Abstract
Patient-derived xenografts (PDXs) of tumors are increasingly becoming important tools for translational research in oncology. The NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic (NOG) mouse is an efficient host for PDXs. Thus as a basis for future development of methods to obtain PDXs from various disease types, we have studied the factors that affect the outcome of transplantation of human colorectal cancer in NOG mice. Of the original donor cases examined, 73% had successful engraftment. The outcome of donor-matched tissues was consistent in most cases, and was thought to show that the condition of the host did not affect engraftment. Next we analyzed the tumor aggressiveness in terms of histology grade of the original tumor and found that they were related to engraftment. Detailed histopathological examination of the transplanted tissues strongly indicated that lymphocytes engrafted with the tumor cells affect engraftment. As a factor related to transplantation of lymphocytes, we studied the human IgG concentration in the serum of tumor-bearing mice, but there was no tendency for higher concentrations to result in unsuccessful engraftment. Finally, we studied the type, density and location of T cells in the original donor tissue to determine the immune contexture and found that the unsuccessful engraftment cases tended to have an adequate or coordinated immune contexture compared to successful engraftment cases. From these results, we concluded that the aggressiveness and the T cell infiltration of the original tumor affect the outcome of transplantation in the NOG mouse.
Keywords: immunoglobulin, NOG mouse, patient-derived xenograft, T lymphocyte, tumor aggressiveness
Introduction
The difficulty of translating preclinical findings to clinical cancer has created a growing demand for preclinical models that are relevant to human cancer [25]. Patient-derived xenografts (PDXs) of human tumors which are prepared by direct transplantation of surgically excised tumors from cancer patients recapitulate many of the characteristics of the original tumor such as morphology and genetics [25] and have provided some promising results that show their correlation with clinical cancer such as tumor growth and prognosis, or prediction of drug efficacy. Therefore they have come to be seen as important tools for translational research [3, 25].
The NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic (NOG) mouse, a type of NOD-scid, Il-2rgnull mouse, is an efficient host for PDXs, including human tumor cells [12, 17, 21, 25]. In the case of tumors, our research group has found that colon cancer stem cells can be maintained and concentrated in tissue lines established by in vivo passage of human tumor tissues in the NOG mouse [15]. This trait is thought to contribute to the stable maintenance of the characteristic hierarchical structure of the original tumor across several generations. The tumor tissue lines can be reproduced and expanded on demand and we believe they will be important for the progress of oncology.
To establish PDXs of human tumors that can be maintained as tissue lines, we have transplanted several types of solid human tumor tissues into the NOG mouse. Although these efforts have succeeded in establishing tissue lines that reflect the morphology of their original patient tumors [9], we have also found that the rate of establishment is not as high as we initially expected [9]. To enhance the efficiency of establishing solid tumor tissue lines, we studied the factors that affect engraftment of tumor tissues and elucidated that one of the hindering factors is an Epstein-Barr virus-related, lymphoproliferative lesion (LPL) which completely eliminated and replaced the original tumor and was responsible for unsuccessful engraftment of approximately 30% of the total number of transplanted cases [7]. However, even when these cases were taken into account, there were still cases that could not be engrafted.
As a basis for future development of methods to obtain PDXs from various disease types and subtypes, we believe it is necessary to investigate the factors that may affect engraftment. To this end, we have analyzed the characteristics of tumors, and also the lymphocytes that are engrafted with the tumor tissues in this study. The aggressiveness of the tumor in terms of the differentiation of tumor cells was found related to engraftment. We have also found that immune cells that are transplanted with the tumor can affect the outcome of transplantation.
Materials and Methods
Extraction of samples from the PLR archive
For analysis, we have selected colorectal cancer (CRC) because tissues from a sufficient number of cases for comparison between successful and unsuccessful engraftment were available in the archive at PharmaLogicals Research, Pte., Ltd. (PLR, Singapore). The original donor tissue (tissue before transplant, obtained by surgery), corresponding xenograft tissue (tissue transplanted into NOG mice) and mouse (host) serum were selected from the archive at PLR and examined retrospectively (Fig. 1a). Tissues with LPL or from animals that were sick or developed bacterial infection were omitted from the study. The total number of tissues selected to assess engraftability was 48 patient (donor) tissues and transplanted tissues of 74 mice (host) (Supplementary Table 1).
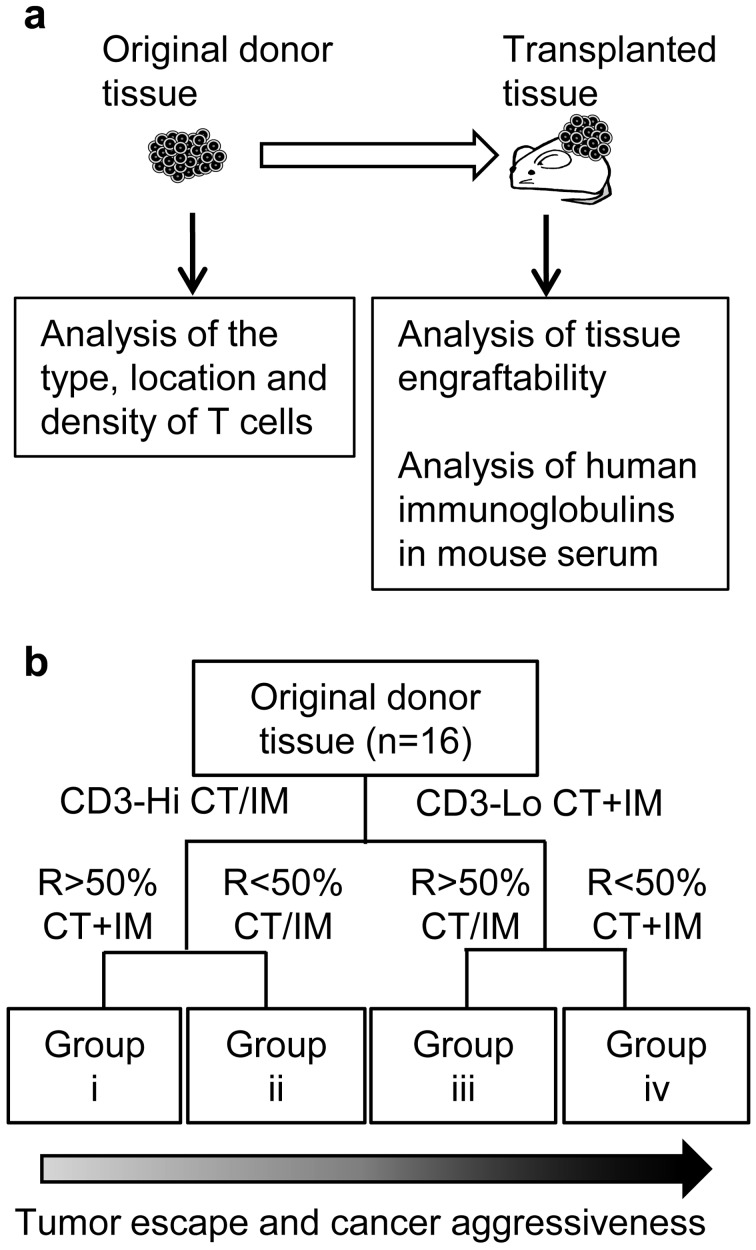
Fig. 1.
Study scheme of archived samples (a). Original donor tissues (tissues before transplant) and xenograft tissues (tissues after transplant) were selected from the tissue archive at PLR and were analyzed in this study. Decision tree for analysis of T cells in original donor tissues (b). The cases were grouped into Groups i to iv according to the analysis of T cells [2]. CT, center of tumor; IM, invasive margin; CT+IM, both CT and IM; CT/IM, CT and/or IM; CD3-Hi, median CD3-positive cell density or higher; CD3-Lo, lower CD3-positive cell density than median. R, ratio of CD8-positive to CD3-positive cell density.
The tissues were fixed in 4% paraformaldehyde and embedded in paraffin by the AMeX method as previously described [22, 24]. The mouse sera were collected by orbital puncture for time course examination or from the abdominal artery at the time of necropsy.
The surgically excised tissues were provided by the patients that gave their informed consent as approved by the ethical committee at PLR and Parkway Laboratory Services in Singapore.
Transplantation of human tumor tissues into NOG mice
The NOG mice used to produce the xenograft tissues were acquired from the breeding facility of the Central Institute for Experimental Animals (Kanagawa, Japan), at the age of 5–6 weeks. After an adaptation period, animals between 6 and 12 weeks of age were submitted to surgical transplantation. The original donor tissues that were judged to contain viable tumor tissue by a pathologist were received as fresh tissues at PLR and transplantation into NOG mice was performed as described previously [9]. Briefly, the tissues were placed immediately after surgical excision in Hanks balanced salt solution containing 5% penicillin, streptomycin, and a neomycin antibiotics mixture. Each tissue was cut into pieces (~5 mm3) using sterilized surgical scissors and transplanted into the flank at a total volume of approximately 200 mm3 with a transplant needle via a small incision in the leg. The tissues were transplanted and observed until the tumor mass was approximately 1cm3 in size, or until the case was judged to have no tumor growth. All transplantations were terminated within 13 months after transplant. At the time of necropsy, the mice were euthanized by exsanguination from the abdominal artery under deep ether anaesthesia and the transplanted tissues were collected.
All animals were housed in plastic cages within a bioBubble system (bioBubble®, CO, USA) in a pathogen-free state. Mice were fed commercial pelleted diet (CE-2; Clea Japan Inc., Tokyo, Japan) and distilled water ad libitum.
All studies and procedures involving animal subjects were approved by the Animal Care and Use Committee at PLR. The animals used in this experiment were treated in accordance with the Animal Research Guideline of PLR.
Analysis of tissue engraftability by histopathological examination
HE-stained slides were prepared of the tissues from the 74 mice selected from the archive and examined histopathologically. The engraftability of the tissues was judged as successful engraftment if viable tumor cells forming ductal or solid tumor nests that are characteristic of CRC were present, and unsuccessful engraftment if there were no tumor cells, or if only a very small number of tumor cells forming incomplete structures were identified.
To determine whether there were any conditions of the host that affected engraftment, tissues from the same donor (donor-matched tissues) that had been transplanted into 2 to 4 hosts were compared. For this analysis, 46 transplanted tissues from 20 donors were examined (Table 1). In donor-matched cases, the original cases were considered to have successful engraftment if 1 or more hosts had successful engraftment.
Table 1. The fate of donor-matched tissues tranplanted into NOG mice.
| Fate | Donor ID | No. of hosts | |
|---|---|---|---|
| Successful | Unsuccessful | ||
| All positive | d1 | 3 | 0 |
| d2 | 2 | 0 | |
| d4 | 2 | 0 | |
| d5 | 2 | 0 | |
| d6 | 2 | 0 | |
| d7 | 2 | 0 | |
| d8 | 2 | 0 | |
| d9 | 2 | 0 | |
| d11 | 2 | 0 | |
| d12 | 2 | 0 | |
| d13 | 4 | 0 | |
| d14 | 4 | 0 | |
| d16 | 2 | 0 | |
| d17 | 3 | 0 | |
| Both outcomes | d3 | 1 | 1 |
| All negative | d10 | 0 | 2 |
| d15 | 0 | 2 | |
| d18 | 0 | 2 | |
| d19 | 0 | 2 | |
| d20 | 0 | 2 | |
The numbers in the table show the number of hosts (mice). Successful, successful engraftment; Unsuccessful, Unsuccessful engraftment.
Next, to examine if the aggressiveness of the original tumor affected engraftment we compared the histopathological grade and disease stage of the original donor cases with the outcome of transplantation. Information concerning the histology grade and disease stage was provided by certified pathologists that carried out the histopathological diagnosis of the original patient tissues. The histology grades were: Grade 1, well differentiated; Grade 2, moderately differentiated; Grade 3, poorly differentiated [4]. The disease staging was based on the TNM staging from Stage I to IV [4].
Finally, we also examined the engrafted tissues in detail to determine the state of the tumor cells such as viability or degeneration and necrosis, the presence of lymphoid cell infiltration, and also changes in stromal components such as fibrosis.
Analysis of human immunoglobulins in NOG mouse serum
Time course changes in the mouse serum immunoglobulins were examined in mice transplanted with human CRC. For this analysis, the serum from a total of 16 mice transplanted with tissue from 9 donors were examined. Human IgG and IgM concentrations in serum collected weekly from Week 1 to 6 after transplantation were measured. To compare the concentrations with the outcome of engraftment, sera from 18 mice transplanted with tissue from 11 donors that had successful engraftment and 12 mice transplanted with tissues from 8 donors that had unsuccessful engraftment were analyzed. The concentrations at Week 3 and 6 were compared.
The concentration of human immunoglobulins in serum of the tumor-bearing NOG mice was determined using a sandwich ELISA method. Briefly, 96-well plates were coated with 100 µL of 0.25 mg/ml anti-human IgG (Polyclonal rabbit anti-human IgG, Dako Cytomation, Glostrup, Denmark) or IgM (Polyclonal rabbit anti-human IgM, Dako Cytomation) overnight at 4°C. Next, 100 µl of the sample serum or the standard solutions were added to the wells and incubated at 37°C for 45 min. Two single-point dilutions were set for the sample serum. 100 µl of anti-human IgG (or IgM)-conjugated with horseradish peroxidase was added and incubated at 37°C for 45 min. The OD was read at 492 nm using a Tecan Sunrise RC Automated Microplate Reader with Magellan Data Reduction Software (Endotoxin Testing Solutions LLC., SC, USA). The concentrations were calculated based on the standard curve.
Analysis of the type, location and density of T cells in tissues before transplantation by immunohistochemical examination
The location, density and functional orientation of the different immune cell populations, or the “immune contexture” in human tumor tissue are related to tumor escape and cancer aggressiveness [2, 5, 10]. Thus we examined the immune contexture by analyzing the type, location and density of T cells based on a method described by Camus et al [2]. HE slides were prepared from all paraffin blocks that had been archived for the 48 original donor cases and examined to determine whether the areas necessary for analysis (invasive margin and center of tumor mass) were included in the tissues. A total of 12 donor cases with successful engraftment and 4 donor cases with unsuccessful engraftment were selected and examined. In addition to confirm that T cells could be engrafted into the NOG mouse, a representative case was examined immunohistochemically for CD3 and 8 in the original donor tissue (before transplant) and in the transplanted tissue.
The density of CD3-positive cells and the ratio of CD8 to CD3-positive cells were determined based on the theory described by Camus et al. (Fig. 1b) [2]. Briefly, cell counts were performed for CD3- and CD8-positive cells at the center of the tumor (CT) and the invasive margin (IM). The cell density was determined by counting the cells in 3 areas representative of the IM and 2 areas for the CT. Each area (183,000 µm2) was designated using a virtual slide software (Image Scope, Aperio Technologies, Inc., CA, USA).
Based on the results, the cases were grouped into 4 groups. Group i was considered to have adequate reaction of the immune cells to suppress tumor cells, Group iv to have inadequate reaction of the immune cells to tumor cells that would allow tumor escape. Group ii and iii were thought to have immune reactions that could be positioned between the two extremes (Fig. 1b) [2].
Immunohistochemical staining for human CD3 and CD8 was performed using the following method. A monoclonal mouse anti-human CD3 antibody (Clone F7.2.38, Dako Cytomation, Glostrup, Denmark) and a monoclonal mouse anti-human CD8 antibody (Clone C8/144B, Dako Cytomation) were applied as the primary antibodies. The tissues were stained by an indirect immunoperoxidase method using the Ventana HX Discovery System (Ventana Medical Systems, AZ, USA). Briefly, the slides were de-waxed and heated for antigen retrieval followed by treatment with protein block (Dako Cytomation) to reduce non-specific staining and 0.3% H2O2 in methanol to block endogenous peroxidase. After incubation with the primary and the universal secondary antibody (Ventana Medical Systems), streptavidin conjugated to horseradish peroxidase (Ventana Medical Systems) was applied and the reaction visualized with a diaminobenzidine solution (Ventana Medical Systems). The slides were counterstained with hematoxylin and coverslipped.
Statistical analysis
The data for immunoglobulin concentrations were analyzed for statistical significance of any differences between the successful group and unsuccessful group at the same timepoint using the Wilcoxon rank sum test at the level of 5%.
Results
Engraftability of CRC tissues in NOG mice
Of the 48 original donor cases that were transplanted, 35 cases were judged by histopathological examination to have successful tumor cell engraftment (73%). We found that for 19 of the 20 donor cases examined, the outcome of the engraftment was the same for all donor-matched tissues (Table 1).
Next we investigated whether the clinical aggressiveness of the tumor had any correlation to engraftment. For grade 3 cases, engraftment was successful in 9/10 (90%) original donor cases, and the ratio of successful cases was considerably lower in grade 2 cases (25/36 cases, 69%) and grade 1 cases (1/2 cases, 50%) (Table 2). There tended to be more successful than unsuccessful engraftment cases with tissues from donors with higher disease stage as well (Table 2).
Table 2. Comparison of tumor grade of the original donor tissue with engraftability.
| Tumor grade | |||
|---|---|---|---|
| G1 | G2 | G3 | |
| Successful | 1 | 25 | 9 |
| Unsuccessful | 1 | 11 | 1 |
The numbers in the table show the number of original donor cases. Successful, successful engraftment; Unsuccessful, unsuccessful engraftment.
Histopathological characteristics of the transplanted tissues
To explore the possibility that any other aspects of the biology of the tumor tissue itself could affect engraftment, we examined the histopathological characteristics of the engrafted tissues.
Of the tissues from 74 hosts, 55 tissues had successful engraftment. Infiltration of lymphocytes was observed in 30/55 successfully engrafted tissues. The infiltration of lymphocytes in 26 of these tissues was diffusely observed in the stroma, with no evidence of degenerative changes in tumor cells. In the other 4 tissues, areas of viable tumor cell growth were observed alongside areas with infiltration of lymphocytes, degeneration and necrosis of tumor cells, and fibrosis (Figs. 2a and b).
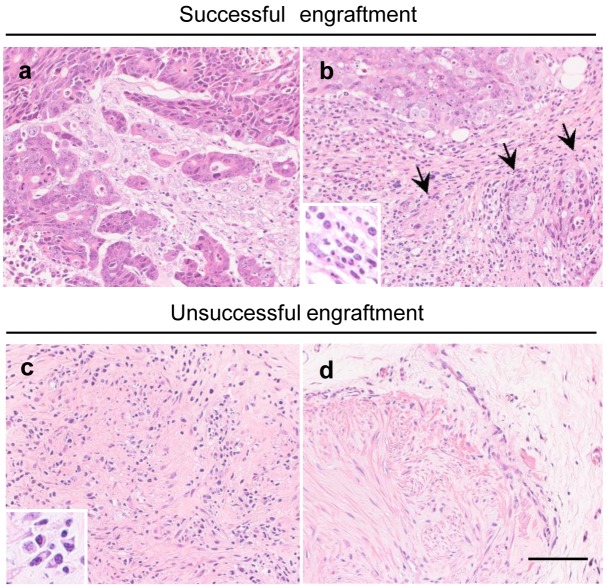
Fig. 2.
Representative images of transplanted tumor cells and lymphocytes, and host tissue reaction (fibrosis) in the transplanted tissues. Two images from the same slide of a successful-engraftment tissue (h13c) with an area of viable tumor growth and scattered infiltration of lymphocytes in the stroma (a), and an area with infiltration of lymphocytes, degeneration and necrosis of tumor cells (arrows), and fibrosis (b) are shown. Images from tissues of unsuccessful-engraftment cases with no tumor cells with (c) or without (d) lymphocyte infiltration are also shown (c, h10b, d, h20b). The inserts show a high magnification of the infiltrating lymphocytes, and plasma cells. Bar=40 µm
Next, the tissues from 19/74 hosts with unsuccessful engraftment were examined. In 6 tissues, there were residual tumor cells surrounded by infiltration of lymphocytes, with degeneration and necrosis of the tumor cells. In the 10 tissues with no remaining tumor cells, fibrosis was observed in the site of transplantation, and in 6 of these tissues, infiltration of lymphocytes was seen within or surrounding the fibrotic areas (Figs. 2c and d). With 3 cases there was no clear fibrosis, with no infiltration of lymphocytes, and only residual atrophic tumor cells.
Thus the main findings were the infiltration of lymphocytes in the site of transplantation associated with the degeneration and necrosis of tumor cells. Since the NOG mouse lacks T and B lymphocytes, we judged that the lymphocytes originated from the donor tissue.
Analysis of human immunoglobulins in NOG mouse serum
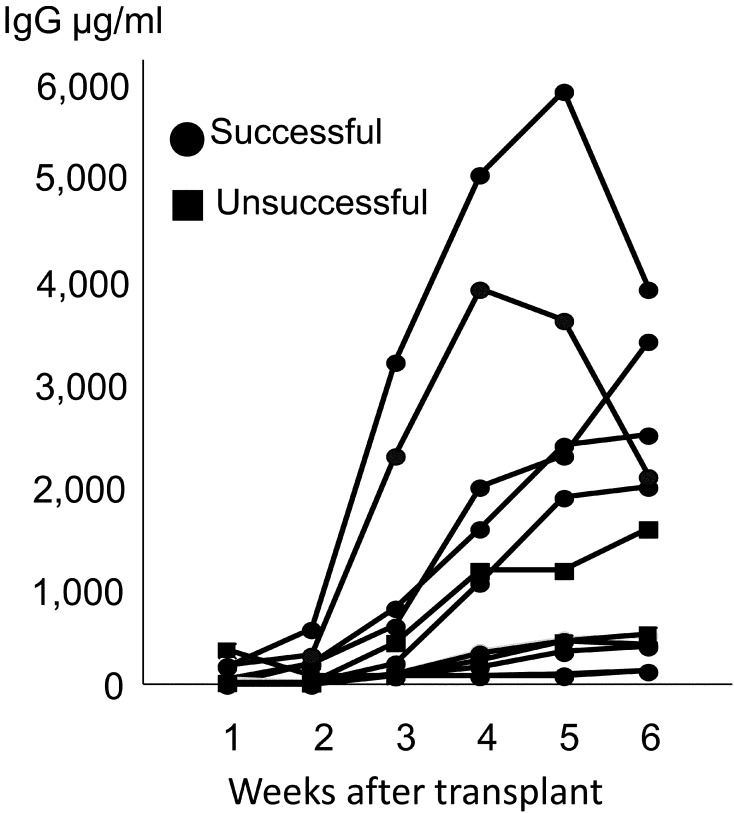
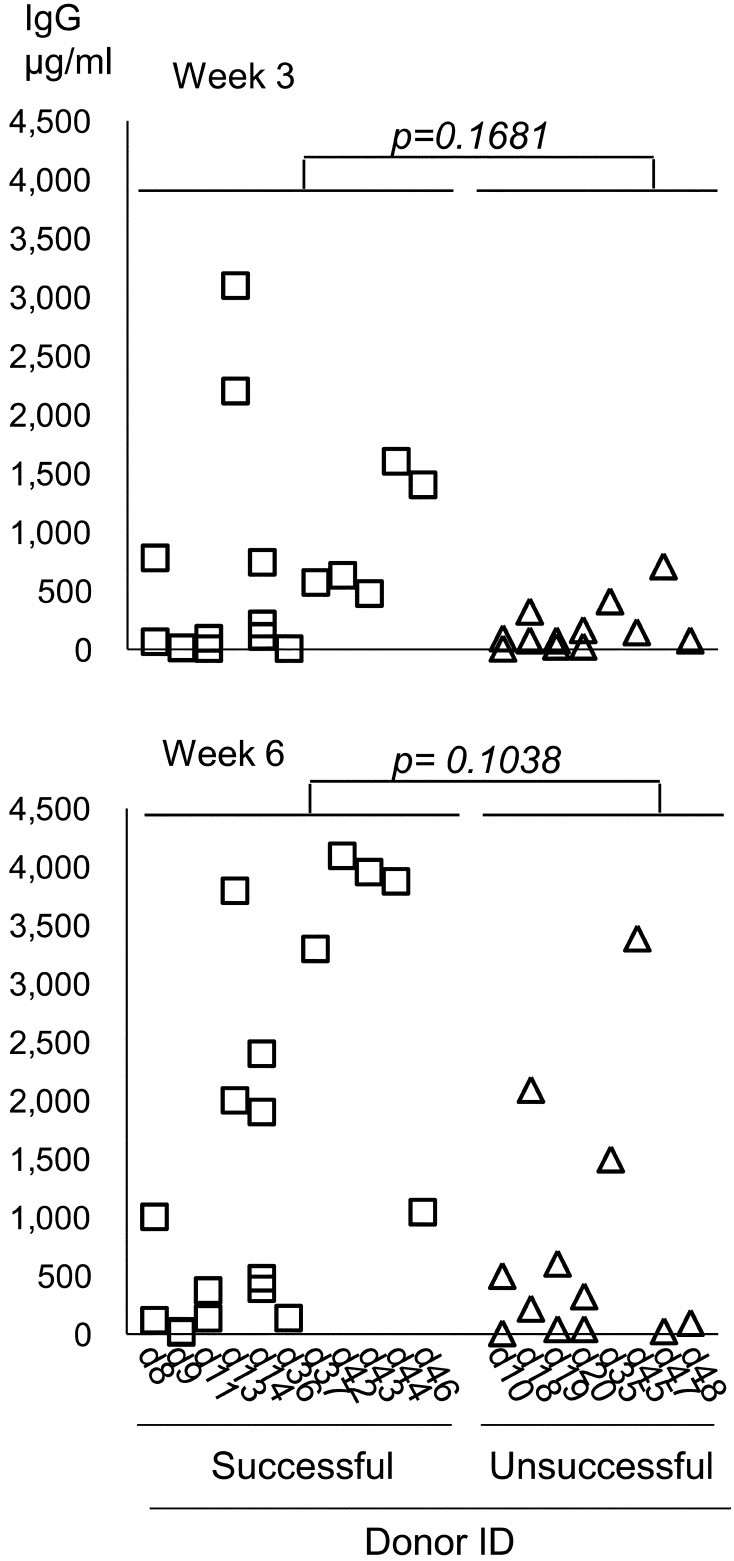
First we examined the time course changes of serum immunoglobulins. A steady rise in serum IgG levels that exceeded 100 µg/ml in at least 1 time point was observed in 13/16 cases. The time course observations of these cases are shown in Fig. 3. The concentrations were low at 1–2 weeks, but were found to steadily rise up to or peak at 4–6 weeks. To determine the relationship of the serum IgG levels with engraftment, we compared the serum IgG concentration between successful and unsuccessful cases at 2 time points (Fig. 4). For successful engraftment cases, the level was up to 3,100 µg/ml at 3 weeks and up to 4,100 µg/ml at 6 weeks. For unsuccessful cases, the levels were up to 709 µg/ml at 3 weeks and up to 3,390 µg/ml at 6 weeks. There was no statistically significant difference between successful and unsuccessful cases at either timepoint for IgG levels. Serum IgM concentrations tended to be much lower, but were similar to IgG in terms of a steady increase over several weeks in most cases and unsuccessful cases had significantly lower levels compared to successful cases (Supplementary Figs. 1 and 2). The present findings show that the concentrations for the unsuccessful cases did not exceed those of the successful cases.
Fig. 3.
Time course observation of IgG concentration in the host serum. Each line represents 1 host.
Fig. 4.
IgG concentration in the host serum compared between unsuccessful-engraftment and successful-engraftment cases at Week 3 and 6 after transplantation of human tumor tissues. Wilcoxon rank sum test. Each column represents one donor, and each symbol one host.
Analysis of the type location and density of T cells in tissues before transplantation by immunohistochemical examination
Both CD3- and CD8-positive cells were confirmed in the tissues before and after transplantation (Fig. 5). As for the analysis in the original donor tissues, grouping was carried out according to the criteria described in Fig. 1b. Cases of unsuccessful engraftment were seen only in Groups i and ii, and for Group i most cases were unsuccesful (Table 3). On the other hand, cases of successful engraftment were seen in all groups (Table 3). Most cases in Group ii and all cases in Groups iii and iv were found to have successful engraftment (Table 3).
Fig. 5.
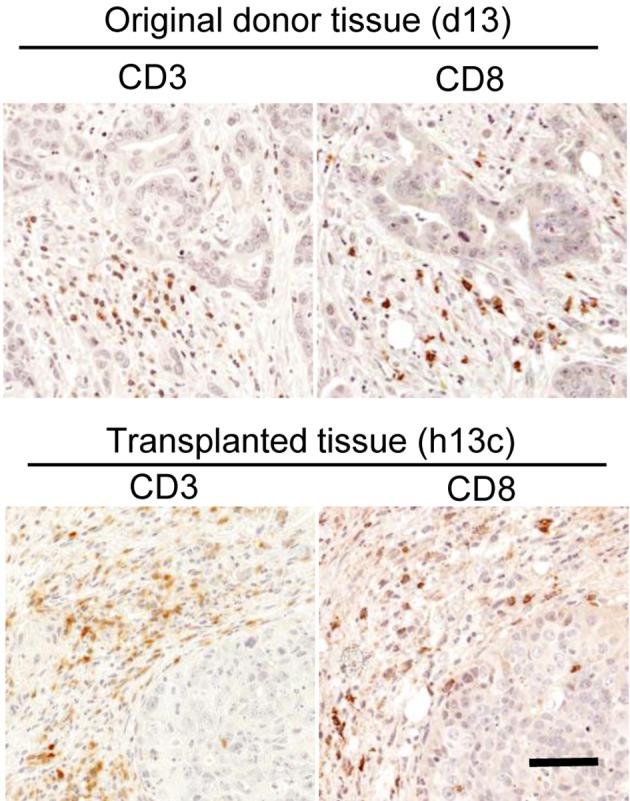
Existence of T cells (CD3 and 8-positive cells) in original donor tissue (d13, upper row) and transplanted tissue (h13c, bottom row). CD3- and CD8-positve lymphocytes were observed both in original donor tissues and transplanted tissues. LSAB method, bar=55 µm.
Table 3. Comparison of the immune contexture with the immune contexture of original donor tissues.
| Immune contexture | Outcome of xenograft | |
|---|---|---|
| Successful | Unsuccessful | |
| Group i | 1 | 3 |
| Group ii | 6 | 1 |
| Group iii | 4 | 0 |
| Group iv | 1 | 0 |
The numbers in the table show the number of original donor cases. Successful, successful engraftment; Unsuccessful, unsuccessful engraftment.
Discussion
One of the most unique and essential points in utilizing PDXs is that they have the potential to recapitulate the heterogeneity of human tumors among individuals [25]. Considering this fact, a low or biased take rate can be problematic for maintaining the heterogeneity of a cancer type in PDX panels [3, 20]. We have recently found that although the NOG mouse is an ideal host for human tissues in many respects, the rate of engraftment was not as high as we expected [9]. Thus we considered it important to study the factors that affect the engraftment of human tumor tissues as a basis for improving the efficiency of engraftment in the NOG mouse.
First, we considered the possibility that the condition of the host may affect engraftment. The tissues from the same donor either consistently engrafted into multiple hosts, or were consistently rejected in multiple hosts. The consistency between hosts agrees with our previous findings in a study of transplanted human non-tumor (thyroid) tissue [8]. Thus the conditions of the host were thought to be stable, and were considered to have no influence on the difference of engraftability in the NOG mouse.
Because of the above evidence we decided to explore the possibility that the characteristics of the tumor may affect engraftment. It is reported that the clinical aggressiveness of tumors, which is shown by parameters such as tumor grade or disease stage is related to engraftability [13, 14]. Our current results agree with these findings, so we judged that the characteristics of the original tumor could indeed affect the outcome of transplantation in NOG mice. However, there was still a difference in outcome among cases with the same tumor grade or disease stage, so we considered that there were other characteristics of the tumor that could affect engraftment.
In order to find these additional factors, we carried out a detailed examination of the transplanted tissues. Histopathologically, we found that there was infiltration of lymphocytes in many of the examined tissues. In positive engraftment tissues, the infiltration of lymphocytes was observed alongside viable tumor cells, and in some cases there were also areas of degeneration and necrosis of tumor cells along with the infiltration of lymphocytes and fibrosis. With unsuccessful engraftment cases, infiltration of lymphocytes and degeneration and necrosis of tumor cells was observed with only residual tumor cells or with no tumor cells at all. These findings were thought to show that the presence of lymphocytes had a role in the process of eliminating tumor cells, and in those cases where the tumor cells survived this process there was successful engraftment, whereas in cases when the tumor cells could not survive there was unsuccessful engraftment. Engraftment of tumor-infiltrating lymphocytes into immunodeficient mice is a well-known phenomenon [23, 26]. Williams et al. suggested that the suppressive effects of lymphocytes on tumor growth may explain the reason for the failure of some transplanted human tumor tissues to grow in scid mice [26]. The aforementioned findings and reports strongly indicate that tumor-infiltrating lymphocytes were transplanted along with the tumor cells and inhibited engraftment by exerting suppressive effects on tumor growth.
There are reports showing that human immunoglobulins can be detected in the serum of immunodeficient mice transplanted with lung cancer [19, 23, 26]. The host serum immunoglobulins are related to suppression of tumor growth in lung cancer although there are some descripancies concerning histological subtype [19, 26]. First we analyzed the serum immunoglobulin concentrations in the present study. We observed a progressive increase in serum human immunoglobulin in most of the NOG mice transplanted with human CRC tissues but we found that there was no tendency for higher concentrations in unsuccessful-engraftment cases. As the serum immunoglobulin concentrations did not correlate well with the outcome of transplantation in the present study, we turned to other factors that involve immune reactions.
Recently a novel theory proposes the involvement of immune cells in the suppression of tumor progression [6, 11]. The location, density and functional orientation of the different immune cell populations, or the “immune contexture” are related to controlling tumor escape and cancer aggressiveness [2, 5, 10]. From this fact we hypothesized that there is a relationship of the immune contexture with the outcome of transplantation. In the current study we examined the immune contexture by analyzing the type, location and density of T cells based on a method described by Camus et al [2]. We have found that unsuccessful-engraftment cases tended to be from donor tissues in Group i or ii. This indicates that there was an adequate or coordinated immune reaction that is tumor suppressive [2, 6]. On the other hand, cases from donor tissues with successful engraftment tended to be from donor tissues in Group iii or iv, which indicates an inadequate or uncoordinated immune reaction that would allow tumor escape [2, 6]. The results were thought to show that the immune contexture in the tissue before transplantation is related to the outcome of engraftment in NOG mice.
In the present study, we have found that the aggressiveness of the tumor and the immune contexture of the lymphocytes that are implanted with the tumor cells are related to engraftment. Further studies to elucidate the characteristics of tumor tissues that are difficult to engraft into NOG mice may serve to find ways to improve engraftment and enhance the efficiency of producing PDX models. We have recently found that tissues from cases of adenomatous goiter, a non-neoplastic disease of the thyroid that is not an aggressive growth, could be engrafted at a rate of approximately 85%, a rate that is higher than that of cancer tissues in the present study [8]. There was no marked infiltration of lymphocytes in these tissues. Judging from this evidence, it may be possible to engraft less aggressive tumors by controlling the infiltration of lymphocytes in the tissue. For example, sorting out the lymphocytes and transplanting only tumor cells may be a solution. Other options may include treating tumor-bearing hosts with immune suppressive compounds such as corticosteroids, calcineurin inhibitors or cyclophosphamide or depleting donor lymphocytes by administering cell surface antigen-specific antibodies in vivo or ex vivo [1, 16]. One potential factor related to engraftability is the reformation of a vascular network in the transplanted tissue [18]. Masuda et al. have shown that human-mouse chimeric blood vessels are formed within transplanted endometrial tissue, and suggested that the chimerism is one of the factors that enables long-term maintenance of the implanted tissues [18]. In our previous experience with transplantation of adenomatous goiter tissue, we have found that some of the blood vessels formed in the transplanted tissue are human which was thought suggestive of chimersim and may have contributed to the long-term maintenance of the tissues [8]. As for the relationship of angiogenesis with the immune response in CRC, Camus et al. have shown that the angiogenic factor VEGF in combination with genes representing a Th1 immune response is a prognostic factor in human CRC [2]. There was no predictive value of VEGF alone, which was thought to show that angiogenesis added on to adaptive immune responses are related to recurrence [2]. From the above evidence, we believe that there may be a role in engraftability for angiogenesis that is linked with the immune contexture, and this would be an important topic to address in future studies. Thus studies to investigate these possibilities would be an important basis for the development of efficient methods to establish PDX models.
Supplementary Material
Acknowledgments
We would like to express our thanks to Dr. M. Watanabe, Dr. T. Watanabe, and Ms. Y.L. Yang for their skillful assistance, Dr. H. Yamada-Okabe and Dr. K. Yoshida for critical discussions concerning human tissue models, and Mr. R. Nomura for his continuous support throughout the study. We would also like to thank Dr. T. Yamazaki for his continuous encouragement and support for research at PLR since 2002 when he realized the importance of human cancer tissue models generated in NOG mice for biological insight including cancer stem cells.
References
- 1.Alpdogan O., van den Brink M.R.2012. Immune tolerance and transplantation. Semin. Oncol. 39: 629–642. doi: 10.1053/j.seminoncol.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camus M., Tosolini M., Mlecnik B., Pagès F., Kirilovsky A., Berger A., Costes A., Bindea G., Charoentong P., Bruneval P., Trajanoski Z., Fridman W.H., Galon J.2009. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 69: 2685–2693. doi: 10.1158/0008-5472.CAN-08-2654 [DOI] [PubMed] [Google Scholar]
- 3.Decaudin D.2011. Primary human tumor xenografted models (‘tumorgrafts’) for good management of patients with cancer. Anticancer Drugs 22: 827–841. doi: 10.1097/CAD.0b013e3283475f70 [DOI] [PubMed] [Google Scholar]
- 4.Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A., III, editors.2010. pp. 1–22. General Information on Cancer Staging and End-Results Reporting. In: Cancer Staging Handbook From the AJCC Cancer Staging Manual, 7th Edition. Springer New York Dordrecht Heidelberg London. [Google Scholar]
- 5.Fridman W.H., Dieu-Nosjean M.C., Pagès F., Cremer I., Damotte D., Sautès-Fridman C., Galon J.2013. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron 6: 117–122. doi: 10.1007/s12307-012-0124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J.2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12: 298–306. doi: 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 7.Fujii E., Kato A., Chen Y.J., Matsubara K., Ohnishi Y., Suzuki M.2014. Characterization of EBV-related lymphoproliferative lesions arising in donor lymphocytes of transplanted human tumor tissues in the NOG mouse. Exp. Anim. 63: 289–296. doi: 10.1538/expanim.63.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii E., Kato A., Chen Y.J., Matsubara K., Ohnishi Y., Suzuki M.2014. Histopathological characteristics of human non-tumor thyroid tissues in a long-term model of adenomatous goiter xenografts in the NOD/Shi-scid, IL-2Rγ(null) mouse. Exp. Toxicol. Pathol. 66: 203–209. doi: 10.1016/j.etp.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Fujii E., Suzuki M., Matsubara K., Watanabe M., Chen Y.J., Adachi K., Ohnishi Y., Tanigawa M., Tsuchiya M., Tamaoki N.2008. Establishment and characterization of in vivo human tumor models in the NOD/SCID/γ(c)(null) mouse. Pathol. Int. 58: 559–567. doi: 10.1111/j.1440-1827.2008.02271.x [DOI] [PubMed] [Google Scholar]
- 10.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., Zinzindohoué F., Bruneval P., Cugnenc P.H., Trajanoski Z., Fridman W.H., Pagès F.2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964. doi: 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 11.Galon J., Mlecnik B., Bindea G., Angell H.K., Berger A., Lagorce C., Lugli A., Zlobec I., Hartmann A., Bifulco C., Nagtegaal I.D., Palmqvist R., Masucci G.V., Botti G., Tatangelo F., Delrio P., Maio M., Laghi L., Grizzi F., Asslaber M., D’Arrigo C., Vidal-Vanaclocha F., Zavadova E., Chouchane L., Ohashi P.S., Hafezi-Bakhtiari S., Wouters B.G., Roehrl M., Nguyen L., Kawakami Y., Hazama S., Okuno K., Ogino S., Gibbs P., Waring P., Sato N., Torigoe T., Itoh K., Patel P.S., Shukla S.N., Wang Y., Kopetz S., Sinicrope F.A., Scripcariu V., Ascierto P.A., Marincola F.M., Fox B.A., Pagès F.2014. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 232: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M., Kobayashi K., Nakahata T.2008. NOD/Shi-scid IL2rγ(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 324: 53–76. [DOI] [PubMed] [Google Scholar]
- 13.John T., Kohler D., Pintilie M., Yanagawa N., Pham N.A., Li M., Panchal D., Hui F., Meng F., Shepherd F.A., Tsao M.S.2011. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin. Cancer Res. 17: 134–141. doi: 10.1158/1078-0432.CCR-10-2224 [DOI] [PubMed] [Google Scholar]
- 14.Julien S., Merino-Trigo A., Lacroix L., Pocard M., Goéré D., Mariani P., Landron S., Bigot L., Nemati F., Dartigues P., Weiswald L.B., Lantuas D., Morgand L., Pham E., Gonin P., Dangles-Marie V., Job B., Dessen P., Bruno A., Pierré A., De Thé H., Soliman H., Nunes M., Lardier G., Calvet L., Demers B., Prévost G., Vrignaud P., Roman-Roman S., Duchamp O., Berthet C.2012. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 18: 5314–5328. doi: 10.1158/1078-0432.CCR-12-0372 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S., Yamada-Okabe H., Suzuki M., Natori O., Kato A., Matsubara K., Jau Chen Y., Yamazaki M., Funahashi S., Yoshida K., Hashimoto E., Watanabe Y., Mutoh H., Ashihara M., Kato C., Watanabe T., Yoshikubo T., Tamaoki N., Ochiya T., Kuroda M., Levine A.J., Yamazaki T.2012. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells 30: 2631–2644. doi: 10.1002/stem.1257 [DOI] [PubMed] [Google Scholar]
- 16.Lee R.A., Gabardi S.2012. Current trends in immunosuppressive therapies for renal transplant recipients. Am. J. Health Syst. Pharm. 69: 1961–1975. doi: 10.2146/ajhp110624 [DOI] [PubMed] [Google Scholar]
- 17.Manz M.G., Di Santo J.P.2009. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat. Immunol. 10: 1039–1042. doi: 10.1038/ni1009-1039 [DOI] [PubMed] [Google Scholar]
- 18.Masuda H., Maruyama T., Hiratsu E., Yamane J., Iwanami A., Nagashima T., Ono M., Miyoshi H., Okano H.J., Ito M., Tamaoki N., Nomura T., Okano H., Matsuzaki Y., Yoshimura Y.2007. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc. Natl. Acad. Sci. USA 104: 1925–1930. doi: 10.1073/pnas.0604310104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizukami M., Hanagiri T., Shigematsu Y., Baba T., Fukuyama T., Nagata Y., So T., Ichiki Y., Sugaya M., Yasuda M., So T., Takenoyama M., Sugio K., Yasumoto K.2006. Effect of IgG produced by tumor-infiltrating B lymphocytes on lung tumor growth. Anticancer Res. 26:(3A): 1827–1831. [PubMed] [Google Scholar]
- 20.Moro M., Bertolini G., Tortoreto M., Pastorino U., Sozzi G., Roz L.2012. Patient-derived xenografts of non small cell lung cancer: resurgence of an old model for investigation of modern concepts of tailored therapy and cancer stem cells. J. Biomed. Biotechnol. 2012: 568567. doi: 10.1155/2012/568567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson T., Greiner D.L., Shultz L.D.2008. Humanized SCID mouse models for biomedical research. Curr. Top. Microbiol. Immunol. 324: 25–51. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y.1986. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am. J. Pathol. 125: 431–435. [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson-Abelson M.R., Sonnenberg G.F., Takita H., Yokota S.J., Conway T.F., Jr, Kelleher R.J., Jr, Shultz L.D., Barcos M., Bankert R.B.2008. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rgamma(null) mice. J. Immunol. 180: 7009–7018. doi: 10.4049/jimmunol.180.10.7009 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M., Katsuyama K., Adachi K., Ogawa Y., Yorozu K., Fujii E., Misawa Y., Sugimoto T.2002. Combination of fixation using PLP fixative and embedding in paraffin by the AMeX method is useful for histochemical studies in assessment of immunotoxicity. J. Toxicol. Sci. 27: 165–172. doi: 10.2131/jts.27.165 [DOI] [PubMed] [Google Scholar]
- 25.Tentler J.J., Tan A.C., Weekes C.D., Jimeno A., Leong S., Pitts T.M., Arcaroli J.J., Messersmith W.A., Eckhardt S.G.2012. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9: 338–350. doi: 10.1038/nrclinonc.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams S.S., Chen F.A., Kida H., Yokata S., Miya K., Kato M., Barcos M.P., Wang H.Q., Alosco T., Umemoto T., Croy B.A., Repasky E.A., Bankert R.B.1996. Engraftment of human tumor-infiltrating lymphocytes and the production of anti-tumor antibodies in SCID mice. J. Immunol. 156: 1908–1915. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.