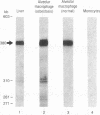
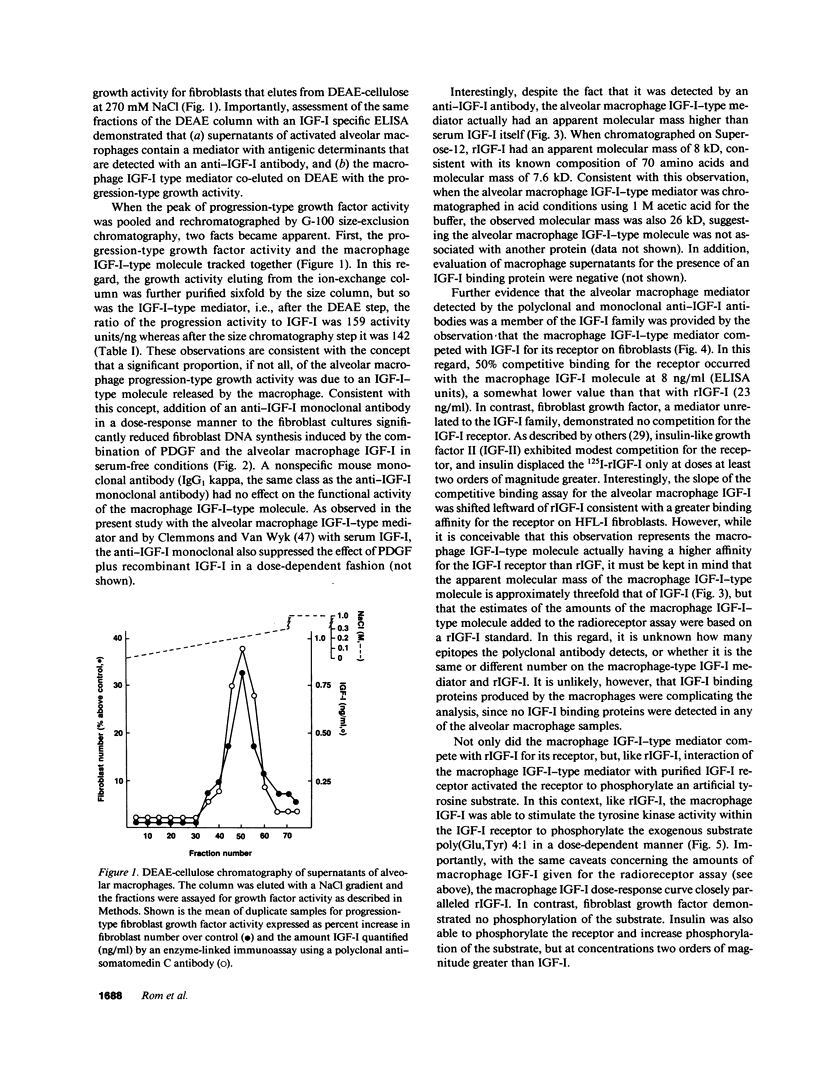
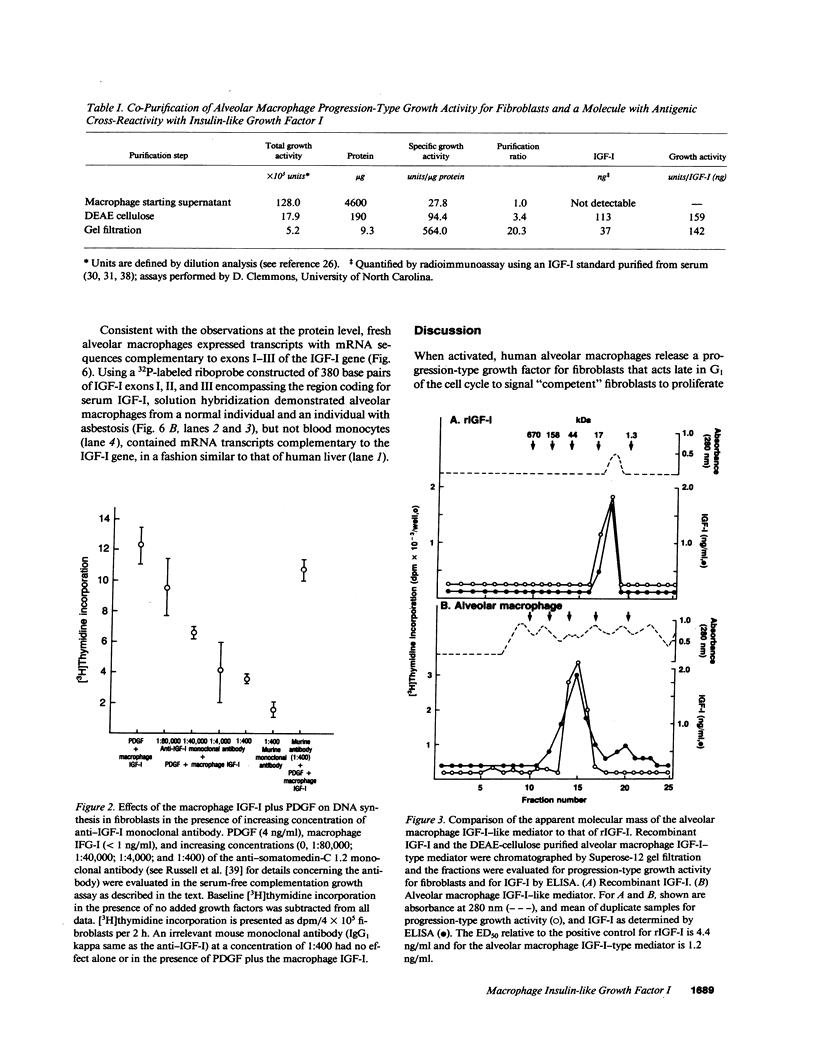
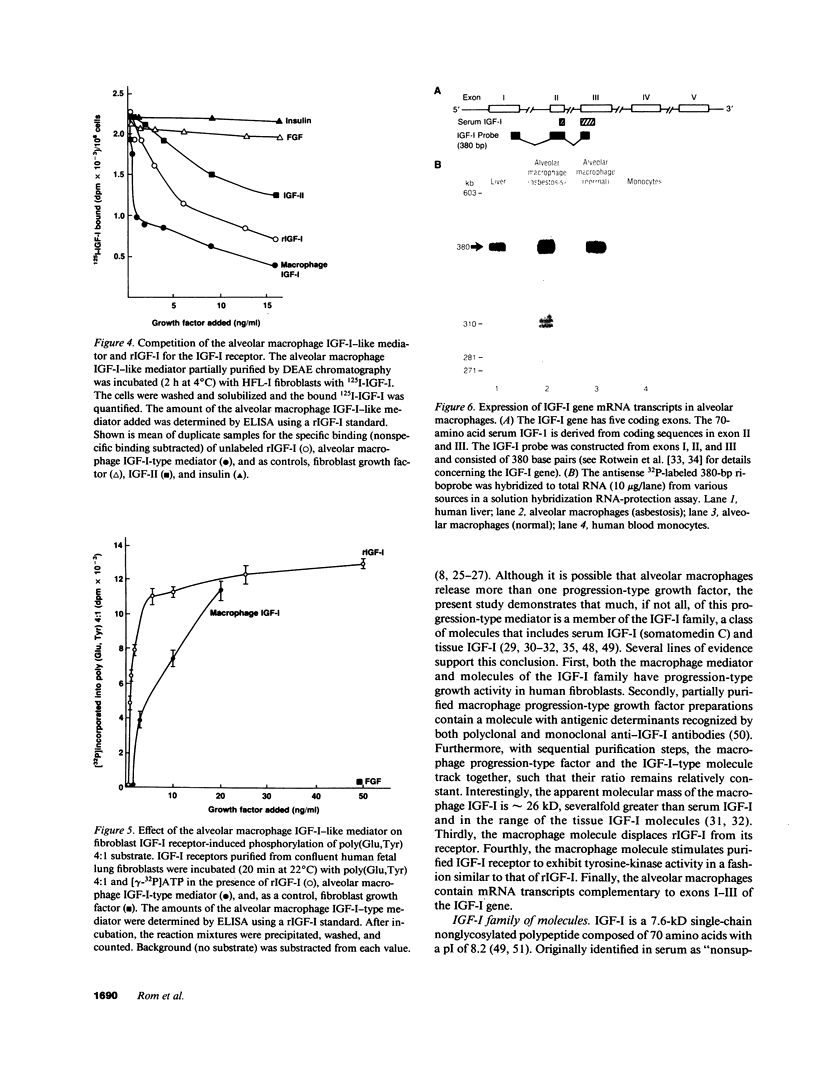
Abstract
Human alveolar macrophages, when activated, release a progression-type growth factor for fibroblasts that signals "competent" fibroblasts to replicate. The present study demonstrates that this growth activity is an insulin-like growth factor I (IGF-I)-type molecule. Partial purification of medium conditioned by activated alveolar macrophages using ion exchange and gel filtration chromatography revealed an IGF-I molecule as detected by an anti-IGF-I polyclonal antibody and that the specific activity of the progression-type growth activity tracked with the amount of IGF-I present. In a serum-free complementation test, the increase in fibroblast proliferation by alveolar macrophage IGF-I was reduced in a dose-response manner with an anti-IGF-I monoclonal antibody. The alveolar macrophage IGF-I displaced 125I-IGF-I from its receptor in a binding assay utilizing human lung fibroblasts and it stimulated type I IGF receptors purified from human lung fibroblasts to phosphorylate a tyrosine-containing artificial substrate. In contrast to the 7.6-kD serum IGF-I, gel chromatography revealed that the alveolar macrophage IGF-I had an apparent molecular mass of 26 kD, similar to other tissue IGF-Is. Alveolar macrophages expressed IGF-I mRNA transcripts as detected by solution hybridization using a 32P-labeled riboprobe complementary to exons I-II-III of the IGF-I gene. In the context of the known functions of the family of IGF-I molecules in cell growth, IGF-I released by activated alveolar macrophages may play a role in acute and chronic inflammatory disorders.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Adelberg S., Crystal R. G. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983 Dec;97(6):1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P., Rennard S., Schoenberger C., Crystal R. Asbestos stimulates alveolar macrophages to release a factor causing human lung fibroblasts to replicate. Chest. 1981 Jul;80(1 Suppl):38–39. doi: 10.1378/chest.80.1_supplement.38s. [DOI] [PubMed] [Google Scholar]
- Blum W. F., Ranke M. B., Bierich J. R. Isolation and partial characterization of six somatomedin-like peptides from human plasma Cohn fraction IV. Acta Endocrinol (Copenh) 1986 Feb;111(2):271–284. doi: 10.1530/acta.0.1110271. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Shaw D. S. Purification and biologic properties of fibroblast somatomedin. J Biol Chem. 1986 Aug 5;261(22):10293–10298. [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J. Evidence for a functional role of endogenously produced somatomedinlike peptides in the regulation of DNA synthesis in cultured human fibroblasts and porcine smooth muscle cells. J Clin Invest. 1985 Jun;75(6):1914–1918. doi: 10.1172/JCI111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover C. A., Rosenfeld R. G., Hintz R. L. Hormonal control of the replication of human fetal fibroblasts: role of somatomedin C/insulin-like growth factor I. J Cell Physiol. 1986 Jul;128(1):47–54. doi: 10.1002/jcp.1041280109. [DOI] [PubMed] [Google Scholar]
- Cook J. J., Haynes K. M., Werther G. A. Mitogenic effects of growth hormone in cultured human fibroblasts. Evidence for action via local insulin-like growth factor I production. J Clin Invest. 1988 Jan;81(1):206–212. doi: 10.1172/JCI113296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- De Troyer A., Desir D., Copinschi G. Regression of lung size in adults with growth hormone deficiency. Q J Med. 1980;49(195):329–340. [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Han V. K., D'Ercole A. J., Lund P. K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987 Apr 10;236(4798):193–197. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Mathews L. S., Norstedt G., Palmiter R. D. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merimee T. J., Zapf J., Hewlett B., Cavalli-Sforza L. L. Insulin-like growth factors in pygmies. The role of puberty in determining final stature. N Engl J Med. 1987 Apr 9;316(15):906–911. doi: 10.1056/NEJM198704093161503. [DOI] [PubMed] [Google Scholar]
- Morell B., Froesch E. R. Fibroblasts as an experimental tool in metabolic and hormone studies. II. Effects of insulin and nonsuppressible insulin-like activity (NSILA-S) on fibroblasts in culture. Eur J Clin Invest. 1973 Mar;3(2):119–123. doi: 10.1111/j.1365-2362.1973.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Mornex J. F., Martinet Y., Yamauchi K., Bitterman P. B., Grotendorst G. R., Chytil-Weir A., Martin G. R., Crystal R. G. Spontaneous expression of the c-sis gene and release of a platelet-derived growth factorlike molecule by human alveolar macrophages. J Clin Invest. 1986 Jul;78(1):61–66. doi: 10.1172/JCI112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissley S. P., Rechler M. M. Somatomedin/insulin-like growth factor tissue receptors. Clin Endocrinol Metab. 1984 Mar;13(1):43–67. doi: 10.1016/s0300-595x(84)80008-0. [DOI] [PubMed] [Google Scholar]
- Ong J., Yamashita S., Melmed S. Insulin-like growth factor I induces c-fos messenger ribonucleic acid in L6 rat skeletal muscle cells. Endocrinology. 1987 Jan;120(1):353–357. doi: 10.1210/endo-120-1-353. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Rom W. N., Bitterman P. B., Rennard S. I., Cantin A., Crystal R. G. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis. 1987 Dec;136(6):1429–1434. doi: 10.1164/ajrccm/136.6.1429. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A. Characterization of the somatomedin-C/insulin-like growth factor I (SM-C/IGF-I) receptor on cultured human fibroblast monolayers: regulation of receptor concentrations by SM-C/IGF-I and insulin. J Clin Endocrinol Metab. 1982 Sep;55(3):434–440. doi: 10.1210/jcem-55-3-434. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A., Conover C. A. Density-associated loss of functional receptors for somatomedin-C/insulinlike growth factor I (SM-C/IGF-I) on cultured human fibroblast monolayers. J Cell Physiol. 1984 Nov;121(2):419–424. doi: 10.1002/jcp.1041210221. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rotwein P., Pollock K. M., Didier D. K., Krivi G. G. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986 Apr 15;261(11):4828–4832. [PubMed] [Google Scholar]
- Rotwein P. Two insulin-like growth factor I messenger RNAs are expressed in human liver. Proc Natl Acad Sci U S A. 1986 Jan;83(1):77–81. doi: 10.1073/pnas.83.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. E., Van Wyk J. J., Pledger W. J. Inhibition of the mitogenic effects of plasma by a monoclonal antibody to somatomedin C. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2389–2392. doi: 10.1073/pnas.81.8.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Rees-Jones R. W., Zick Y., Nissley S. P., Rechler M. M. Characterization of insulin-like growth factor I-stimulated tyrosine kinase activity associated with the beta-subunit of type I insulin-like growth factor receptors of rat liver cells. J Biol Chem. 1985 Aug 15;260(17):9793–9804. [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Smith E. P., Svoboda M. E., Van Wyk J. J., Kierszenbaum A. L., Tres L. L. Partial characterization of a somatomedin-like peptide from the medium of cultured rat Sertoli cells. Endocrinology. 1987 Jan;120(1):186–193. doi: 10.1210/endo-120-1-186. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Scott J., Bell G. I., Shows T. B. Localization of insulin-like growth factor genes to human chromosomes 11 and 12. 1984 Aug 30-Sep 5Nature. 310(5980):784–786. doi: 10.1038/310784a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk J. J., Svoboda M. E., Underwood L. E. Evidence from radioligand assays that somatomedin-C and insulin-like growth factor-I are similar to each other and different from other somatomedins. J Clin Endocrinol Metab. 1980 Jan;50(1):206–208. doi: 10.1210/jcem-50-1-206. [DOI] [PubMed] [Google Scholar]
- Vassilopoulou-Sellin R., Phillips L. S. Extraction of somatomedin activity from rat liver. Endocrinology. 1982 Feb;110(2):582–589. doi: 10.1210/endo-110-2-582. [DOI] [PubMed] [Google Scholar]
- Vetter U., Zapf J., Heit W., Helbing G., Heinze E., Froesch E. R., Teller W. M. Human fetal and adult chondrocytes. Effect of insulinlike growth factors I and II, insulin, and growth hormone on clonal growth. J Clin Invest. 1986 Jun;77(6):1903–1908. doi: 10.1172/JCI112518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger B., Kelley D. G., Engleman W., Kuhn C., 3rd, McDonald J. A. Human alveolar macrophage fibronectin: synthesis, secretion, and ultrastructural localization during gelatin-coated latex particle binding. J Cell Biol. 1981 Sep;90(3):711–720. doi: 10.1083/jcb.90.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Saltini C., Sellers S., Tocci M. J., Bayne E. K., Schmidt J. A., Crystal R. G. Evaluation of alveolar macrophages in normals and individuals with active pulmonary sarcoidosis for the spontaneous expression of the interleukin-1 beta gene. Cell Immunol. 1987 Jul;107(2):479–488. doi: 10.1016/0008-8749(87)90255-3. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Kaufmann U., Eigenmann E. J., Froesch E. R. Determination of nonsuppressible insulin-like activity in human serum by a sensitive protein-binding assay. Clin Chem. 1977;23(4):677–682. [PubMed] [Google Scholar]
- Zapf J., Schmid C., Froesch E. R. Biological and immunological properties of insulin-like growth factors (IGF) I and II. Clin Endocrinol Metab. 1984 Mar;13(1):3–30. doi: 10.1016/s0300-595x(84)80006-7. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]