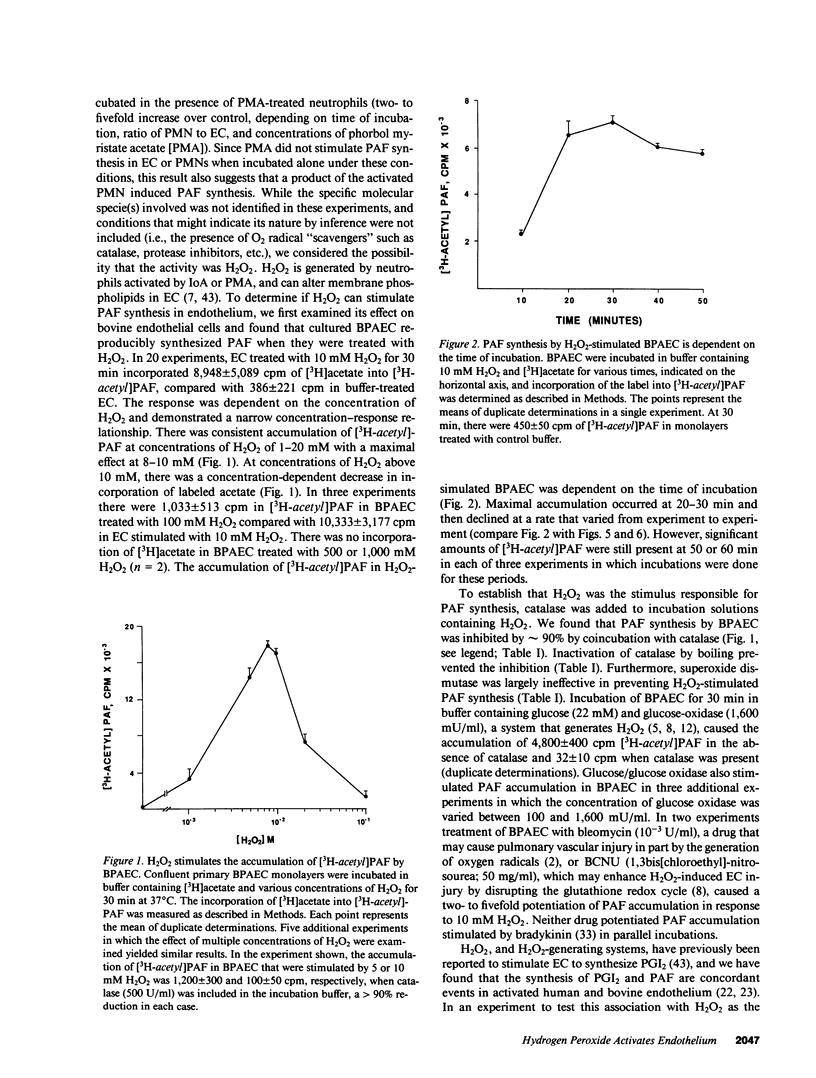
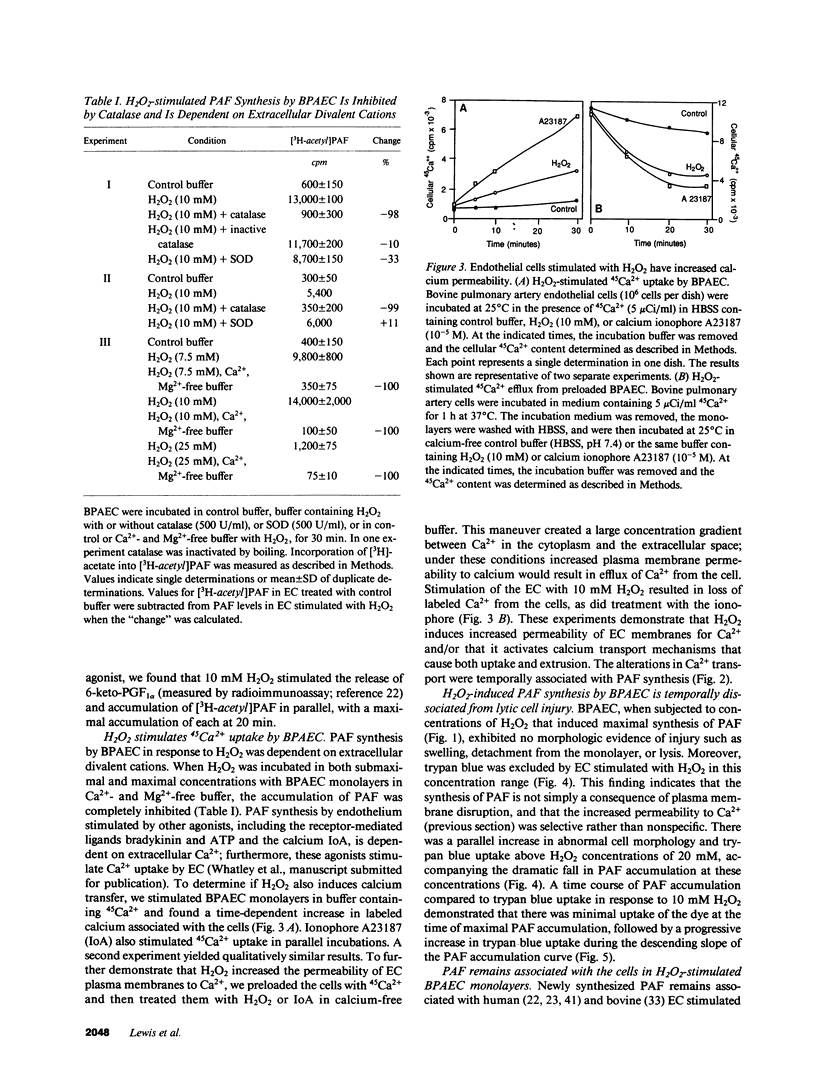
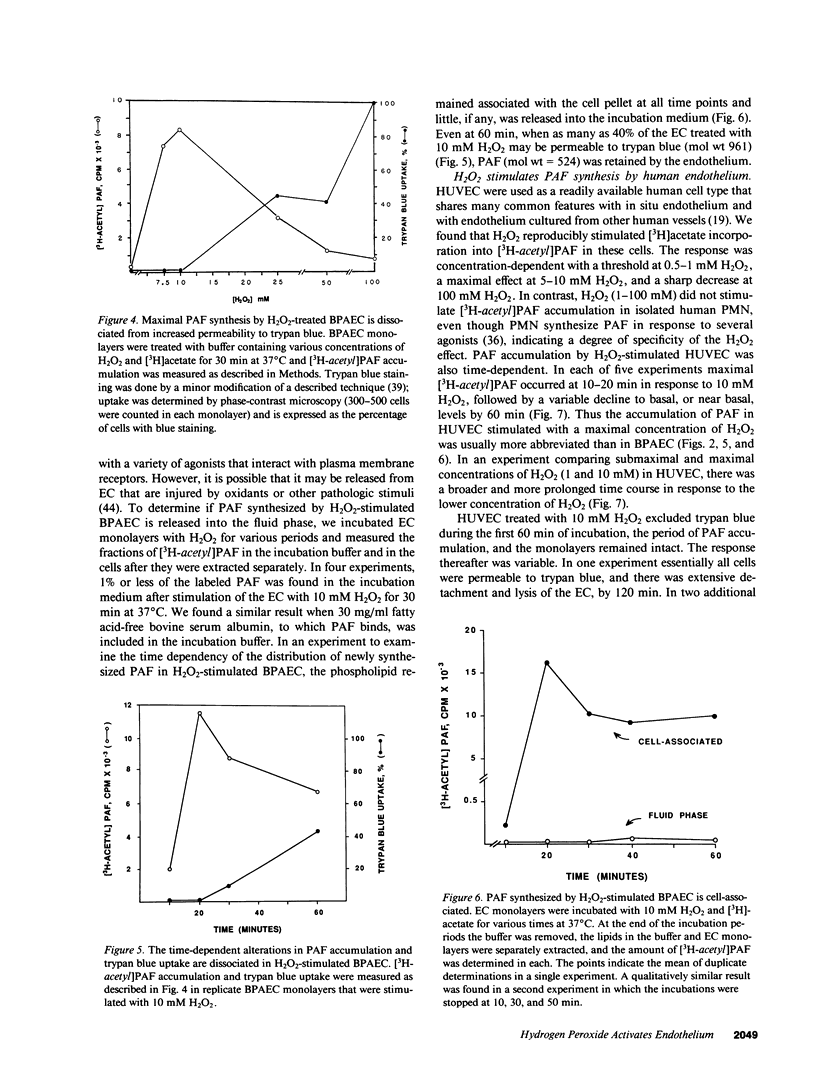
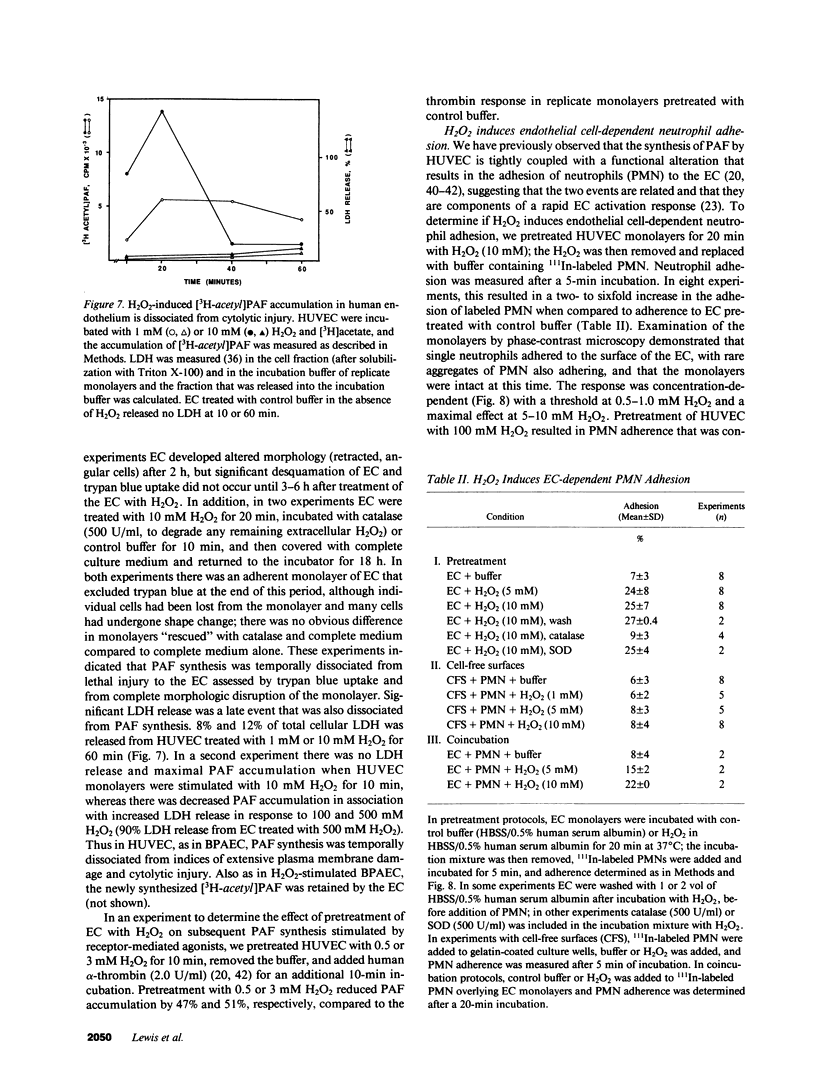
Abstract
Oxidant-induced damage to the intima of pulmonary and systemic vessels is thought to be an important mechanism of injury in a variety of syndromes of vascular damage. Hydrogen peroxide (H2O2) is an active oxygen metabolite that may induce intimal injury by cytolytic attack or by inducing biochemical and functional alterations in the endothelial cells (EC); however, mechanisms involved in noncytolytic perturbation of EC are largely unknown. We found that H2O2 stimulated the synthesis of platelet-activating factor (PAF) by primary cultures of bovine pulmonary artery endothelium (BPAEC) and by human umbilical vein endothelium (HUVEC). In each cell type the incorporation of [3H]acetate into [3H-acetyl]PAF was concentration- and time-dependent and was temporally dissociated from severe plasma membrane disruption and cytolytic cell injury; the newly synthesized PAF remained associated with the EC. H2O2 caused permeabilization of EC to 45Ca2+ and an increase in intracellular Ca2+, suggesting that a transmembrane Ca2+ flux is the signal that initiates PAF synthesis. H2O2 also induced the endothelial cell-dependent adhesion of neutrophils to HUVEC monolayers. This response was rapid, with an onset within minutes and a subsequent time course that paralleled the time course of PAF accumulation, and was dependent on extracellular Ca2+ but not on de novo protein synthesis. These studies demonstrate that H2O2 can induce two rapid activation responses of endothelium, PAF synthesis and EC-dependent neutrophil adhesion, events that may be important in physiologic and pathologic inflammation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977 Oct;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Bozeman P. M., Hoidal J. R., Shepherd V. L. Oxidant-mediated inhibition of ligand uptake by the macrophage mannose receptor. J Biol Chem. 1988 Jan 25;263(3):1240–1247. [PubMed] [Google Scholar]
- Burghuber O., Mathias M. M., McMurtry I. F., Reeves J. T., Voelkel N. F. Lung edema due to hydrogen peroxide is independent of cyclooxygenase products. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):900–905. doi: 10.1152/jappl.1984.56.4.900. [DOI] [PubMed] [Google Scholar]
- Burhop K. E., van der Zee H., Bizios R., Kaplan J. E., Malik A. B. Pulmonary vascular response to platelet-activating factor in awake sheep and the role of cyclooxygenase metabolites. Am Rev Respir Dis. 1986 Sep;134(3):548–554. doi: 10.1164/arrd.1986.134.3.548. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Breviario F., Aglietta M., Sanavio F., Bosia A., Dejana E. Studies on the mechanism of interleukin 1 stimulation of platelet activating factor synthesis in human endothelial cells in culture. Biochim Biophys Acta. 1987 Jan 19;927(1):43–54. doi: 10.1016/0167-4889(87)90064-4. [DOI] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Camussi G., Pawlowski I., Bussolino F., Caldwell P. R., Brentjens J., Andres G. Release of platelet activating factor in rabbits with antibody-mediated injury of the lung: the role of leukocytes and of pulmonary endothelial cells. J Immunol. 1983 Oct;131(4):1802–1807. [PubMed] [Google Scholar]
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220–8226. [PubMed] [Google Scholar]
- Dejana E., Colella S., Languino L. R., Balconi G., Corbascio G. C., Marchisio P. C. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987 May;104(5):1403–1411. doi: 10.1083/jcb.104.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Dobrina A., Patriarca P. Neutrophil-endothelial cell interaction. Evidence for and mechanisms of the self-protection of bovine microvascular endothelial cells from hydrogen peroxide-induced oxidative stress. J Clin Invest. 1986 Aug;78(2):462–471. doi: 10.1172/JCI112598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki Y., Mojarad M., Saga T., Tai H. H., Said S. I. Platelet-activating factor raises airway and vascular pressures and induces edema in lungs perfused with platelet-free solution. Am Rev Respir Dis. 1984 May;129(5):742–746. doi: 10.1164/arrd.1984.129.5.742. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Callahan K. S. Role of hydrogen peroxide in the neutrophil-mediated release of prostacyclin from cultured endothelial cells. J Clin Invest. 1984 Aug;74(2):442–448. doi: 10.1172/JCI111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. L., Prescott S. M., Majerus P. W. The effects of mepacrine and p-bromophenacyl bromide on arachidonic acid release in human platelets. Arch Biochem Biophys. 1982 Apr 15;215(1):237–244. doi: 10.1016/0003-9861(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Jaffe E. A. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- Kenzora J. L., Pérez J. E., Bergmann S. R., Lange L. G. Effects of acetyl glyceryl ether of phosphorylcholine (platelet activating factor) on ventricular preload, afterload, and contractility in dogs. J Clin Invest. 1984 Oct;74(4):1193–1203. doi: 10.1172/JCI111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., O'Flaherty J. T., McCall C. E., Wykle R. L., Bond M. G. Platelet-activating factor effects on pulmonary ultrastructure in rabbits. Exp Mol Pathol. 1983 Feb;38(1):100–108. doi: 10.1016/0014-4800(83)90102-8. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Prescott S. M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. W., O'Flaherty J. T., Wykle R. L. The molecular species distribution of platelet-activating factor synthesized by rabbit and human neutrophils. J Biol Chem. 1984 Dec 10;259(23):14554–14559. [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 May;82(9):3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Platelet-activating factor and leukotriene biosynthesis is inhibited in polymorphonuclear leukocytes depleted of arachidonic acid. J Biol Chem. 1986 Jun 15;261(17):7592–7595. [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Polymorphonuclear leukocyte-dependent plasma leakage in the rabbit skin is enhanced or inhibited by prostacyclin, depending on the route of administration. Am J Pathol. 1986 Jul;124(1):66–73. [PMC free article] [PubMed] [Google Scholar]
- Reinders J. H., de Groot P. G., Dawes J., Hunter N. R., van Heugten H. A., Zandbergen J., Gonsalves M. D., van Mourik J. A. Comparison of secretion and subcellular localization of von Willebrand protein with that of thrombospondin and fibronectin in cultured human vascular endothelial cells. Biochim Biophys Acta. 1985 Mar 21;844(3):306–313. doi: 10.1016/0167-4889(85)90131-4. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem. 1987 Mar 25;262(9):4223–4230. [PubMed] [Google Scholar]
- Starkebaum G., Harlan J. M. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986 Apr;77(4):1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Simon L. M. Lung cell oxidant injury. Enhancement of polymorphonuclear leukocyte-mediated cytotoxicity in lung cells exposed to sustained in vitro hyperoxia. J Clin Invest. 1982 Aug;70(2):342–350. doi: 10.1172/JCI110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Test S. T., Weiss S. J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984 Jan 10;259(1):399–405. [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBenthuysen K. M., McMurtry I. F., Horwitz L. D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 1987 Jan;79(1):265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J., Furcht L. T., Jacob H. S., Moldow C. F. Inflamed fibronectin: an altered fibronectin enhances neutrophil adhesion. Blood. 1983 Nov;62(5):1063–1069. [PubMed] [Google Scholar]
- Voelkel N. F., Chang S. W., McDonnell T. J., Westcott J. Y., Haynes J. Role of membrane lipids in the control of normal vascular tone. Am Rev Respir Dis. 1987 Jul;136(1):214–217. doi: 10.1164/ajrccm/136.1.214. [DOI] [PubMed] [Google Scholar]
- Weisfeldt M. L. Reperfusion and reperfusion injury. Clin Res. 1987 Jan;35(1):13–20. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley R. E., Zimmerman G. A., McIntyre T. M., Prescott S. M. Endothelium from diverse vascular sources synthesizes platelet-activating factor. Arteriosclerosis. 1988 May-Jun;8(3):321–331. doi: 10.1161/01.atv.8.3.321. [DOI] [PubMed] [Google Scholar]
- Whorton A. R., Montgomery M. E., Kent R. S. Effect of hydrogen peroxide on prostaglandin production and cellular integrity in cultured porcine aortic endothelial cells. J Clin Invest. 1985 Jul;76(1):295–302. doi: 10.1172/JCI111960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Klein-Knoeckel D. Human endothelial cells inhibit granulocyte aggregation in vitro. J Immunol. 1986 May 15;136(10):3839–3847. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates neutrophil adherence by an endothelial cell-dependent mechanism: characterization of the response and relationship to platelet-activating factor synthesis. Ann N Y Acad Sci. 1986;485:349–368. doi: 10.1111/j.1749-6632.1986.tb34596.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Renzetti A. D., Hill H. R. Granulocyte adherence in pulmonary and systemic arterial blood samples from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1984 May;129(5):798–804. doi: 10.1164/arrd.1984.129.5.798. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Whatley R. E., McIntyre T. M., Prescott S. M. Production of platelet-activating factor, a biologically active lipid, by vascular endothelial cells. Am Rev Respir Dis. 1987 Jul;136(1):204–207. doi: 10.1164/ajrccm/136.1.204. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Wiseman G. A., Hill H. R. Human endothelial cells modulate granulocyte adherence and chemotaxis. J Immunol. 1985 Mar;134(3):1866–1874. [PubMed] [Google Scholar]


