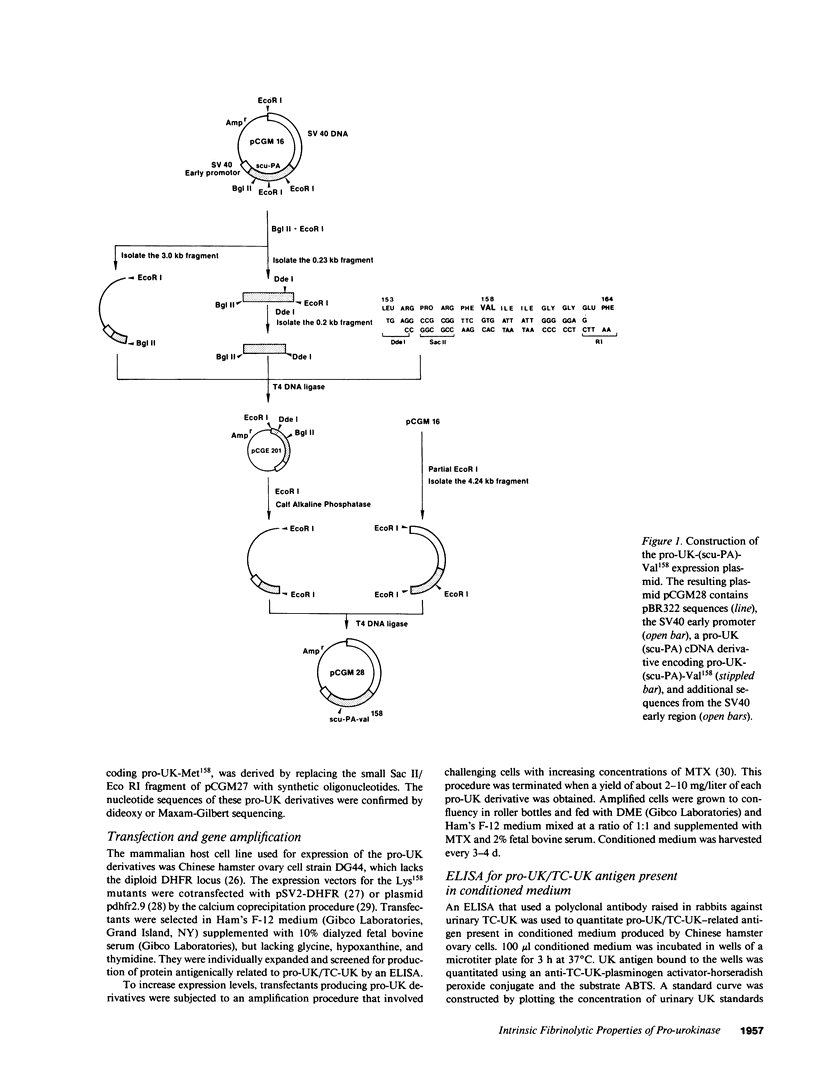
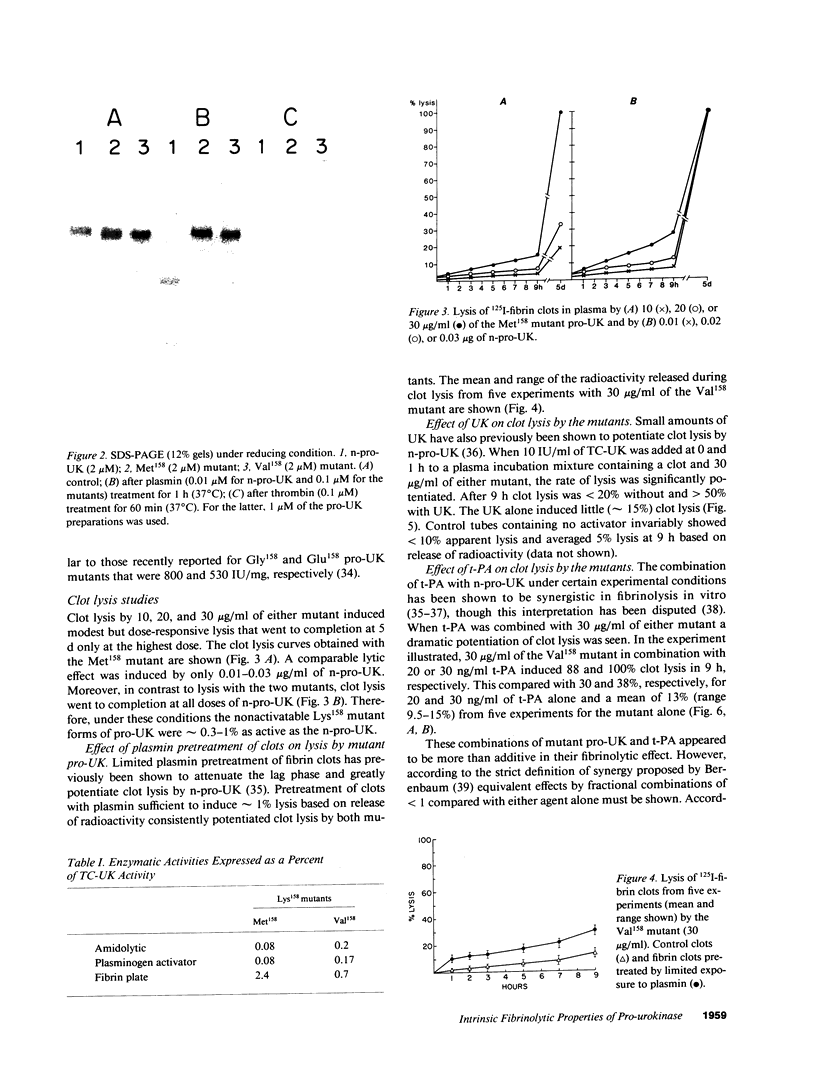
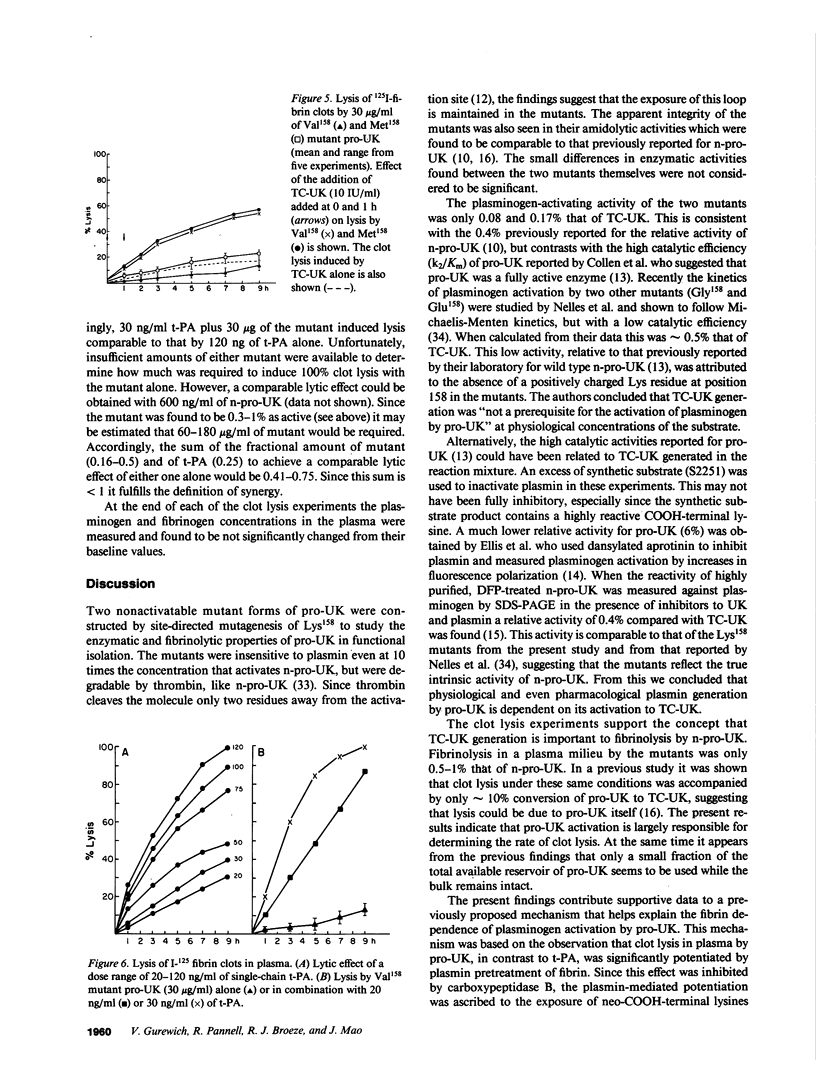
Abstract
Two plasmin-resistant mutant forms of pro-urokinase (pro-UK) constructed by site-directed mutagenesis of Lys158 to Val158 and Met158 were used to evaluate the intrinsic enzymatic and fibrinolytic properties of pro-UK as distinct from those of its two-chain UK (TC-UK) derivative. Both mutants, while resistant to plasmin activation, were as sensitive as pro-UK to degradation by thrombin. Since thrombin cleaves a peptide bond only two residues from the activation site, the integrity of this loop was maintained in the two mutants. The amidolytic and plasminogen-activating activities of the mutants averaged 0.14 and 0.12% that of TC-UK, respectively. The fibrin plate activities were 2,400 IU/ml and 700 IU/mg for the Met158 and Val158 mutants or about 1.5% that of TC-UK. These findings attest to a discrete but low intrinsic activity for pro-UK and suggest that the higher values reported in the literature may be related to UK contaminants or plasmin-induced TC-UK generation during the assay. Clot lysis by the mutants required doses greater than 100-fold higher than those of pro-UK to induce a comparable effect. From this it appears that pro-UK activation is a major determinant of the rate of clot lysis occurring with pro-UK. Clot lysis by the mutants was potentiated by plasmin pretreatment of the fibrin and by the addition of small amounts of TC-UK or tissue plasminogen activator (t-PA). Combinations of t-PA and the mutants were synergistic in their fibrinolytic effects. These findings mirror those previously obtained with pro-UK. We concluded that the previously described potentiation of pro-UK-induced clot lysis by UK or t-PA is mediated primarily by pro-UK itself rather than by a promotion of its activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin Exp Immunol. 1977 Apr;28(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Castellino F. J., Powell J. R. Human plasminogen. Methods Enzymol. 1981;80(Pt 100):365–378. doi: 10.1016/s0076-6879(81)80031-6. [DOI] [PubMed] [Google Scholar]
- Collen D., De Cock F., Demarsin E., Lijnen H. R., Stump D. C. Absence of synergism between tissue-type plasminogen activator (t-PA), single-chain urokinase-type plasminogen activator (scu-PA) and urokinase on clot lysis in a plasma milieu in vitro. Thromb Haemost. 1986 Aug 20;56(1):35–39. [PubMed] [Google Scholar]
- Collen D., Zamarron C., Lijnen H. R., Hoylaerts M. Activation of plasminogen by pro-urokinase. II. Kinetics. J Biol Chem. 1986 Jan 25;261(3):1259–1266. [PubMed] [Google Scholar]
- Crouse G. F., McEwan R. N., Pearson M. L. Expression and amplification of engineered mouse dihydrofolate reductase minigenes. Mol Cell Biol. 1983 Feb;3(2):257–266. doi: 10.1128/mcb.3.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L., Scott R. W., Baker J. B. Purification of human fibroblast urokinase proenzyme and analysis of its regulation by proteases and protease nexin. J Biol Chem. 1984 May 25;259(10):6241–6247. [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation by single-chain urokinase in functional isolation. A kinetic study. J Biol Chem. 1987 Nov 5;262(31):14998–15003. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Pannell R. A comparative study of the efficacy and specificity of tissue plasminogen activator and pro-urokinase: demonstration of synergism and of different thresholds of non-selectivity. Thromb Res. 1986 Oct 15;44(2):217–228. doi: 10.1016/0049-3848(86)90137-4. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Pannell R. Inactivation of single-chain urokinase (pro-urokinase) by thrombin and thrombin-like enzymes: relevance of the findings to the interpretation of fibrin-binding experiments. Blood. 1987 Mar;69(3):769–772. [PubMed] [Google Scholar]
- Gurewich V., Pannell R., Louie S., Kelley P., Suddith R. L., Greenlee R. Effective and fibrin-specific clot lysis by a zymogen precursor form of urokinase (pro-urokinase). A study in vitro and in two animal species. J Clin Invest. 1984 Jun;73(6):1731–1739. doi: 10.1172/JCI111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Buse G., Flohé L. Structural relationship between human high and low molecular mass urokinase. Hoppe Seylers Z Physiol Chem. 1982 Feb;363(2):133–141. doi: 10.1515/bchm2.1982.363.1.133. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Chang T. S., Verderber E. Tissue plasminogen activator and urokinase mediate the binding of Glu-plasminogen to plasma fibrin I. Evidence for new binding sites in plasmin-degraded fibrin I. J Biol Chem. 1985 Apr 10;260(7):4432–4440. [PubMed] [Google Scholar]
- Husain S. S., Gurewich V., Lipinski B. Purification and partial characterization of a single-chain high-molecular-weight form of urokinase from human urine. Arch Biochem Biophys. 1983 Jan;220(1):31–38. doi: 10.1016/0003-9861(83)90383-1. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Fujikawa K., Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J Biol Chem. 1986 Mar 15;261(8):3486–3489. [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Interaction of plasminogen activators and inhibitors with plasminogen and fibrin. Semin Thromb Hemost. 1982 Jan;8(1):2–10. doi: 10.1055/s-2007-1005038. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moir D., Mao J., Schumm J. W., Vovis G. F., Alford B. L., Taunton-Rigby A. Molecular cloning and characterization of double-stranded cDNA coding for bovine chymosin. Gene. 1982 Jul-Aug;19(1):127–138. doi: 10.1016/0378-1119(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles L., Lijnen H. R., Collen D., Holmes W. E. Characterization of recombinant human single chain urokinase-type plasminogen activator mutants produced by site-specific mutagenesis of lysine 158. J Biol Chem. 1987 Apr 25;262(12):5682–5689. [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Pannell R., Black J., Gurewich V. Complementary modes of action of tissue-type plasminogen activator and pro-urokinase by which their synergistic effect on clot lysis may be explained. J Clin Invest. 1988 Mar;81(3):853–859. doi: 10.1172/JCI113394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell R., Gurewich V. Activation of plasminogen by single-chain urokinase or by two-chain urokinase--a demonstration that single-chain urokinase has a low catalytic activity (pro-urokinase). Blood. 1987 Jan;69(1):22–26. [PubMed] [Google Scholar]
- Pannell R., Gurewich V. Pro-urokinase: a study of its stability in plasma and of a mechanism for its selective fibrinolytic effect. Blood. 1986 May;67(5):1215–1223. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Käs E., Carothers A. M., Chasin L. A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983 Jun;33(2):405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. R. B. The fibrinolytic activity of urine. Br J Exp Pathol. 1951 Dec;32(6):530–537. [PMC free article] [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]