Abstract
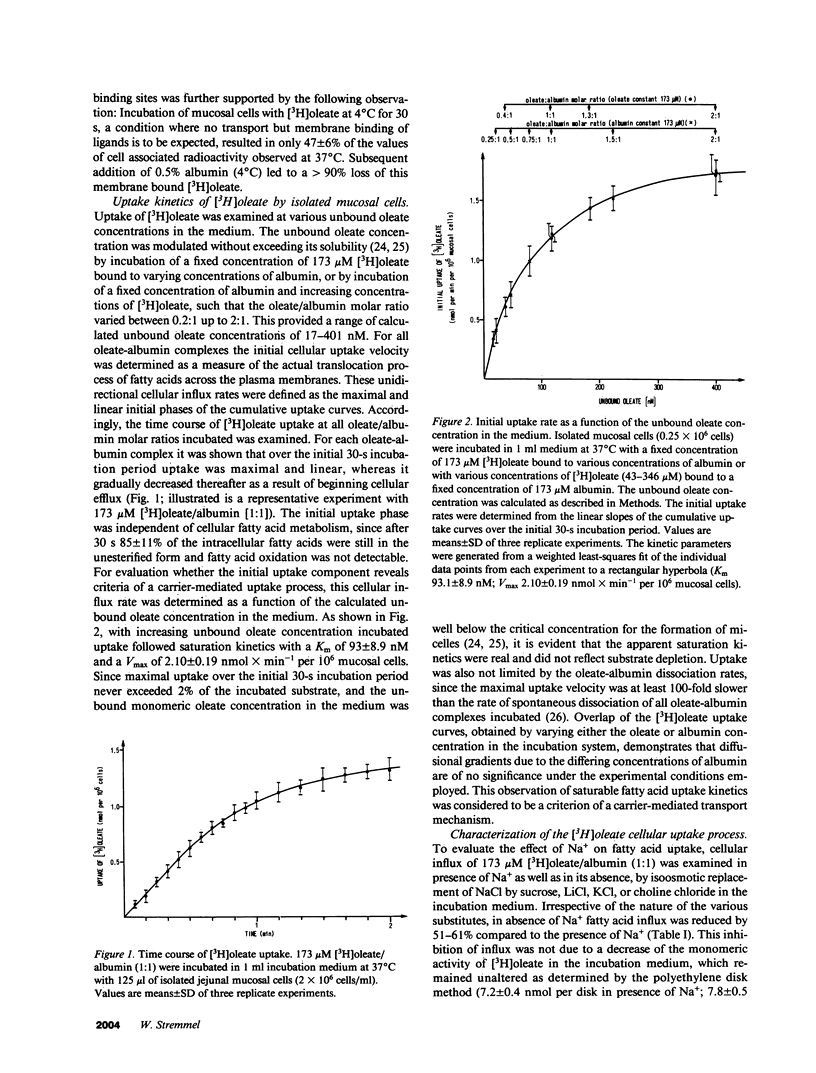
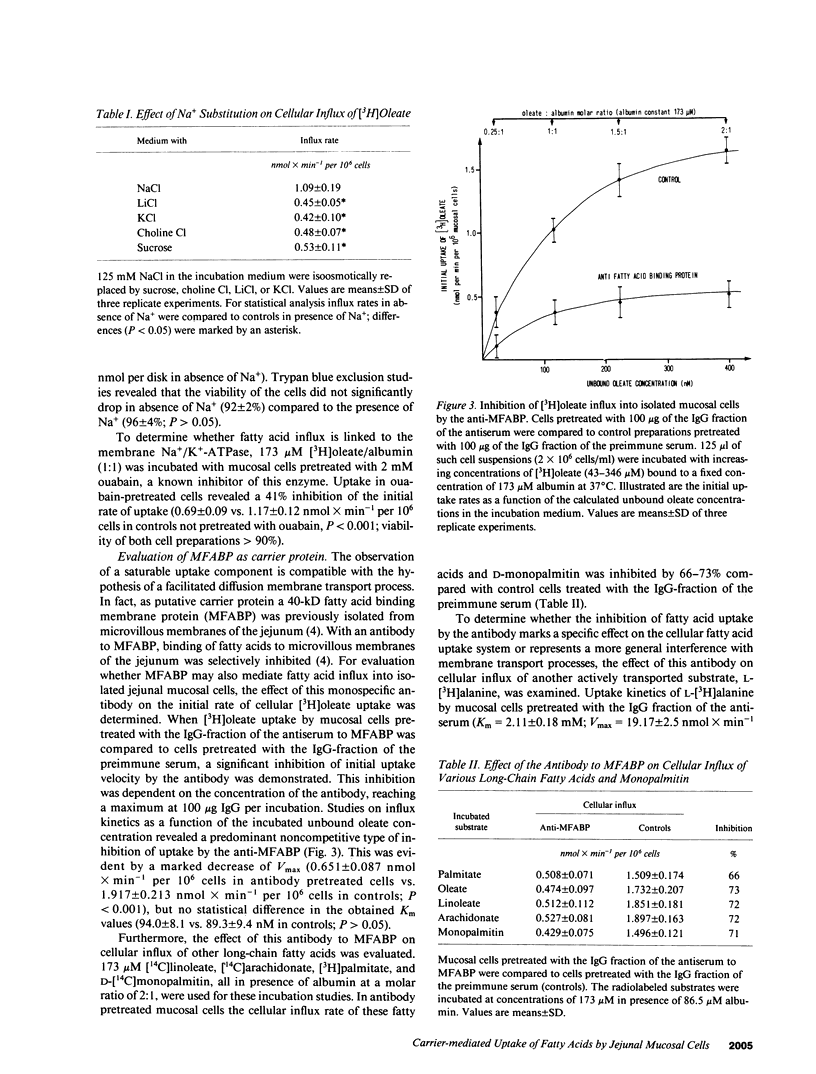
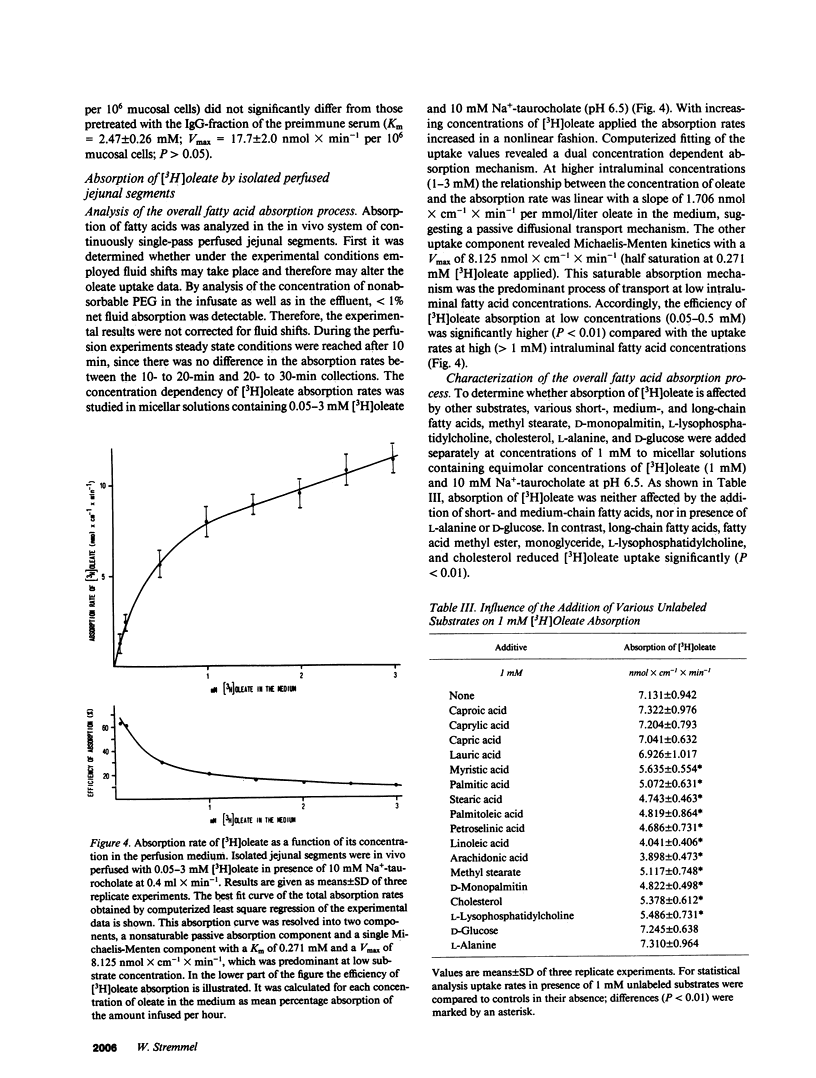
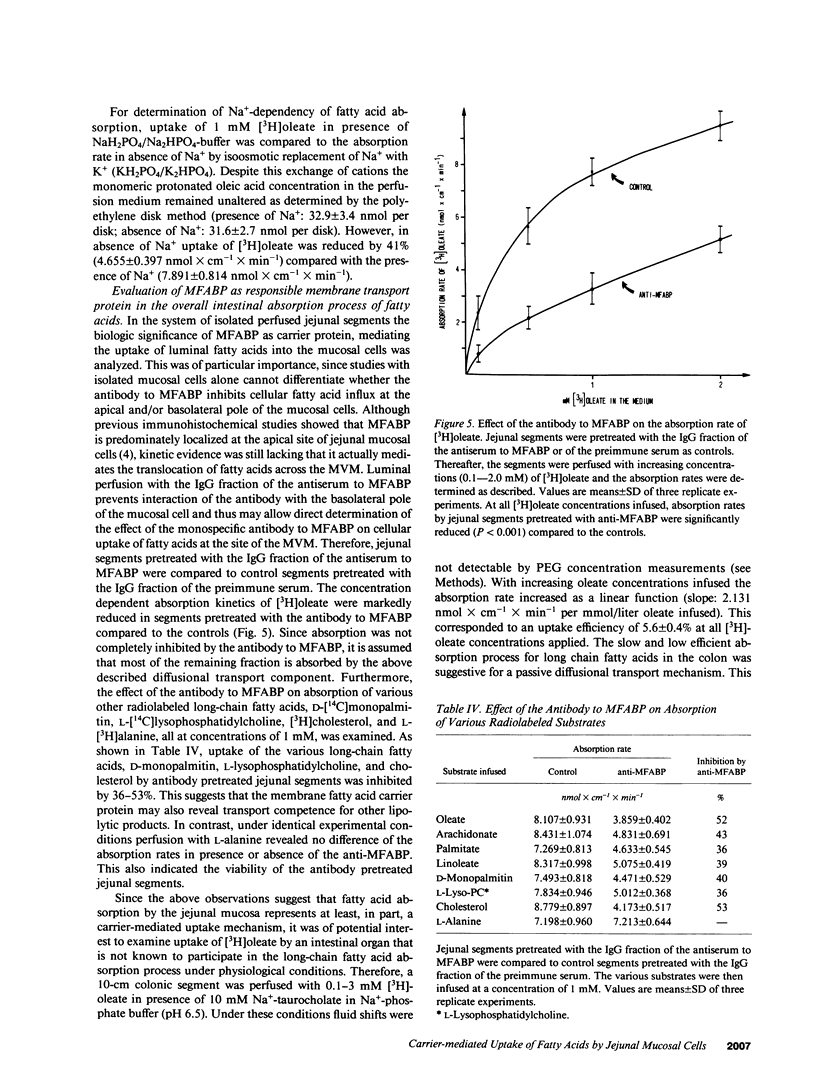
The previous identification of a membrane fatty acid binding protein (MFABP) in brush border plasma membranes of the jejunum suggested that mucosal cell uptake of fatty acids might represent a carrier-mediated transport system. For evaluation of this hypothesis cellular influx kinetics (V0) of [3H]-oleate were examined in isolated rat jejunal mucosal cells. With increasing unbound oleate concentration in the medium V0 was saturable (Km = 93 nM; Vmax = 2.1 nmol X min-1 per 10(6) cells) and temperature dependent with an optimum at 37 degrees C. Pretreatment of the cells with a monospecific antibody to MFABP significantly inhibited V0 of oleate, other long-chain fatty acids, and D-monopalmitin, but not of L-alanine. Moreover, in the in vivo system of isolated perfused jejunal segments the physiologic significance of MFABP in the directed overall intestinal absorption process of fatty acids was documented. In the presence of the anti-MFABP oleate absorption was markedly reduced, whereas uptake of L-alanine remained unaltered. By antibody inhibition studies it was suggested that this membrane carrier also reveals transport competence for various other long-chain fatty acids, D-monopalmitin, L-lysophosphatidylcholine, and cholesterol. These data support the hypothesis that absorption of fatty acids is mediated by a fatty acid binding membrane protein.
Full text
PDF


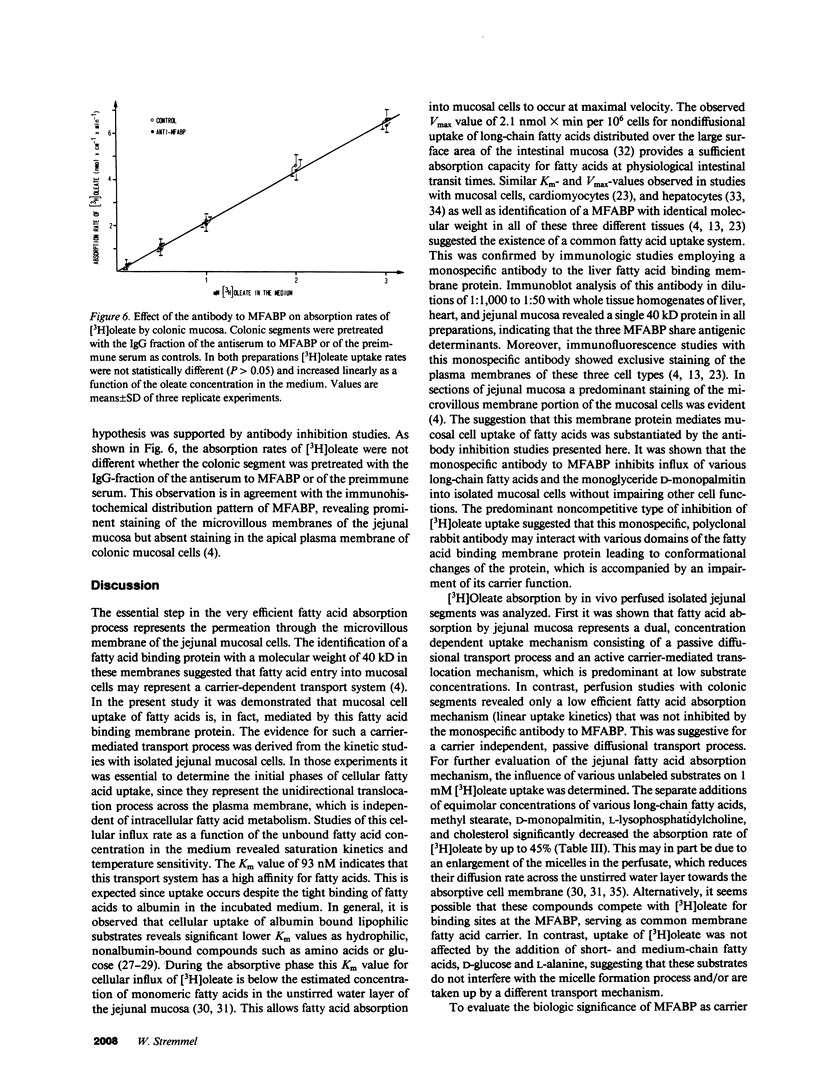


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R., Gibb L., Ansel A., Hohl C., Kruger F. A., Brierley G. P. Calcium tolerance of isolated rat heart cells. J Mol Cell Cardiol. 1980 Dec;12(12):1383–1395. doi: 10.1016/0022-2828(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Carey M. C., Small D. M., Bliss C. M. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- Chow S. L., Hollander D. Linoleic acid absorption in the unanesthetized rat: mechanism of transport and influence of luminal factors on absorption. Lipids. 1979 Apr;14(4):378–385. doi: 10.1007/BF02533421. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Hartmann F., Owen R., Bissell D. M. Characterization of isolated epithelial cells from rat small intestine. Am J Physiol. 1982 Feb;242(2):G147–G155. doi: 10.1152/ajpgi.1982.242.2.G147. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Groseclose R. The mechanism of Na+-dependent D-glucose transport. J Biol Chem. 1980 May 25;255(10):4453–4462. [PubMed] [Google Scholar]
- KOCHWA S., ROSENFIELD R. E., TALLAL L., WASSERMAN L. R. Isoagglutinins associated with ABO erythroblastosis. J Clin Invest. 1961 May;40:874–883. doi: 10.1172/JCI104322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W., Solberg D., Carter S., Hughes M., Phan D., Zollman F., Diamond J. Uptake pathways for amino acids in mouse intestine. Am J Physiol. 1986 Oct;251(4 Pt 1):G501–G508. doi: 10.1152/ajpgi.1986.251.4.G501. [DOI] [PubMed] [Google Scholar]
- Kessler M., Semenza G. The small-intestinal Na+, D-glucose cotransporter: an asymmetric gated channel (or pore) responsive to delta psi. J Membr Biol. 1983;76(1):27–56. doi: 10.1007/BF01871452. [DOI] [PubMed] [Google Scholar]
- Kuhl W. E., Spector A. A. Uptake of long-chain fatty acid methyl esters by mammalian cells. J Lipid Res. 1970 Sep;11(5):458–465. [PubMed] [Google Scholar]
- Lewis L. D., Fordtran J. S. Effect of perfusion rate on absorption, surface area, unstirred water layer thickness, permeability, and intraluminal pressure in the rat ileum in vivo. Gastroenterology. 1975 Jun;68(6):1509–1516. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Sallee V. L. Apparent monomer activity of saturated fatty acids im micellar bile salt solutions measured by a polyethylene partitioning system. J Lipid Res. 1974 Jan;15(1):56–64. [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Berk P. D. Hepatocellular influx of [14C]oleate reflects membrane transport rather than intracellular metabolism or binding. Proc Natl Acad Sci U S A. 1986 May;83(10):3086–3090. doi: 10.1073/pnas.83.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Debuch H. The lipids of the Golgi apparatus subfractions from rat liver. Biochim Biophys Acta. 1979 May 25;573(2):301–307. doi: 10.1016/0005-2760(79)90063-8. [DOI] [PubMed] [Google Scholar]
- Stremmel W. Fatty acid uptake by isolated rat heart myocytes represents a carrier-mediated transport process. J Clin Invest. 1988 Mar;81(3):844–852. doi: 10.1172/JCI113393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Strohmeyer G., Berk P. D. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985 Mar;75(3):1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Berk P. D. Hepatocellular uptake of oleate is energy dependent, sodium linked, and inhibited by an antibody to a hepatocyte plasma membrane fatty acid binding protein. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3584–3588. doi: 10.1073/pnas.83.11.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P. D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Theilmann L. Selective inhibition of long-chain fatty acid uptake in short-term cultured rat hepatocytes by an antibody to the rat liver plasma membrane fatty acid-binding protein. Biochim Biophys Acta. 1986 Jun 11;877(1):191–197. doi: 10.1016/0005-2760(86)90134-7. [DOI] [PubMed] [Google Scholar]
- Stremmel W. Translocation of fatty acids across the basolateral rat liver plasma membrane is driven by an active potential-sensitive sodium-dependent transport system. J Biol Chem. 1987 May 5;262(13):6284–6289. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Gollan J. L., Ockner R. K. The role of albumin in hepatic uptake processes. Prog Liver Dis. 1982;7:71–85. [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976 Jul;58(1):97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosilait W. D., Nagy P. A method of computing drug distribution in plasma using stepwise association constants: clofibrate acid as an illustrative example. Comput Programs Biomed. 1976 Oct;6(3):142–148. doi: 10.1016/0010-468x(76)90020-9. [DOI] [PubMed] [Google Scholar]


