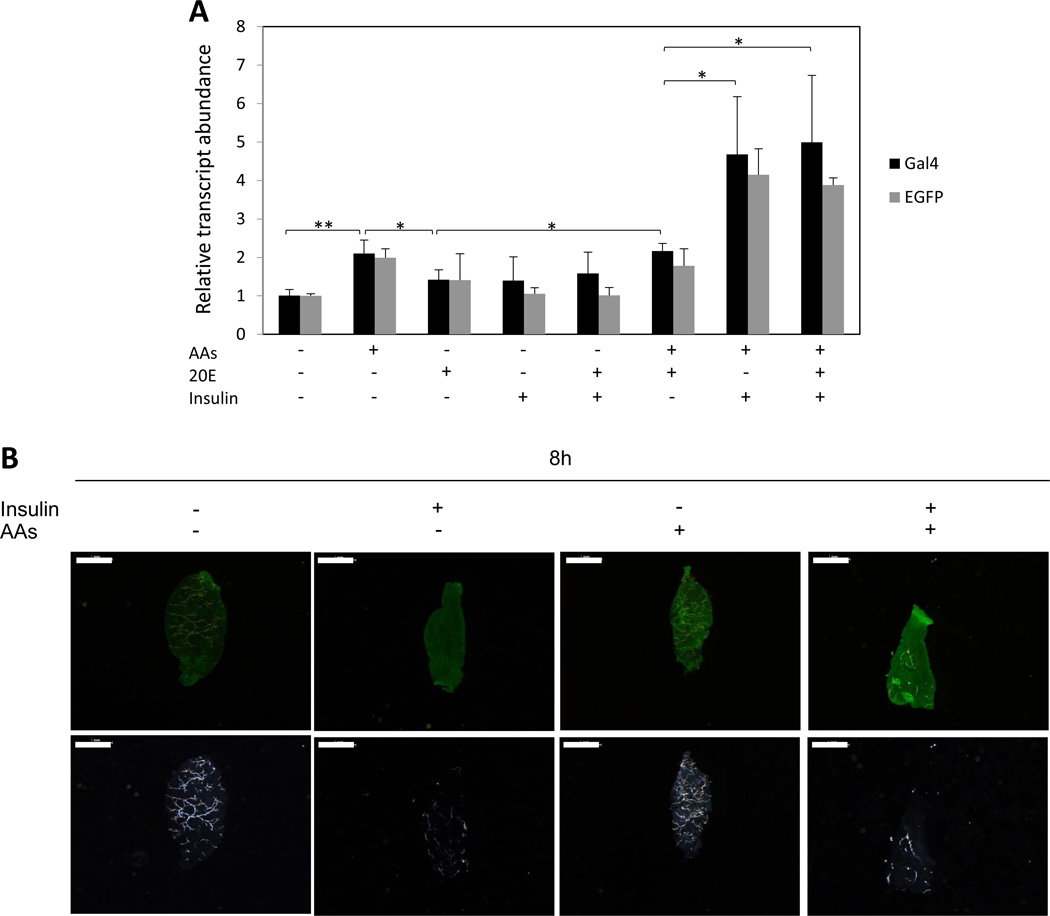
To elucidate the signals involved in regulation of CP-Gal4>UAS-EGFP transgene expression, we first used an in vitro organ culture assay (Fig. 5). Digestive systems (midgut) isolated from the CP-Gal4>UAS-EGFP transgenic female mosquitoes were incubated in the presence or absence of specific factors in a culture medium. Results were evaluated by means of quantitative reverse-transcriptase PCR (qRT-PCR), using primers for the CP-Gal4 (Gal4) and UAS-EGFP (EGFP) transgenes (Table S3). The tissue incubated in the medium without AAs showed a low, baseline level expression of the CP-Gal4>UAS-EGFP transgene transcript, while addition of AAs to the medium resulted in a statistically significant increase in transcript levels (Fig. 5A). Insulin alone did not increase the CP-Gal4>UAS-EGFP transgene transcript level; however, the transcript level rose significantly after incubation in medium containing both AAs and insulin together. Testing expression of both the CP-Gal4 and the UAS-EGFP transgene has permitted us to demonstrate that their expression is clearly co-regulated.
Figure 5.
Effects of AAs, insulin and 20E on expression of the CP-Gal4>UAS-EGFP transgene. (A) Midguts were isolated from non-blood fed CP-Gal4>UAS-EGFP female mosquitoes and incubated under different indicated conditions in vitro for 8 h. Levels of the CP-Gal4>UAS-EGFP transgene transcript were monitored using Gal4 and EGFP primers (Table S3) by means of qRT-PCR. Control transcript level was set at 1, while transcript levels for other treatments were expressed relative to the control. Values are the means of three replicates (± SEM). The experiment was repeated three times. * Indicates statistical significance <0.05. ** Indicates statistical significance <0.01. (B) Detection of EGFP protein in midguts isolated from non-blood fed CP-Gal4>UAS-EGFP female mosquitoes by means of fluorescent immunocytochemistry. The midguts were cultured for 8h under indicated conditions. Immunocytochemistry was performed using the anti- EGFP antibody, followed by incubation with an anti-rabbit fluorescent secondary antibody. Images were obtained using Leica M165FC fluorescent stereomicroscope using GFP-B filter or transparent filter (White light) and LAS V4.0 software. Scale bar: 1mm.
When we examined the EGFP protein level using immunocytochemistry, we observed a substantial increase in the signal only after incubation of midguts from CP-Gal4>UAS-EGFP hybrid female mosquitoes in the medium supplemented with AAs and insulin (Fig. 5B). Thus, in vitro organ culture experiments have shown that both insulin and AAs are required for transcription and translation of the CP-Gal4-driven EGFP in the midgut of the transgenic mosquitoes. Moreover, they exhibit a strong additive effect.
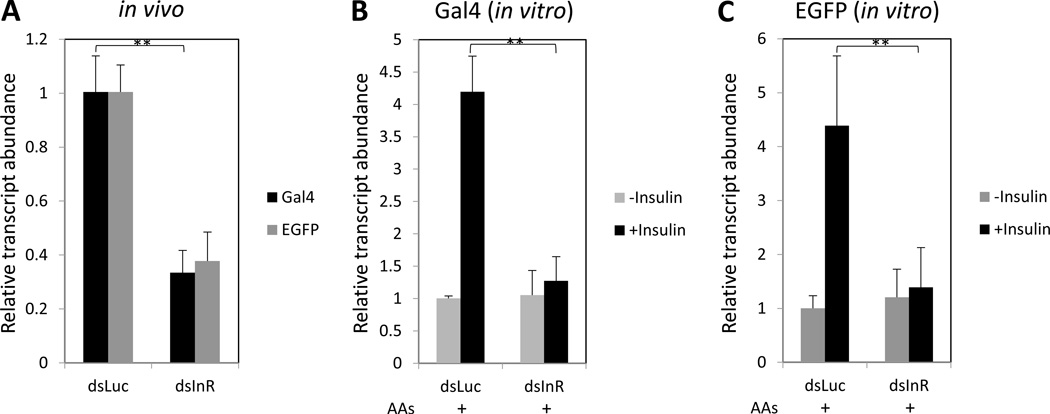
To substantiate in vitro experiments, we investigated the in vivo effect of RNAi depletions of potential factors involved in regulation of the CP-Gal4>UAS-EGFP transgene in the mosquito midgut. Because insulin has been identified as a principal regulator of the transgene expression, we examined the effect of RNAi depletion of the InR. There is a single gene encoding InR in the Ae. aegypti genome (Brown et al., 2008). RNAi depletion of InR in vivo resulted in a significant decrease of the CP-Gal4>UAS-EGFP transgene transcript level in midguts at 24h PBM, as indicated by testing with Gal4 and EGFP probes (Fig. 6A). To confirm that the effect observed after InR RNAi depletions correlated with the responsiveness to insulin, we incubated midguts isolated from the non-blood fed CP-Gal4>UAS-EGFP transgenic female mosquitoes at 72 h post-eclosion treated with Luciferase double-stranded RNA (dsLuc) or double-stranded insulin receptor RNA (dsInR) in the amino acid-containing culture media in the presence or absence of insulin (Fig. 6B–C). Addition of insulin elevated the CP-Gal4>UAS-EGFP transgene transcript in the dsLuc-treated mosquitoes. In contrast, no response to insulin was observed in dsInR-treated mosquitoes (Fig. 6B–C). Thus, in vivo RNAi experiment results were in agreement with those from the in vitro tissue culture, strongly suggesting that insulin plays a major role in regulating CP gene expression.
Figure 6.
Insulin signaling pathway controls expression of the CP-Gal4>UAS-EGFP transgene. (A) RNAi of Insulin Receptor (dsInR) in vivo. RNAi for Luciferase (dsLuc) served as a control. The CP-Gal4>UAS-EGFP females were injected with dsRNAs at 1 day post-eclosion, blood fed and examined at 24 h PBM. Transcript abundance was examined using Gal4 and EGFP primers by means of qRT-PCR. Control dsLuc transcript level was standardized to 1, while transcript levels for other RNAi treatments were expressed relative to the control. (B–C). Activation of the CP-Gal4>UAS-EGFP transgene expression by insulin in vitro was prevented by dsInR. The CP-Gal4>UAS-EGFP females were injected with dsRNAs at 1-day post-eclosion, and an in vitro assay was performed as in Figure 5. Midguts were isolated from non-blood fed CP-Gal4>UAS-EGFP females and incubated in the AA-containing culture medium. Expression of the CP-Gal4>UAS-EGFP transgene was monitored by means of qRT-PCR using probes for Gal4 (B) and EGFP (C) (primer sequences in Table S3).
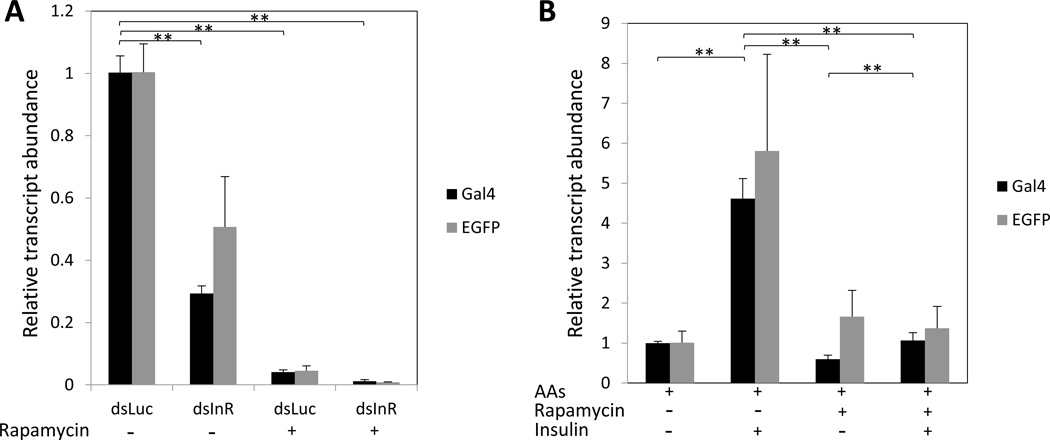
Target of Rapamycin kinase (TOR) is a conserved nutritional sensor that mediates both insulin and AA signaling (Grewal, 2008). We tested whether TOR inhibition affects the expression of the CP-Gal4>UAS-EGFP transgene transcript. We injected 3-day-old, CP-Gal4>UAS-EGFP female mosquitoes with the TOR inhibitor rapamycin at different concentrations and examined at 24 h PBM. Results indicated that the transgene transcript expression was inhibited by rapamycin in a dose-dependent manner (Fig. S3A), and rapamycin did not affect survival of mosquitoes (Fig. S3B). Rapamycin significantly decreased the transcript level of this gene in the CP-Gal4>UAS-EGFP female mosquitoes treated with dsLuc (Fig. 7A). RNAi depletion of InR significantly decreased the level of the CP-Gal4>UAS-EGFP transgene transcript when compared with that of the dsLuc. However, this transcript level was completely diminished in transgenic mosquitoes co-treated with rapamycin and dsInR (Fig. 7A). Rapamycin also prevented elevation of this transcript by insulin in the mosquito digestive system in an in vitro culture assay (Fig. 7B).
Figure 7.
Effect of TOR inhibitor rapamycin on the expression of the CP-Gal4>UAS-EGFP transgene. (A). In vitro application of AAs and insulin activated CP-Gal4>UAS-EGFP expression, and rapamycin was capable of blocking this activation. The transcript level was set at 1 for the “no treatment” sample (control), and transcript levels for the other treatments were expressed relative to the control. (B). qRT-PCR analysis of CP-Gal4>UAS-EGFP transgene expression in midguts at 24 h PBM from CP-Gal4>UAS-EGFP hybrid females after different treatments. 20 pmol of rapamycin was injected in each experiment. Transcript levels were standardized to 1 for the dsLuc sample (control), and transcript levels for the other treatment were expressed relative to the control.
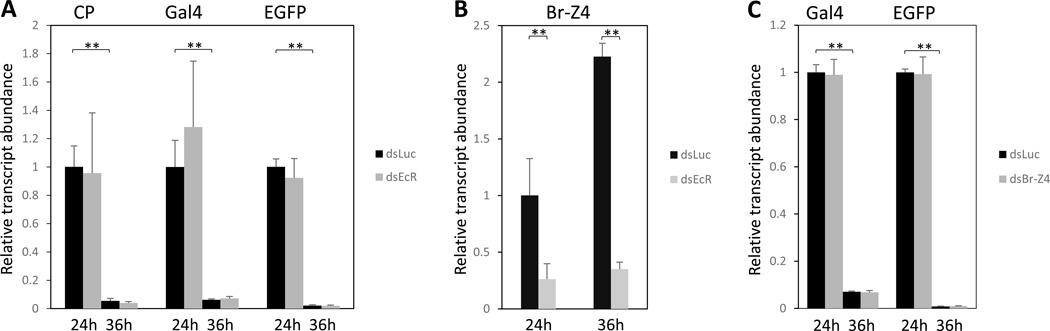
20E is a major regulator of the PBM reproductive events; however, its role in regulating digestive enzymes is not understood. To investigate the possible influence of the ecdysone regulatory cascade on the CP-Gal4>UAS-EGFP transgene expression, we first investigated the effect of 20E using an in vitro organ culture assay, as described above. Supplementing the medium with 20E did not elevate its level, regardless of the presence or absence of AAs (Fig. 5A). Next, we used in vivo ecdysone receptor (EcR) RNAi depletion. The double-stranded RNA for EcR (dsEcR) was designed using the region common to both EcR-A and EcR-B isoforms. This depletion had no effect on the transgene transcript level, which was similar to that of the dsLuc control (Fig. 8). The dsEcR treatments also had no effect on the transcript abundance of the endogenous CP gene examined in parallel with that of the CP-Gal4>UAS-EGFP transgene transcript at 24 h and 36 h PBM in vivo (Fig. 8).
Figure 8.
RNAi of the EcR (dsEcR) and Br-Z4 (dsBr-Z4) gene in the CP-Gal4>UAS-EGFP female mosquitoes. (A–C). The procedure was performed as in Figure 6A. RNAi for Luciferase (dsLuc) served as a control. The effect of the EcR and Br-Z4 knockdown on the CP-Gal4>UAS-EGFP transgene expression was monitored by means of qRT-PCR using Gal4 and EGFP primers. The transcript levels of the endogenous CP gene (A) and Br-Z4 gene (B) after dsEcR treatment were also examined. Times at which examinations were done are marked by 24h and 36h PMB.
Analysis of the CP gene promoter has revealed the presence of a putative binding site—tgtttag—for Broad isoform Z4 (Br-Z4). Br is a downstream gene in the 20E hierarchy, although it is reportedly not entirely dependent on 20E signaling and can act independently (Brennan et al., 2001; Gancz et al., 2011). In our experiments, EcR RNAi depletion significantly reduced the level of Br-Z4 transcript, suggesting that this gene is under the control of 20E hierarchy (Fig. 8B). However, to further investigate a possible involvement of Br-Z4 in regulation of the CP-Gal4>UAS-EGFP transgene, we conducted Br-Z4 RNAi depletion. The double-stranded RNA of Br-Z4 (dsBr-Z4) treatment did not affect the transcript abundance of the transgene gene examined at 24 h and 36 h PBM in vivo (Fig. 8C). It is possible that Br-Z4 plays an important role in regulation of CP gene expression during other stages of the mosquito life cycle. Effects of RNAi depletions of tested factors on their own transcript levels are presented in Figure S2.
Thus, our study using the CP-Gal4>UAS-EGFP transgene has shown that insulin and AA/TOR signaling pathways, but not 20E, are involved in regulating the expression of the gene encoding the late-phase blood digestive enzyme CP.
4. Discussion
In this study, we expanded the development of the binary Gal4/UAS system for the yellow fever mosquito Ae. aegypti and used the 5' upstream region of the CP gene, which is induced by a blood meal in the midgut of female mosquitoes (Isoe et al, 2009a; Edwards et al., 2000), to genetically engineer the transgenic CP-Gal4 driver line. After a blood meal, the CP gene expression and enzymatic activity of CP increases rapidly and reaches its peak at 24 h (Edwards et al., 2000; Noriega et al., 2002). The 1.1-kb 5' upstream region of the CP gene is sufficient to lead transgene expression with a profile similar to that of the endogenous CP gene (Franz et al., 2006). The important feature of this driver is that the CPT exhibits specific expression in the female mosquito midgut, and is activated by blood feeding. We generated two Ae. aegypti CP-Gal4 driver lines. However, only the M16-1 line expressed the transgene in a manner consistent with that of the endogenous CP, from which the regulatory DNA was derived. In contrast, the F4-4 line showed a different transgene expression profile, in which the CPT transcript was expressed prior to blood-meal activation and reached its peak at 12 h PBM rather than 24 h. The integration transgene site for F4-4 located 8 kb upstream of a predicted gene, AAEL004671, which encodes (s)-2-hydroxy-acid oxidase. The expression pattern in the F4-4 line was possibly affected by its integration location. Transgene miss-expression has previously been reported for midgut-specific transgenes in An. gambiae and Ae. aegypti (Lynd and Lycett, 2012; Franz et al., 2011). Integration of a transgene in a specific site in the genome is critical for its correct expression. For example, integration near a certain transcription enhancer or repressor could lead to an unintended transgene expression pattern (Wallrath and Elgin, 1995), and integration near a transcription repressor or heterochromatic region could result in low levels of transgene expression (O'Kane and Gehring, 1987). In some transposable-element-generated mosquito lines, transgenes have reportedly been poorly expressed because of the position effect (Nimmo et al., 2006; Labbe et al., 2010). Franz et al. described that when the CP gene promoter region was used to drive EGFP reporter expression in Ae. aegypti, only two lines from a total number of nine transgenic mosquitoes lines robustly expressed the EGFP reporter (Franz et al., 2011). Lycett et al. (2012) found that the alpha-tubulin-1b gene promoter directed transgene expression in the head, ventral nerve cord and testes of one An. gambiae transgenic line; however, expression of the same transgene was observed in larval and adult muscles, fat body, cuticle and midgut secretory cells of a different transgenic line. The lack of more advanced techniques for mosquito transformation similar to those of Drosophila complicates a challenging task of mosquito genetic transformation.
Multiple enzymes are involved in blood digestion in the midgut of female mosquitoes. These include trypsin-like and chymotrypsin-like serine endoproteases, amino- and carboxypeptidases (Isoe et al., 2009a; Isoe et al., 2009b). In Ae. aegypti, mRNA of the early trypsin (AaET) gene is expressed and accumulated in the midgut cells prior to blood feeding, while the synthesis of the AaET protein occurs just after blood feeding (Noriega et al., 1996). The transcript and protein levels of a digestive endoprotease, late trypsin (AaLT), reach their peaks by 24 h post blood meal (PBM) (Isoe et al., 2009b; Barillas-Mury et al., 1993). Recent studies have revealed seven additional midgut serine proteases (AaSPs); the expression profiles of genes encoding these proteases are similar to that of the AaLT gene (Isoe et al., 2009a; Brackney et al., 2010). The RNAi and enzymatic analyses have demonstrated differential roles of midgut serine proteases. Knockdown of AaSPVI caused a 78% decrease in the late-phase, trypsin-like activity, while knockdowns of AaLT, AaSPVI and AaSPVII inhibited degradation of the serum albumin (Isoe et al., 2009a). Carboxypeptidases represent another important group of mosquito gut proteases (Isoe et al, 2009b). The gene encoding Ae. aegypti carboxypeptidase A has an expression profile similar to that of the AaLT gene (Edwards et al., 2000). However, the regulation of the CP gene has not been studied.
Previous studies on amino acids suggest that these blood components serve as nutritional signal in digestive protease introduction (Caroci and Noriega, 2003). Amino acids are key stimuli, which play a role in regulating the translation of AaET (Brandon et al., 2008). Eight insulin-like peptides (ILPs) have been reported in Ae. aegypti, among which AaILP3 binds the InR (Riehle et al., 2006; Wen et al., 2010). Gulia-Nuss et al. reported that amino acids and insulin signaling pathways regulate Ae. aegypti late phase gene expression, blood digestion and egg maturation (Gulia-Nuss et al., 2011). InR RNAi knockdown or TOR inhibition reduces the AaLT gene expression and the activity of this enzyme (Gulia-Nuss et al., 2011).
Using the Ae. aegypti CP-Gal4>UAS-EGFP line with the M16- 1 Gal4 driver, we established that the expression of the UAS-EGFP transgene matched that of the endogenous CP gene as well as that of the CPT. In vitro organ-culture experiments demonstrated a synergistic action of insulin and AAs in activating expression of the CP-Gal4>UAS-EGFP transgene. These observations were in agreement with in vivo RNAi depletion experiments. RNAi silencing of InR resulted in a significant decrease in the CP-Gal4>UAS-EGFP transgene transcript level. Application of rapamycin, a specific inhibitor of TOR, had a strong inhibitory effect on the CP-Gal4>UAS-EGFP transgene transcript expression, indicating importance of the TOR kinase in regulation of the CP gene. Moreover, the transcript level was completely diminished in transgenic mosquitoes co-treated with rapamycin and dsInR. This inhibition was significantly stronger than that caused by InR RNAi depletion alone, suggesting additive effects of TOR and insulin. Treatment with rapamycin also prevented elevation of this transcript after incubation with either AAs or insulin in vitro, confirming independent effects of these regulators. The TOR kinase pathway has been reported to integrate extracellular signals such as insulin and AAs (Nave et al. 1999; Sarbassov et al. 2005). Thus, insulin and AA/TOR signaling pathways are involved in regulating expression of the CP gene. Taken together, our investigation along with previous studies has revealed important roles of insulin and nutrition-driven AA/TOR pathways in regulating the expression of late-phase digestive enzymes in the mosquito midgut.
20E is the major regulator of vitellogenic events in female mosquitoes (Raikhel et al., 2005). It regulates expression of genes encoding yolk protein genes in the fat body. However, our experiments revealed no apparent role of this hormone in controlling the CP gene in the female mosquito midgut. The level of the CP-Gal4>UAS-EGFP transgene transcript was not affected as a result of in vitro midgut incubations in the presence of 20E. Moreover, EcR RNAi silencing had no effect on the transcript level of this transgene. The absence of ecdysone response elements (EcRE) or binding sites for any other known 20E downstream factors (other than that of BrZ4) within the CP gene promoter further corroborates these results. RNAi silencing has shown that BrZ4 is not involved in regulation of the CP gene in the gut of female mosquitoes. Thus, the presence of the BrZ4 recognition site in the CP promoter could be explained by a possible contribution of this transcription factor in control of CP gene expression during other stages of development.
In conclusion, this study contributes toward the improvement of genetics tools for mosquitoes. We developed an Ae. aegypti CP-Gal4 driver line that is capable of directing a female, midgut-specific and blood-meal-induced expression of the UAS/target transgene. Using the CP-Gal4>UAS-EGFP system, we have demonstrated that activation of the CP gene depends on insulin/InR and nutrient-driven AA/TOR pathways. In contrast, 20E, which is the major regulator of mosquito vitellogenesis, plays no role in CP gene activation. Establishment of the Ae. aegypti CP-Gal4/UAS system permits functional testing of various midgut-specific genes involved in blood digestion and pathogen interaction.
Supplementary Material
We generated CP-Gal4 Aedes aegypti mosquitoes with a midgut-specific expression.
The CP-Gal4 drives expression in the female mosquito midgut after blood feeding.
Insulin and Target-of-Rapamycin regulate the carboxypeptidase CP gene.
RNA interference silencing of the insulin receptor has confirmed this finding.
Acknowledgements
We thank Dr. Kenneth E. Olson for providing the pBluescript-AeCPA promoter plasmid. This work was supported by NIH grant RO1 AI113729.
Abbreviations
- CP
carboxypeptidase A
- EGFP
enhanced green fluorescent protein
- AAs
amino acids
- 20E
20-hydroxyecdysone
- TOR
Target-of-Rapamycin
- RNAi
RNA interference
- InR
insulin receptor
- MG
midgut
- PBM
post blood meal
- WT
wild type
- PCR
polymerase chain reactions
- CPT
CP-Gal4 transgene
- PV
previtellogenic
- RT-PCR
reverse-transcriptase polymerase chain reaction
- FB
fat body
- OV
ovaries
- MT
Malpighian tubules
- qRT-PCR
quantitative reverse transcriptase PCR
- dsLuc
double-stranded RNA of Luciferase
- dsInR
double-stranded RNA of insulin receptor
- EcR
Ecdysone Receptor
- dsEcR
double-stranded RNA of Ecdysone Receptor
- Br-Z4
Broad isoform Z4
- dsBr-Z4
double-stranded RNA of Br-Z4
- EcRE
ecdysone response element
- ILPs
insulin-like peptides
- BLAST
basic local alignment search tool
- PBS
Phosphate Buffered Saline
- dsRNA
double-stranded RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bo Zhao, Email: bzhao002@ucr.edu.
Vladimir A. Kokoza, Email: vladimir.kokoza@ucr.edu.
Tusar T. Saha, Email: tsaha001@ucr.edu.
Stephanie Wang, Email: swang024@ucr.edu.
Sourav Roy, Email: sourav.roy@ucr.edu.
References
- Adelman ZN, Anderson MA, Morazzani EM, Myles KM. A transgenic sensor strain for monitoring the RNAi pathway in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2008;38:705–713. doi: 10.1016/j.ibmb.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. doi: 10.1126/science.1191864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillas-Mury CV, Wells MA. Cloning and sequencing of the blood meal-induced late trypsin gene from the mosquito Aedes aegypti and characterization of the upstream regulatory region. Insect Mol. Bio. 1993;2:7–12. doi: 10.1111/j.1365-2583.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Brackney DE, Isoe J, Black WCIV, Zamora J, Foy BD, Miesfeld RL, Olson KE. Expression profiling and comparative analyses of seven midgut serine proteases from the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 2010;56:736–744. doi: 10.1016/j.jinsphys.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brandon MC, Pennington JE, Isoe J, Zamora J, Schillinger AS, Miesfeld RL. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2008;38(10):916–922. doi: 10.1016/j.ibmb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Li TR, Bender M, Hsiung F, Moses K. Broad-complex, but not ecdysone receptor, is required for progression of the morphogenetic furrow in the Drosophila eye. Development. 2001;128:1–11. doi: 10.1242/dev.128.1.1. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroci SA, Noriega GF. Free amino acids are important for the retention of protein and non-protein meals by the midgut of Aedes aegypti females. J Insect Physiol. 2003;49(9):839–844. doi: 10.1016/S0022-1910(03)00134-3. [DOI] [PubMed] [Google Scholar]
- Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat. Biotechnol. 2005;23:1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- Cho KH, Cheon HM, Kokoza V, Raikhel AS. Regulatory region of the vitellogenin receptor gene sufficient for high-level, germ line cell-specific ovarian expression in transgenic Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2006;36:273–281. doi: 10.1016/j.ibmb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Moskalyk LA, Donelly-Doman M, Vlaskova M, Noriega FG, Walker VK, Jacobs-Lorena M. Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol. Biol. 2000;9:33–38. doi: 10.1046/j.1365-2583.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AW, Jasinskiene N, Sanchez-Vargas I, Isaacs AT, Smith MR, Khoo CCH, Heersink MS, James AA, Olson KE. Comparison of transgene expression in Aedes aegypti generated by mariner Mos 1 transposition and ΦC31 site-directed recombination. Insect Mol. Biol. 2011;20:587–598. doi: 10.1111/j.1365-2583.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancz D, Lengil T, Gilboa L. Coordinated regulation of niche and stem cell precursors by hormone signaling. Plos Biology. 2011;9:e1001202. doi: 10.1371/journal.pbio.1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS. Insulin/TOR signaling in growth and homeostasis: A view from the fly world. Int. J. Biochem. Cell Biol. 2008;41(5):1006–1010. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the Target of Rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. Plos One. 2011;6(5):e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM, Harrell RA., II Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol. Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- Handler AM, McCombs SD, Fraser MJ, Saul SH. The lepidopteran transposon vector, piggyBac mediates germ-line transformation in the Mediterranean fruit fly. Proc. Natl. Acad. Sci. USA. 1998;95:7520–7525. doi: 10.1073/pnas.95.13.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley KO, Nutt SL, Amaya E. Targeted gene expression in transgenic Xenopus using the binary Gal4/UAS system. Proc. Natl. Acad. Sci. USA. 2002;99:1377–1382. doi: 10.1073/pnas.022646899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev. Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- Imamura M, Nakai J, Inoue S, Quan GX, Kanda T, Tamura T. Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics. 2003;165:1329–1340. doi: 10.1093/genetics/165.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe J, Kunz S, Manhart C, Wells MA, Miesfeld RL. Regulated expression of microinjected DNA in adult Aedes aegypti mosquitoes. Insect Mol. Biol. 2007;16:83–92. doi: 10.1111/j.1365-2583.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- Isoe J, Rascón AA, Jr, Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2009a;39:903–912. doi: 10.1016/j.ibmb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe J, Zamora J, Miesfeld RL. Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem. Mol. Biol. 2009b;39:68–73. doi: 10.1016/j.ibmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, Raikhel AS. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Raikhel AS. Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system. Insect Biochem. Mol. Biol. 2011;41:637–644. doi: 10.1016/j.ibmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M, Arnosti DN. Information display by transcriptional enhancers. Development. 2003;130:6569–6575. doi: 10.1242/dev.00890. [DOI] [PubMed] [Google Scholar]
- Labbe GMC, Nimmo DD, Alphey L. piggybac- and ΦC31-mediated genetic transformation of the Asian tiger mosquito, Ae. albopictus (Skuse) PLoS Negl. Trop. Dis. 2010;4(8):e788. doi: 10.1371/journal.pntd.0000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett GJ, Amenya D, Lynd A. The Anopheles gambiae alpha-tubulin-1b promoter directs neuronal, testes and developing imaginal tissue specific expression and is a sensitive enhancer detector. Insect Mol. Biol. 2012;21:79–88. doi: 10.1111/j.1365-2583.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- Lynd A, Lycett GJ. Development of the Bi-Partite Gal4-UAS System in the African Malaria Mosquito, Anopheles gambiae. PLoS ONE. 2012;7(2):e31552. doi: 10.1371/journal.pone.0031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, Jacobs-Lorena M. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA. 2000;97:10895–10898. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;334(2):427–431. [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo DD, Alphey L, Meredith JM, Eggleston P. High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol. Biol. 2006;15:129–136. doi: 10.1111/j.1365-2583.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Petris E, Muller HM, Cronin A, Cateruccia F, Crisanti A. Analysis of two novel midgut-specific promoters driving transgene expression in Anopheles stephensi mosquitoes. PLoS One. 2011;6(2):e16471. doi: 10.1371/journal.pone.0016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega FG, Edgar KA, Bechet R, Wells MA. Midgut exopeptidase activities in Aedes aegypti are induced by blood feeding. J. Insect Physiol. 2002;48:205–212. doi: 10.1016/s0022-1910(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Pennington JE, Barillas-Mury C, Wang XY, Wells MA. Early trypsin, an Aedes aegypti female specific protease, is post-transcriptionally regulated by the blood meal. Insect Mol. Bio. 1996;5:25–29. doi: 10.1111/j.1365-2583.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- O'Kane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Moreadith RW, Leder P. Binary system for regulating transgene expression in mice: targeting int-2 gene expression with yeast GAL4/UAS control elements. Proc. Natl. Acad. Sci. USA. 1991;88:698–702. doi: 10.1073/pnas.88.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanos PA, Windbichler N, Menchinelli M, Burt A, Crisanti A. The vasa regulatory region mediates germline expression and maternal transmission of proteins in the malaria mosquito Anopheles gambiae: a versatile tool for genetic strategies. BMC Mol Biol. 2009;10:65. doi: 10.1186/1471-2199-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel AS, Brown MR, Belles X. Endocrine Control of Reproductive Processes. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Vol. 3, Endocrinology. Elsevier Press; 2005. pp. 433–491. [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr. Opin. Cell. Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Schinko JB, Weber M, Viktorinova I, Kiupakis A, Averof M, Klinger M, Wimmer EA, Bucher G. Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev. Biol. 2010;10:53–64. doi: 10.1186/1471-213X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath LL, Elgin SC. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9(10):1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol. Cell Endocrinol. 2010;328:47–55. doi: 10.1016/j.mce.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.