Abstract
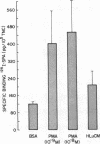
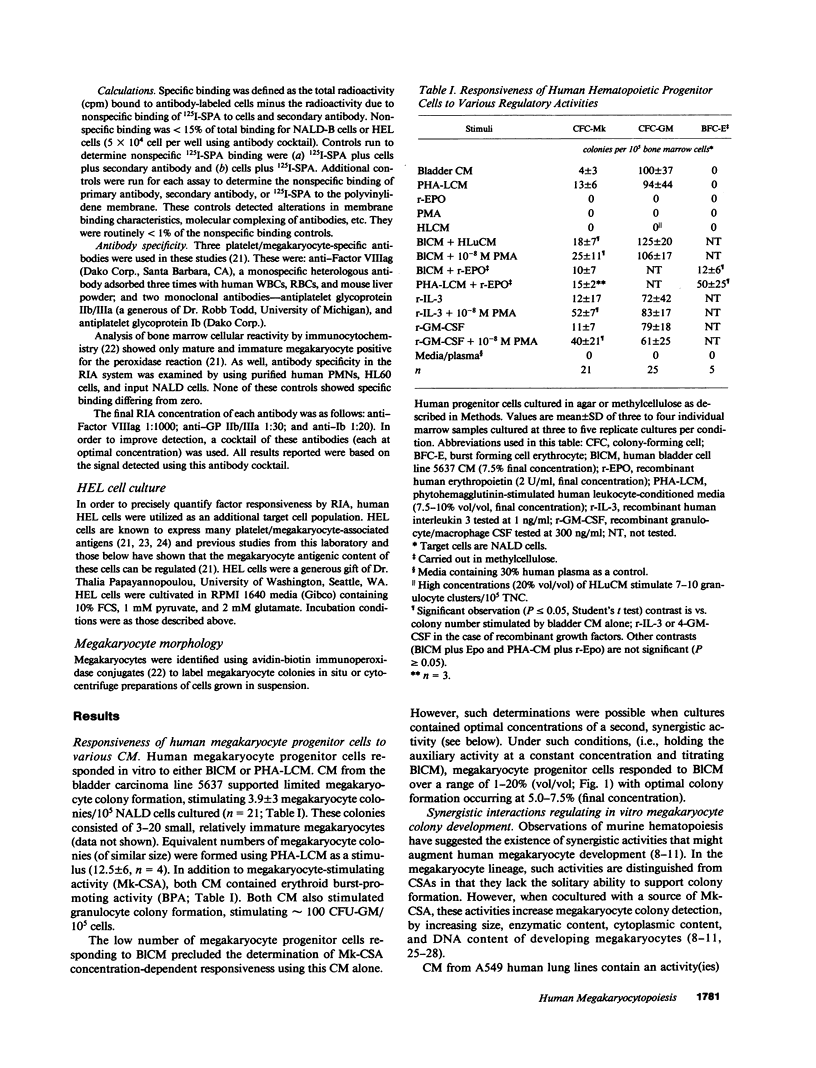
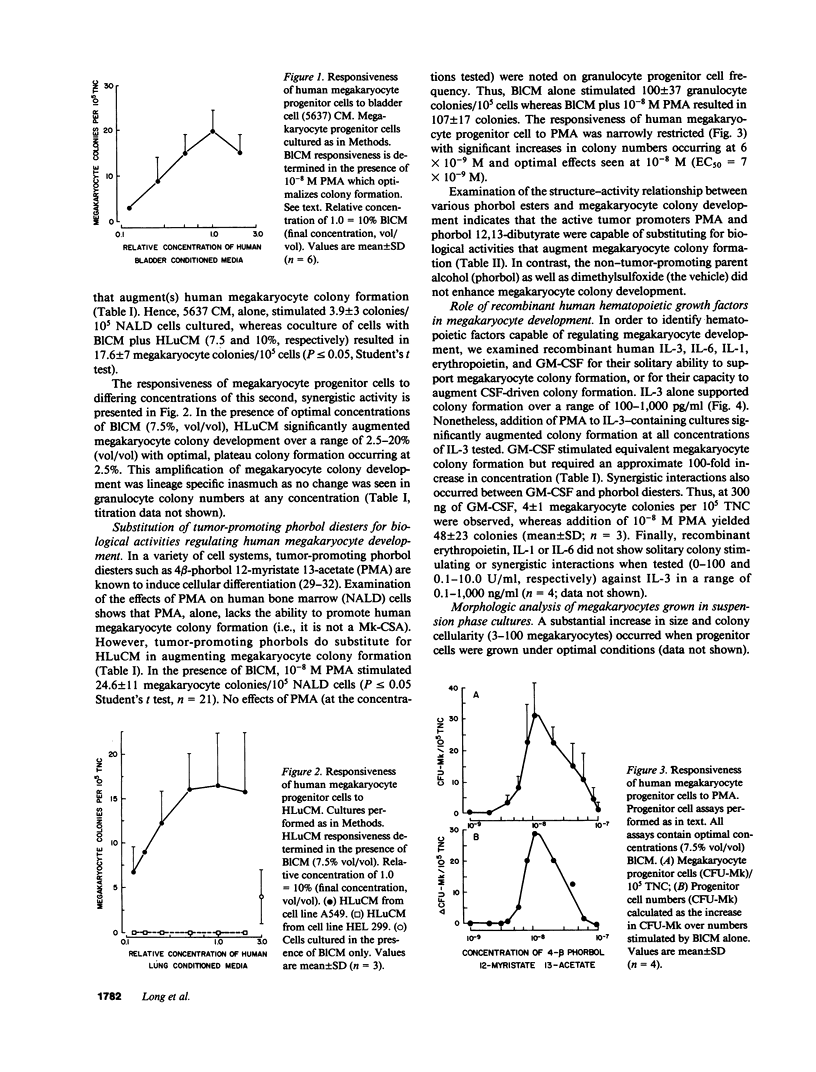
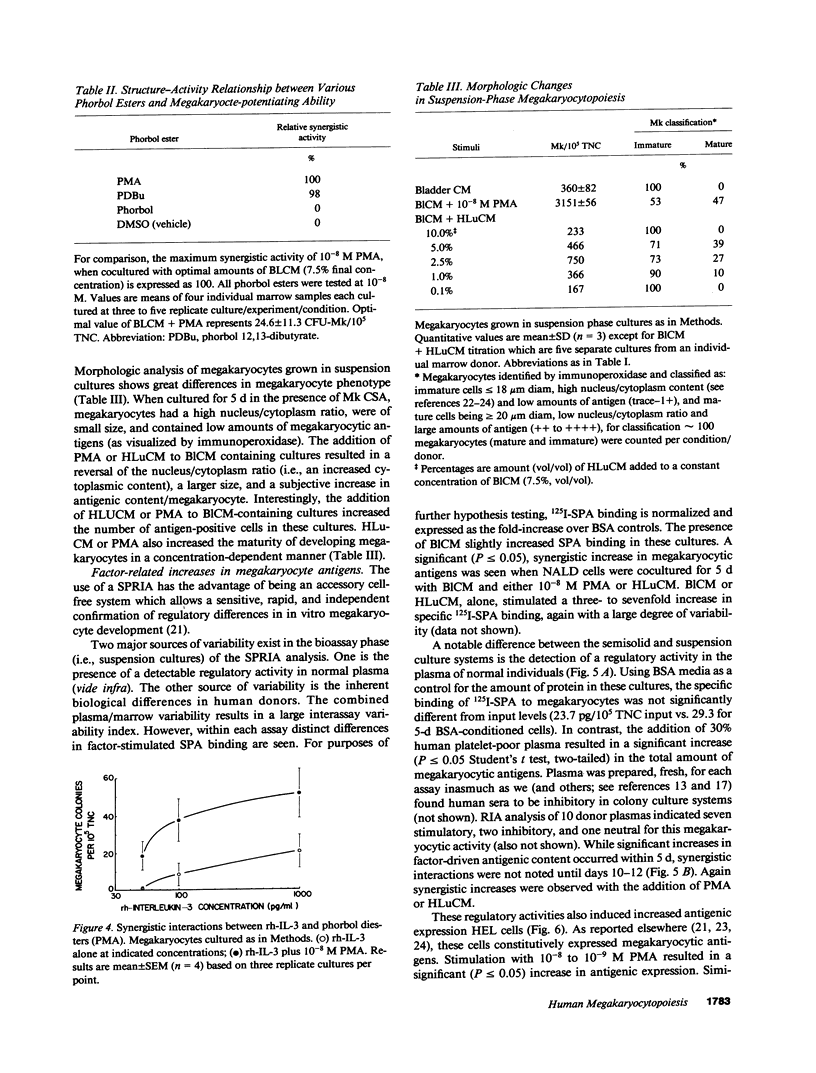
Little information exists concerning differing levels of regulation occurring during human megakaryocyte development. We hypothesize that megakaryocytic proliferation and maturation is controlled by two, synergistic regulatory factors. One, megakaryocyte colony-stimulating activity, is an obligate requirement for colony formation and drives the development of relatively immature cells. Megakaryocyte colony-stimulating activity is a functional component of the human recombinant proteins, interleukin 3 or GM-CSF. Human recombinant growth factors, interleukin 1, interleukin 6, or crythropoietin, do not effect megakaryocyte development either alone or in combination with interleukin 3. Full maturation requires a second synergistic activity which increases megakaryocyte number, size, and cytoplasmic and antigenic content. In culture, this synergistic regulator augments maturation by increasing the number of colonies, colony cellularity, and size. In suspension cultures, this cofactor increases megakaryocyte cytoplasmic and antigenic content, and shifts the morphological distribution from immature to mature megakaryocytes. Finally, this activity also increases the number of antigen positive megakaryocytes, either by stimulating proliferation or conversion of antigen-negative to antigen-positive cells. Comparative studies of megakaryocytic regulation suggests that this in vitro regulator mimicks some of the known effects of thrombopoietin in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979 Dec;54(6):1446–1450. [PubMed] [Google Scholar]
- Ebbe S. Biology of megakaryocytes. Prog Hemost Thromb. 1976;3:211–229. [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Marks P. A., Rifkind R. A. Tumor promoters enhance myeloid and erythroid colony formation by normal mouse hemopoietic cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4152–4155. doi: 10.1073/pnas.77.7.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz A. M., Bruno E., Elwell J., Hoffman R. In vitro studies of megakaryocytopoiesis in thrombocytotic disorders of man. Blood. 1983 Feb;61(2):384–389. [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [PubMed] [Google Scholar]
- Harker L. A., Finch C. A. Thrombokinetics in man. J Clin Invest. 1969 Jun;48(6):963–974. doi: 10.1172/JCI106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Kinetics of thrombopoiesis. J Clin Invest. 1968 Mar;47(3):458–465. doi: 10.1172/JCI105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Regulation of thrombopoiesis. Am J Physiol. 1970 May;218(5):1376–1380. doi: 10.1152/ajplegacy.1970.218.5.1376. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Mazur E., Bruno E., Floyd V. Assay of an activity in the serum of patients with disorders of thrombopoiesis that stimulates formation of megakaryocytic colonies. N Engl J Med. 1981 Sep 3;305(10):533–538. doi: 10.1056/NEJM198109033051001. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Yang H. H., Bruno E., Straneva J. E. Purification and partial characterization of a megakaryocyte colony-stimulating factor from human plasma. J Clin Invest. 1985 Apr;75(4):1174–1182. doi: 10.1172/JCI111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Kimura H., Burstein S. A., Thorning D., Powell J. S., Harker L. A., Fialkow P. J., Adamson J. W. Human megakaryocytic progenitors (CFU-M) assayed in methylcellulose: physical characteristics and requirements for growth. J Cell Physiol. 1984 Jan;118(1):87–96. doi: 10.1002/jcp.1041180115. [DOI] [PubMed] [Google Scholar]
- Levin J., Evatt B. L. Humoral control of thrombopoiesis. Blood Cells. 1979 Mar 23;5(1):105–121. [PubMed] [Google Scholar]
- Long M. W., Gragowski L. L., Heffner C. H., Boxer L. A. Phorbol diesters stimulate the development of an early murine progenitor cell. The burst-forming unit-megakaryocyte. J Clin Invest. 1985 Aug;76(2):431–438. doi: 10.1172/JCI111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Heffner C. H. Detection of human megakaryocyte antigens by solid-phase radioimmunoassay. Exp Hematol. 1988 Jan;16(1):62–70. [PubMed] [Google Scholar]
- Long M. W., Smolen J. E., Szczepanski P., Boxer L. A. Role of phorbol diesters in in vitro murine megakaryocyte colony formation. J Clin Invest. 1984 Nov;74(5):1686–1692. doi: 10.1172/JCI111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Williams N. Differences in the regulation of megakaryocytopoiesis in the murine bone marrow and spleen. Leuk Res. 1982;6(5):721–728. doi: 10.1016/0145-2126(82)90089-3. [DOI] [PubMed] [Google Scholar]
- Long M. W., Williams N., Ebbe S. Immature megakaryocytes in the mouse: physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood. 1982 Mar;59(3):569–575. [PubMed] [Google Scholar]
- Long M. W., Williams N. Immature Megakaryocytes in the Mouse: Morphology and quantitation by acetylcholinesterase staining. Blood. 1981 Nov;58(5):1032–1039. [PubMed] [Google Scholar]
- Long M. W., Williams N., McDonald T. P. Immature megakaryocytes in the mouse: in vitro relationship to megakaryocyte progenitor cells and mature megakaryocytes. J Cell Physiol. 1982 Sep;112(3):339–344. doi: 10.1002/jcp.1041120305. [DOI] [PubMed] [Google Scholar]
- Mazur E. M., Hoffman R., Bruno E. Regulation of human megakaryocytopoiesis. An in vito analysis. J Clin Invest. 1981 Sep;68(3):733–741. doi: 10.1172/JCI110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E. M., de Alarcón P., South K., Miceli L. Human serum megakaryocyte colony-stimulating activity increases in response to intensive cytotoxic chemotherapy. Exp Hematol. 1984 Sep;12(8):624–628. [PubMed] [Google Scholar]
- Messner H. A., Jamal N., Izaguirre C. The growth of large megakaryocyte colonies from human bone marrow. J Cell Physiol Suppl. 1982;1:45–51. doi: 10.1002/jcp.1041130410. [DOI] [PubMed] [Google Scholar]
- Niederhuber J. E., Allen P. Role of I-region gene products in macrophage induction of an antibody response. II. Restriction at the level of T cell in recognition of I-J-subregion macrophage determinants. J Exp Med. 1980 May 1;151(5):1103–1113. doi: 10.1084/jem.151.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W., Friday T. J., Charsha D. E. Effects of thrombocytopenia on megakaryocytopoiesis. Br J Haematol. 1969 Jul;17(1):91–101. doi: 10.1111/j.1365-2141.1969.tb05667.x. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W., Friday T. J., Charsha D. E. Effects of thrombocytopenia on megakaryocytopoiesis. Br J Haematol. 1969 Jul;17(1):91–101. doi: 10.1111/j.1365-2141.1969.tb05667.x. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Murphy J. R., Jackson C. W. Stimulation of megakaryocytopoiesis by acute thrombocytopenia in rats. Blood. 1976 Nov;48(5):765–775. [PubMed] [Google Scholar]
- Odell T. T., Shelton C. Increasing stimulation of megakaryocytopoiesis with decreasing platelet count. Proc Soc Exp Biol Med. 1979 Sep;161(4):531–533. doi: 10.3181/00379727-161-40590. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Ihle J. N., McGrath E. The effect of interleukin 3 and GM-CSA-2 on megakaryocyte and myeloid clonal colony formation. Blood. 1985 Jan;65(1):214–217. [PubMed] [Google Scholar]
- Rabellino E. M., Levene R. B., Leung L. L., Nachman R. L. Human megakaryocytes. II. Expression of platelet proteins in early marrow megakaryocytes. J Exp Med. 1981 Jul 1;154(1):88–100. doi: 10.1084/jem.154.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg L. A., Jr, Jamal N., Messner H. A. Characterization of human megakaryocytic colony formation in human plasma. J Cell Physiol. 1985 Jul;124(1):67–74. doi: 10.1002/jcp.1041240112. [DOI] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H. Kinetic analysis of megakaryocyte numbers and ploidy levels in developing colonies from mouse bone marrow cells. Cell Tissue Kinet. 1982 Sep;15(5):483–494. doi: 10.1111/j.1365-2184.1982.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Ralph P., Nakoinz I. Cell interactions influencing murine marrow megakaryocytes: nature of the potentiator cell in bone marrow. Blood. 1981 Jan;57(1):157–163. [PubMed] [Google Scholar]