Abstract
Validation of gene-chip microarray results is one of the challenges in genomic studies. The successful use of a custom-designed 96-well polymerase chain reaction (PCR) array to study the unexpected inflammatory effect of environmental titanium dioxide (TiO2) particles on the lungs of pregnant mice, with similar results not seen in control mice, is reported. In our approach, selection of candidate genes for the custom PCR array was informed by prior gene-chip microarray profiling. Results demonstrated multiple upregulation of genes in the lungs of pregnant but not control mice produced by TiO2 exposure. Customized PCR array is a flexible tool that offers the ability to combine the “blind” genome-wide scan with a hypothesis-driven approach, by including both the “candidate” genes for validation positively identified by the microarray and biologically relevant “suspects” that failed to be found in the genomic data. Compared to conventional gene-by-gene qPCR or manufacturer-preset pathway kits, this technique provides a cost-effective and time-saving method of analysis and allows for a strong, easily detectable signal. Genes with confirmed differential expression were further used for pathway analysis and indicated involvement in several biologically relevant pathways including allergy mediator signaling in dendritic cells. Finally, an analytical network was created that will inform further mechanistic studies. The dual purpose of the work was to demonstrate that the novel custom PCR array is a convenient approach to validate the microarray results, and to obtain biologically significant data on TiO2-induced inflammation by following the PCR array with pathway analysis, which provided feasible hypotheses to support future experimental studies.
Titanium dioxide particles (TiO2) are known to be immunologically “inert” and typically used as control substances in immunotoxicity studies (Sigaud et al., 2007). This is not true during pregnancy (Fedulov et al., 2008). Pregnant mice showed robust and persistent acute inflammation after exposure to TiO2 comparable to that induced by the positive control, diesel exhaust particles (DEP). In contrast, nonpregnant female mice had the expected minimal response to “inert” TiO2 48 h after exposure. Therefore, pregnancy enhanced lung inflammatory responses to otherwise relatively innocuous inert particles of TiO2 and carbon black. Moreover, this leads to serious consequences for the developing fetus and results in increased allergic susceptibility, particularly seen as allergic airway disease in offspring (Fedulov et al., 2008). There are unpublished observations to suggest that dendritic cells (DC) in the neonates of allergic mothers or mothers exposed to TiO2 acquire allergy-skewing capabilities; however, it is unclear how this happens and what signaling pathways in the DCs are altered. To address the mechanism, gene-chip microarray expression profiling of lung tissue was conducted from pregnant and control mice exposed to TiO2 particles or vehicle alone. This approach is becoming more and more common in environmental toxicology (Gwinn & Weston, 2008); however, genome-wide scanning has its challenges.
One of the challenges is the need to individually validate a large number of genes that may show some evidence of differential expression in the genome-wide data scans, as underscored by previous studies (Rajeevan et al., 2001a; Jayapal & Melendez, 2006; Provenzano & Mocellin, 2007; Gaj et al., 2008). A method recognized for its reliability as well as efficiency is that of quantitative real-time polymerase chain reaction (qPCR). Alternative validation assays include Northern/Southern blots and conventional reverse-transcription PCR; however, each of these methods requires substantial amounts of RNA and are more labor-intensive than qPCR (Rajeevan et al., 2001b). The sensitivity and precision afforded by qPCR is particularly important in determining the significance of small fold changes in expression, where older endpoint PCR methods of analysis may not be as viable. An additional challenge is the need to interrogate a large number of genes, preferably of the same RNA sample, in a reasonable amount of time. Thus, various qPCR-based approaches are being developed to address this issue.
In this study, to investigate the mechanism underlying the pregnancy-enhanced response to TiO2 in the lung, total lung RNA was isolated and gene-chip microarray profiling was performed (Fedulov et al., 2007). Genomic data have been submitted to the Gene Expression Omnibus (GEO) database and has been assigned Series Record number GSE7475. The screening identified a number of genes with differential expression upon TiO2 exposure. In this study, the use of a novel high-throughput customized real-time PCR-based approach is demonstrated to validate the “hit list” of microarray expression comparisons independent of whether the genes of interest form a particular pathway(s). While sometimes a pathway-based PCR kit may be used for gene array validation (e.g., “inflammatory genes” etc.), in our approach, pathway enrichment analysis follows (not precedes) the validation and suggests that TiO2-induced inflammation affects the ability of dendritic cells to respond to the allergy mediators histamine and prostaglandin E2 (PGE2), which may explain the pro-allergic skew in utero.
MATERIALS AND METHODS
Animals
BALB/c mice weighing 25–30 g were obtained from Charles River Laboratories (Cambridge, MA). All mice were housed in a clean barrier facility where animals are maintained at 22 to 24°C using a 12-h dark/light cycle with an independent pressure-gradient-enabled ventilation system. Animal care complied with the Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the Institutional Review Board.
Particle Characterization
Respirable-size TiO2 particles with a mean particle size of ~1 μm were a gift from Dr. J. Brain (Harvard School of Public Health). DEP were from the U.S. Environmental Protection Agency (EPA) stock generated between 2004 and 2005, and were generously provided by Dr. Ian Gilmour (U.S. EPA). The stock was obtained using a 30-kW (40 hp) 4-cylinder indirect injection Deutz diesel engine (model BF4M1008) under load of a 22.3-kW (30 hp) Saylor–Beall air compressor (model 707), running on readily available road-taxed diesel fuel. Details of the generation and particle characteristics (“EPA DEP mix”) are described in Shinyashiki et al. (2009).
Particle samples were baked at 165°C for 3 h to eliminate endotoxin, aliquoted, and stored frozen at −80°C.
Exposure Protocol
To test whether pregnancy altered the normally minimal inflammatory response to “‘inert” particles, TiO2 and DEP suspensions (50 μg/mouse in 50 μl) or PBS solution (vehicle) were administered by single intranasal insufflation to pregnant E14 or control mice under light isoflurane anesthesia (21). The mice were subjected to pathologic analysis 48 h later (see Figure 1).
FIGURE 1.
Exposure protocol.
Tissue Isolation
Total lung RNA extraction and isolation were performed using a Qiagen RNAeasy Mini kit according to manufacturer’s instructions (Qiagen, Valencia, CA). Briefly, mice were euthanatized with an ip injection of pentobarbital, and lungs were immediately removed and placed into the RLT buffer (Qiagen). After rotor–stator homogenization and standard column spin RNA cleanup procedure with on-column DNA digestion, the RNA was eluted in 30–50 μl nuclease-free water (Invitrogen). RNA purity and quality were analyzed by Agilent Bioanalyzer 2100 scan (Agilent, Santa Clara, CA).
Gene-Chip Microarray and Data Analysis
The hybridization was carried out at the Harvard Partners Genomic Center Microarray facility (Cambridge, MA) using the Affymetrix GeneChip platform and Affymetrix mouse 430 2.0 chips (Affymetrix, Santa Clara, CA). Signal intensities and detection calls were extracted using dChip (v. 2006). Chip images were evaluated for overall quality; PM/MM pairs were evaluated for outliers to judge hybridization performance. Hybridization quality was found to be consistent with the manufacturer’s requirements. Probe sets were filtered based on detection call to exclude those in which “P” call was not present in all four samples in any one group; this also excluded probe sets with all “A” calls. The filtration resulted in about 24,000 probe sets. RMA values for this list were extracted using RMAExpress (v. 0.4.1) with background correction, normalization, and log2 transformation and were analyzed using TIGR tMEV (v. 4.1). Resampling with bootstrapping using the Support Tree feature indicated appropriate unsupervised sample clustering with 90 to 100% support level (not shown). High-level analysis was performed in tMEV 4.1 and included significance analysis for microarrays (SAM) at false discovery rate (FDR) of 0, analysis of variance (ANOVA), and non-parametric comparisons. Multiple testing correction is achieved in the TIGR tMEV 4.1 program by the Benjamini–Hochberg FDR method. The data were published in the GEO repository (Fedulov et al., 2007).
RT2 Profiler PCR Array Analysis
Primers were synthesized by the manufacturer (SABioscience, Frederick, MD) based on the list of gene names, symbols, UniGene, and Genbank IDs. Primers are adsorbed on the bottom of each well in a 96-well optical micro-plate, 1 primer pair per well, 96 pairs on a plate. RNA samples were reverse transcribed using the RT2 First Strand Kit (SABioscience), which includes an additional step of DNAse digestion to prevent contamination of cDNA with any genomic DNA. PCR was performed using 25 μl of the following mixture: 1275 μl of 2× SuperArray RT2 qPCR Master Mix, 38 μl first-strand cDNA synthesis reaction, and 1435 μl of double-distilled nuclease-free water in the wells of a 96-well microtiter plate (Bio-Rad). The temperature protocol included a start cycle for 10 min at 95°C, 40 cycles of amplification (15 s at 95°C, 15 s at 60°C, and 15 s at 72°C), followed by a melt curve. Thermal cycling and fluorescence detection were performed using a Bio-Rad iCycler (Bio-Rad). The Ct values of the target cDNAs were normalized by the average Ct of 6 housekeeping genes (GAPDH, ACTB, 18srRNA, GUSB, B2M and HPRT1) within the same 96-well microtiter plate. Negative controls (NTC, NRT) remained unamplified throughout the study. Calculations were conducted using a downloadable PCR array data analysis tool provided by the manufacturer.
Replicates
For the custom PCR array, five biological replicates per group were used, and each was measured in duplicate (technical replicates). For the original genome-wide microarray analysis four biological replicates per group were used. For measurement of inflammation in the lungs as a quality control of the exposure, three to five biological replicates were used for each exposure cohort, and each lavage was counted twice.
RESULTS
Gene Selection Strategy
Based on gene-chip microarray results, genes of interest with measurable fold change and significance were selected, while additional genes were included for biological relevance. Specifically, the comparisons were made for the expression in the pregnant TiO2 group versus the pregnant vehicle group, and in the control (nonpregnant) TiO2 versus the control vehicle groups; gene expression was considered different when the cutoff value for fold up- or downregulation was F > 1.5, with a p-value cutoff of <.005 in the Mann–Whitney test. From these lists we included 19 top genes (by fold change) with significantly changed expression in the pregnant mice upon TiO2 exposure compared to vehicle, as well as 19 genes changed in the control mice, and 22 genes changed in both groups (pregnant and control). This choice was supplemented with biologically biased hypothesis-driven genes (n = 20); six random “negative controls” with essentially identical expression between the groups were also included. The list was amended with six housekeeping genes for normalization and four necessary no-template control and no-reverse-transcription (NTC/NRT) controls, as well as reverse-transcription control (RTC) and a positive PCR control (PPC) to form a 96-well layout for the custom PCR arrays.
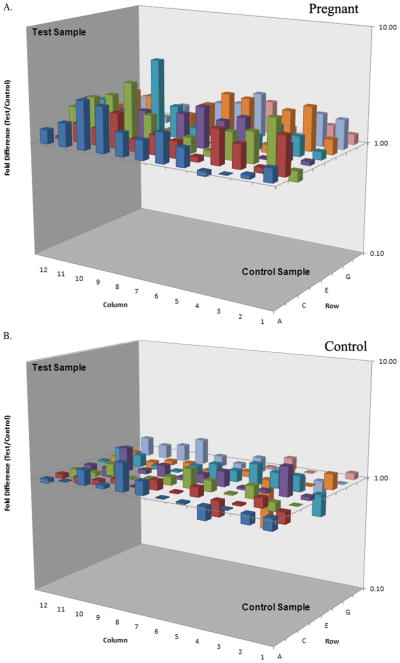
As evident from Figure 2, our selection strategy of candidate genes informed the PCR array very well. While most genes were substantially upregulated upon TiO2 exposure in pregnancy, as predicted, the particles produced minimal change in normal mice.
FIGURE 2.
Test sample is TiO2 exposure, control sample is vehicle exposure. Panel A is pregnant, notice multiple gene upregulation compared to normal control mice (panel B below). The x-axis and the y-axis are the axes of the 96-well plate (one gene per well); the y-axis is the fold change of expression in TiO2 vs vehicle: upregulated genes are represented by columns above the plane (value of 1 or no change), downregulated genes are below the plane.
This approach permits both an unbiased way to validate microarray results (if a user was to configure the array enitrely based on genomic analysis results), and a hypothesis-driven approach, either with or without prior genomic analysis; in our case the choice was to combine these options.
After removal of the housekeeping genes, “negative controls,” and the genes that did not validate as being differentially expressed, the remaining genes were used in the form of a data matrix as an input for the Metacore pathway analysis portal (Table 1). This list is primarily comprised of genes with changes in expression unique to pregnant mice upon exposure to TiO2: Differences were seen for 11 of the 20 “hypothesis-driven” genes and for 7 genes validated from the microarray. Another nine genes changed in pregnant lungs were also different in nonpregnant control mice; although not “exclusive,” they were allowed in the pathway analysis list as they may be involved in particular pathways of interest, along with those uniquely expressed by pregnant mice. Their omission would preclude proper idenitifcation of these pathways in a network. Of the six “negative” genes that were below the p-value threshold in the microarray results, which were also included as controls in the PCR array, two were differentially expressed in the pregnant TiO2 versus PBS group and were also included in Table 1. The remaining four genes were involved exclusively in normal, but not pregnant, mice. Two-thirds of the final gene list was informed by the initial genome-wide scan and one-third was comprised of biologically interesting “suspects” that were not revealed in the microarray data.
TABLE 1.
Genes Identified for Input in the Metacore GeneGo Pathway Analysis Portal
| GenBank number | Symbol | Inclusion | Description |
|---|---|---|---|
| NM_007630 | Ccnb2 | Cyclin B2 | |
| NM_013488 | Cd4 | * | CD4 antigen |
| NM_009855 | Cd80 | * | CD80 antigen |
| NM_019388 | Cd86 | * | CD86 antigen |
| NM_178119 | Centg2 | Centaurin, gamma 2 | |
| NM_009890 | Ch25h | Cholesterol 25-hydroxylase | |
| NM_009639 | Crisp3 | Cysteine-rich secretory protein 3 | |
| NM_007802 | Ctsk | Cathepsin K | |
| NM_007865 | Dll1 | Delta-like 1 (Drosophila) | |
| AK155063 | ENSMUSG 00000074917 | NOD-derived CD11c+ dendritic cells cDNA, hypothetical protein | |
| NM_028784 | F13a1 | Coagulation factor XIII, A1 subunit | |
| NM_008102 | Gch1 | GTP cyclohydrolase 1 | |
| NM_053110 | Gpnmb | Glycoprotein (transmembrane) nmb | |
| NM_030691 | Igsf6 | Immunoglobulin superfamily, member 6 | |
| NM_008351 | Il12a | * | Interleukin 12A |
| NM_008352 | Il12b | * | Interleukin 12B |
| NM_021283 | Il4 | * | Interleukin 4 |
| NM_010558 | Il5 | * | Interleukin 5 |
| NM_021334 | Itgax | * | Integrin alpha X |
| NM_033325 | Loxl2 | Lysyl oxidase-like 2 | |
| NM_019781 | Pex14 | Peroxisomal biogenesis factor 14 | |
| NM_011089 | Pira2 | Paired-Ig-like receptor A4 | |
| NM_009037 | Rcn1 | Reticulocalbin 1 | |
| NM_009104 | Rrm2 | Ribonucleotide reductase M2 | |
| NM_009127 | Scd1 | Stearoyl-Coenzyme A desaturase 1 | |
| NM_030710 | Slamf6 | SLAM family member 6 | |
| NM_024249 | Slc38a10 | Solute carrier family 38, member 10 | |
| NM_011407 | Slfn1 | Schlafen 1 | |
| NM_173869 | Stfa2l1 | Stefin A2 like 1 | |
| NM_011905 | Tlr2 | * | Toll-like receptor 2 |
| NM_021297 | Tlr4 | * | Toll-like receptor 4 |
| NM_013693 | Tnf | * | Tumor necrosis factor |
| NM_172643 | Zbtb41 | Zinc finger and BTB domain containing 41 homolog |
Note. Genes identified for input in the Metacore GeneGo pathway analysis portal. Genes included for biological relevance are marked with an asterisk. Inclusion of others was informed by prior microarray results.
MetaCore (GeneGo, St Joseph, MI) is an online database and an interactive visual medium designed for pathway analysis of biological data. Among other features, it includes a mapping analysis tool, which uses graphic pathway maps created on the basis of known protein interactions (information is derived from PubMed and other sources). For any list of genes suggested by the user, the system returns pathways known to contain said genes, and visualizes interactions between them.
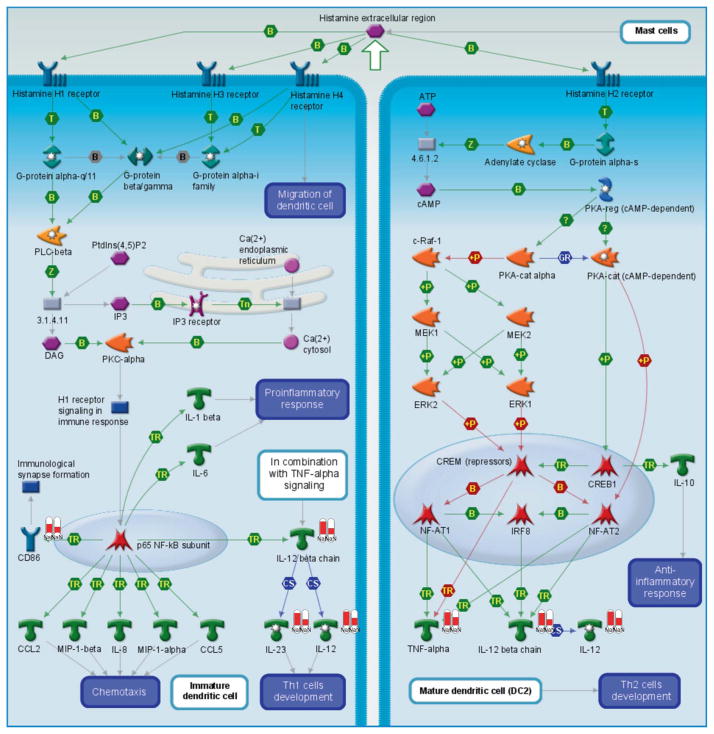
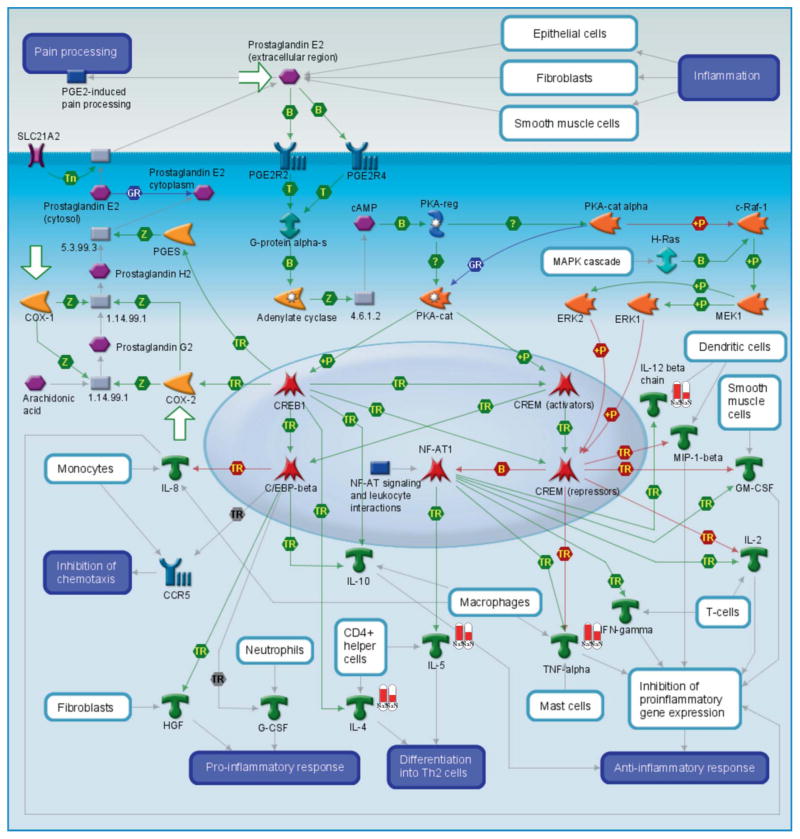
Mapping analysis of the “short list” of hits in MetaCore suggested that the number 1 and 2 pathways involving the genes from Table 1 were “dendritic cell response to histamine” (Figure 3) and “prostaglandin E2 signaling” (Figure 4). These pathways are highly relevant to our study and provide an additional (although indirect) confirmation of the biological relevance of genomic results.
FIGURE 3.
Pathway map of the histamine receptor response in dendritic cell. Many of the genes identified as significantly different in our dataset are involved in this pathway. These differentially expressed factors from experimental data are depicted as white columns with red bars, the height of the red bar is proportionate to the fold change as a result of TiO2 exposure in pregnant (left) vs normal (right) mice.
FIGURE 4.
Pathway map of the Prtostaglandin E2 receptor signaling. Many of the genes identified as significantly different in our dataset are involved in this pathway. These differentially expressed factors from experimental data are depicted as white columns with red bars, the height of the red bar is proportionate to the fold change as a result of TiO2 exposure in pregnant (left) vs normal (right) mice.
Network analysis resulted in an analytical network (auto-expand mode) featured in Figure 5. This network is entirely interactive online and can not be statically saved. A detailed examination of this network will provide insight that informs future mechanistic studies.
FIGURE 5.
Overview of the analytical “auto-expand” network.
DISCUSSION
The exciting and unexpected result that traditionally “inert” TiO2 particles are capable of producing enhanced airway inflammation that predisposes offspring to allergy is consistent with a small body of literature indicating that TiO2 can in fact be pro-allergic in some settings. For example, specially coated TiO2 particles were shown to produce pulmonary inflammation in 8-wk-old male Sprague-Dawley rats (Warheit et al., 2005). Moreover, TiO2 particles were found to induce pulmonary inflammation with activation of antigen-presenting cells and production of certain chemokines in samples from human lung and male Wistar rats (Drumm et al., 1999, Renwick et al., 2004). TiO2 was also associated with increased production of interleukin (IL)-13 by mast cells (Ahn et al., 2005) and were shown to elevate IL-25 and IL-13 production by lung antigen-presenting cells (Kang et al., 2005). While it is typical to use TiO2 particles as an “innocuous” negative control, these publications along with our results indicate that TiO2 is not always immunologically inert. Thus, it is important to reveal pathways involved in enhanced response to TiO2 during pregnancy.
Our genome profiling efforts identified multiple genes differentially upregulated in pregnant but not in normal mice (and v/v) upon exposure to TiO2. A traditional approach in genomic studies suggests individual validation of expression results using real-time PCR. Validation of microarray results is one of the challenges in today’s genomic and epigenomic studies. This is a tedious process requiring substantial time and expense. Although multiple-gene PCR techniques have been available for some time, they require an informed prediction for the selection of genes to be tested (e.g., inflammatory cytokine panel, growth factors collection, etc.). In some studies, it can be a moderately reasonable approach (Gaj et al., 2008); however, for our purpose this was inapplicable.
Here a high-throughput method of PCR analysis based on a custom-ordered RT Profiler RT-PCR array was successfully employed, which was proven to provide a much better signal (Figure 2), at comparable cost, than a preformatted set of primer pairs. Time requirements to set up an array plate include (from the RNA sample) approximately 3 min for pipetting, and about 2.5 h of unattended PCR run, followed by about 5–10 min for analysis, which we found to be at least 2 times less than conventional real-time PCR. As a result of this microarray-informed selection of candidate genes and further dissection of those identified by PCR-array, it was confirmed that TiO2 exposure involves multiple gene upregulation in lung tissue. This upregulation is now characterized in detail in Table 1. Pathway analysis suggested that primarily DCs in the lung were affected, including abnormal expression of factors involved in histamine and PGE2 response (Figures 3 and 4). These molecules are known to participate in allergic processes. They are present at increased levels in blood and tissues of allergic subjects and act on antigen-presenting cells including DCs in a way that skews them toward maintaining the allergic sensitization (Schroeder, 2009; McIlroy et al., 2006). Identification of these pathways is especially valuable given prior data that neonates of TiO2 exposed mothers are pro-allergic (Fedulov et al., 2008) and that there is a crucial role of neonatal DCs in this phenomenon (Fedulov, personal communication). Evidently, TiO2 exposure during pregnancy alters the DC response to mediators of allergy, which may explain why fetal/neonatal DCs become “pro-allergic.” Conventional microchip data analysis did not allow for this observation because of substantial levels of false-positive and false-negative hits resulting in noise levels too high for the MetaCore mapping tool (network analysis not shown). Therefore, supplementation of the pathway analysis input list with biologically relevant candidates increased the power of the study, despite the fact that some of those candidates were found to be falsely negative in the microarray.
Finally, network analysis is now complete (Figure 5) and informs mechanistic studies, in particular the choice of an isolated cell type for genomic profiling, which will further enhance signal to noise ratio.
In summary, it was possible to combine a “blind” genome-wide scan with a hypothesis-driven approach in a custom-designed PCR array to demonstrate pregnancy-dependent enhancement of lung inflammatory responses to TiO2 particles. This was accomplished by including both the “candidate” genes for validation as positively identified by the microarray, and biologically relevant “suspects” that failed to be found in the genomic data.
Acknowledgments
This work was Supported by the National Institutes of Health (ES015425, ES017588 ES 00002 and HL007118).
References
- Ahn MH, Kang CM, Park CS, Park SJ, Rhim T, Yoon PO, Chang HS, Kim SH, Kyono H, Kim KC. Titanium dioxide particle-induced goblet cell hyperplasia: association with mast cells and IL-13. Respir Res. 2005;6:34. doi: 10.1186/1465-9921-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm K, Schindler H, Buhl R, Kustner E, Smolarski R, Kienast K. Indoor air pollutants stimulate interleukin-8-specific mRNA expression and protein secretion of alveolar macrophages. Lung. 1999;177:9–19. doi: 10.1007/pl00007628. [DOI] [PubMed] [Google Scholar]
- Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulov AV, Yang Z, Mariani T, Kobzik L. Pulmonary exposure to titanium dioxide particles is enhanced during pregnancy. Gene Expression Omnibus (GEO) database entry, Record number GSE7475. 2007 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7475.
- Gaj S, Eijssen L, Mensink RP, Evelo CT. Validating nutrient-related gene expression changes from microarrays using RT(2) PCR-arrays. Genes Nutr. 2008;3:153–157. doi: 10.1007/s12263-008-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn MR, Weston A. Application of oligonucleotide microarray technology to toxic occupational exposures. J Toxicol Environ Health A. 2008;71:315–324. doi: 10.1080/15287390701738509. [DOI] [PubMed] [Google Scholar]
- Jayapal M, Melendez AJ. DNA microarray technology for target identification and validation. Clin Exp Pharmacol Physiol. 2006;33:496–503. doi: 10.1111/j.1440-1681.2006.04398.x. [DOI] [PubMed] [Google Scholar]
- Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, Rhim TY, Park CS. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–296. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- McIlroy A, Caron G, Blanchard S, Frémaux I, Duluc D, Delneste Y, Chevailler A, Jeannin P. Histamine and prostaglandin E up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-gamma-induced CXCL10 production by immature human dendritic cells. Immunology. 2006;117:507–516. doi: 10.1111/j.1365-2567.2006.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano M, Mocellin S. Complementary techniques: Validation of gene expression data by quantitative real time PCR. Adv Exp Med Biol. 2007;593:66–73. doi: 10.1007/978-0-387-39978-2_7. [DOI] [PubMed] [Google Scholar]
- Rajeevan MS, Vernon SD, Taysavang N, Unger ER. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J Mol Diagnostics. 2001a;3:26–31. doi: 10.1016/S1525-1578(10)60646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeevan MS, Ranamukhaarachchi DG, Taysavang N, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001b;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–161. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- Shinyashiki M, Eiguren-Fernandez A, Schmitz DA, Di Stefano E, Li N, Linak WP, Cho SH, Froines JR, Cho AK. Electrophilic and redox properties of diesel exhaust particles. Environ Res. 2009;109:239–244. doi: 10.1016/j.envres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Sigaud S, Goldsmith CA, Zhou H, Yang Z, Fedulov A, Imrich A, Kobzik L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol. 2007;223:1–9. doi: 10.1016/j.taap.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: Impact of surface treatments on particle toxicity. Toxicol Sci. 2005;88:514–524. doi: 10.1093/toxsci/kfi331. [DOI] [PubMed] [Google Scholar]