Abstract
Fructose reacts spontaneously with proteins in the brain to form advanced glycation end products (AGE) that may elicit neuroinflammation and cause brain pathology, including Alzheimer’s disease. We investigated whether fructose is eliminated by oxidative metabolism in neocortex. Injection of [14C]fructose or its AGE-prone metabolite [14C]glyceraldehyde into rat neocortex in vivo led to formation of 14C-labeled alanine, glutamate, aspartate, GABA, and glutamine. In isolated neocortical nerve terminals, [14C]fructose labeled glutamate, GABA, and aspartate, indicating uptake of fructose into nerve terminals and oxidative fructose metabolism in these structures. Hexokinase 1, which channels fructose into glycolysis, was highly expressed, and enzyme activity was similar with fructose or glucose as substrates, whereas the fructose-specific ketohexokinase was weakly expressed. The fructose transporter Glut5 was expressed at only ~4% of the level of neuronal glucose transporter Glut3, suggesting transport across plasma membranes of brain cells as the limiting factor in removal of extracellular fructose. Fructose may be formed from glucose through the polyol pathway. The genes encoding enzymes of this pathway, aldose reductase and sorbitol dehydrogenase, were expressed in rat neocortex. We conclude that fructose is transported into neocortical cells, including nerve terminals, and that it is metabolized and thereby detoxified primarily through hexokinase activity.
Keywords: Alzheimer’s disease, brain metabolism, fructose, Glut5, glyceraldehyde, ketohexokinase
Introduction
Sugars and some of their metabolites may be harmful to cells by reacting non-enzymatically with proteins to form advanced glycation end products (AGEs). AGE formation may alter protein function and turnover and elicit inflammatory responses upon activation of AGE receptors (Srikanth et al., 2011). Fructose, a major constituent of the Western diet (Lustig et al., 2012), and glyceraldehyde, one of several possible fructose metabolites, are highly prone to AGE formation (Suárez et al., 1988; Schalkwijk et al., 2004). Early studies suggested that the brain was protected from fructose in the blood stream by a virtual lack of fructose transport across the blood-brain barrier (Oldendorf, 1971; Thurston et al.,1972), but a recent study found extracellular levels of fructose in the hypothalamic area of the rat (~7 μmol/L) that were similar to serum levels (~9 μmol/L) and that responded to changes in serum levels (Page et al., 2013). Recently, the fructose transporter Glut5 was shown to be expressed in the choroid plexus, which could be an entry point for fructose into the brain (Ueno et al., 2014). Further, feeding rats, especially old rats, with fructose was shown to increase the expression of Glut5 in hippocampus (Shu et al., 2006), suggesting increasing fructose entry into the brain with age. In addition to entry into the brain from the circulation, fructose may be formed within the brain from glucose through the polyol pathway, in which glucose is reduced to sorbitol by aldose reductase. Sorbitol, in turn, is oxidized to fructose by sorbitol dehydrogenase (Lyssiotis et al., 2013). Accumulation of fructose was demonstrated in the brains of hyperglycemic, diabetic rats more than forty years ago (Stewart et al., 1967). More recently, diabetic rats were found to have increased AGE levels in the brain (Aragno et al., 2005). AGE formation in the brain increases with age and appears to be accelerated in brains of patients with Alzheimer’s disease (Choei et al., 2004; Lüth et al., 2005; Srikanth et al., 2011), in which AGEs may be related to formation of amyloid plaques (Fawyer et al., 2012). Long term effects of fructose exposure was recently shown when maternal fructose exposure during gestation and lactation caused changes in forebrain mitochondria of male, aging offspring, affecting oxidative phosphorylation (Mortensen et al., 2014).
With respect to removal of fructose from the brain, studies in cultured brain cells and in brain slices showed that fructose to some degree may support ATP levels and axonal and synaptic activity (Saitoh et al., 1994; Wada et al., 1998; Meakin et al., 2007; Izumi and Zorumski, 2009), suggesting metabolism of fructose as an energy substrate. Some level of metabolism of fructose as an energy substrate has been detected in cultured astrocytes (Wiesinger et al., 1990) and cerebellar granule cells (Maher et al., 1996), and in cerebellar slices (Funari et al., 2005). So far, studies in vivo and studies addressing the handling of fructose by the neocortex, a brain area that is susceptible to Alzheimer pathology, are lacking.
In this study we wished to establish whether and how neocortex metabolizes fructose in vivo. Such metabolism could represent a way of detoxifying fructose. We show here that infusion of 14C-labeled fructose into rat neocortex in vivo causes radiolabeling of amino acids that are associated with glycolysis (alanine) or the tricarboxylic acid (TCA) cycle (glutamate, aspartate, glutamine, GABA). Radiolabeling of glutamate, GABA, and aspartate also occurred when isolated nerve terminals were exposed to 14C-labeled fructose in vitro. Genes associated with fructose transport and oxidative metabolism were expressed in rat neocortex. Taken together, these results point to a capacity for fructose detoxification in neocortex through hexokinase activity, with only minor involvement of the ketohexokinase pathway and formation of AGE-prone glyceraldehyde, but uptake of fructose across the cell membrane was identified as a limiting factor for fructose removal, as Glut5 was expressed at only 4% of the level of the neuronal glucose transporter Glut3.
Materials and methods
Animals and chemicals
Male Wistar rats, 8-week old, ~230 g bodyweight (Taconic, Ry, DK), had free access to food and tap water; air humidity was 50%, and the light/dark cycle was 12 hours. Experiments took place between 10 a.m. and 2 p.m. Animal treatment was in strict accordance with EU guidelines (Directive 2010/63/EU), and experiments were approved by the institutional ethics committee at University of Oslo, Oslo, Norway. D-[U-14C]Fructose, and D-[U-14C]glyceraldehyde, both compounds 150 μCi/μmol, were from American Radiolabeled Chemicals (St Louis, MO, USA). D-[U-14C]glucose, 281 μCi/μmol, was from Amersham Biosciences (Amersham, Buckinghamshire, UK). Prior to experiments, the ethanol-water mixture that the [14C]fructose and [14C]glucose were dissolved in was removed by freeze drying and replaced with sodium chloride, 0.15 mol/L. The [14C]glyceraldehyde, which was dissolved in water, was not freeze-dried to avoid polymerization of glyceraldehyde. Instead, NaCl, 3 mol/L, was added prior to experiments to yield a final concentration of 148 mmol NaCl/L. D-[U-13C]fructose and D-[U-13C]glucose, 98% and 99% 13C enrichment, respectively, were from Omicron (South Bend, IN, USA). 13C Nuclear magnetic resonance (NMR) spectroscopy (see below) of D-[U-13C]fructose and D-[U-13C]glucose did not reveal the presence of other 13C-labeled compounds.
Intracerebral injection of 14C-labeled fructose or glyceraldehyde
Wistar rats were anesthetized with (per kg bodyweight) fentanyl, 0.2 mg, fluanisone, 10 mg, and midazolam, 5 mg, intraperitoneally. Fifty μL of a solution containing lidocaine, 20 mg/mL, and adrenalin, 12.5 μg/mL, were injected subcutaneously over the skull. A skin incision was made, and a hole was drilled 3 mm laterally and 1 mm anteriorly to bregma to allow infusion of 1.67 μL (0.75 μCi) D-[U-14C]fructose or D-[U-14C]glyceraldehyde into the neocortex over 5 minutes (1 mm below dura). The animals were decapitated 1 or 10 minutes after completion of the infusion, the heads were cooled in liquid nitrogen for 7 seconds, and the cortex (10–20 mg) around the injection site was harvested and homogenized in 1 mL perchloric acid, 3.5% (vol/vol), with α-aminoadipate, 50 μmol/L, as an internal amino acid concentration standard.
Intracerebral injection of 13C-labeled fructose or glucose
Wistar rats were anesthetized, mounted in a stereotactic frame, and underwent surgery as described above. A solution of [U-13C]fructose or [U-13C]glucose, 50 mmol/L in NaCl, 140 mmol/L, was infused into neocortex at 0.3 μL/min for 15 minutes. We used this high concentration to reduce differences in dilution for the two substrates: extracellular glucose is ~3 mmol/L in the anesthetized rat (Ronne-Engström et al., 1995), whereas fructose is present at low micromolar concentration (Page et al., 2013). Thereafter the animals were decapitated, heads were cooled in liquid nitrogen for 7 seconds, and the cortex around the injection site was harvested and homogenized in 1 mL perchloric acid, 3.5% (vol/vol). Blood was collected from the severed neck vessels and centrifuged to obtain serum, which was mixed 1:1 with perchloric acid, 3.5%. Proteins were removed by centrifugation, and the supernatants were neutralized with KOH, 9 mol/L. The water phase was lyophilized to dryness and redissolved in D2O with dioxane 0.1% (vol/vol) as an internal concentration standard for 13C NMR spectroscopy.
Isolation of nerve terminals. Exposure to isotopically labeled fructose or glucose
Nerve terminals (synaptosomes) were prepared from rat forebrains as described (Gray and Whittaker, 1962). Briefly, frontal, parietal, and temporal neocortex from male Wistar rats was sampled, avoiding the underlying white matter. A 10% (weight/volume) homogenate in ice-cold sucrose, 0.32 mol/L, approximately 2 mL per rat brain, was made in a glass homogenizer with a teflon pistil (500 rpm, 8 strokes). The homogenate was centrifuged at 1 000 × g for 10 minutes. The supernatant was mixed 1:1 with sucrose, 1.3 mol/L, and centrifuged for 30 minutes at 17 000 × g. The pellet was resuspended in 3 mL sucrose, 0.32 mol/L, and 0.6 mL was layered on top of a discontinuous sucrose gradient consisting of a bottom layer of sucrose, 1.2 mol/L (0.3 mL) and a middle layer of sucrose, 0.8 mol/L (0.3 mL) before centrifugation at 65 000 × g for 20 minutes. Synaptosomes were recovered from the interphase between the two lower sucrose phases. All centrifugations were done at 4°C. Synaptosomes (50 μL) were exposed to 1 μCi [U-14C]fructose (13 μmol/L) or [U-14C]glucose (7 μmol/L) in 450 μL of a buffer saturated with O2 and containing (in mmol/L) NaCl, 140, KCl, 4, NaH2PO4, 1.4, MgCl2, 0.8, CaCl2, 1.2, pH 7.3. Incubation took place at 37°C for 10 or 20 minutes, and the reaction was stopped by adding 500 μL ice-cold perchloric acid, 3.5%, with α-aminoadipate, 25 μmol/L. In other experiments synaptosomes (100 μL) were exposed to [U-13C]fructose or [U-13C]glucose (0.1–10 mmol/L) in 900 μL of the above oxygenated buffer. After 1 or 3 hours at 37°C, samples were centrifuged, and supernatants were lyophilized to dryness and resuspended in D2O with dioxane 0.2% (vol/vol) as an internal concentration standard for 13C NMR spectroscopy.
Amino acid analysis
After homogenization of brain and synaptosome samples in perchloric acid, proteins were removed by centrifugation. Supernatants were neutralized with KOH, 9 mol/L. The water phase was lyophilized to dryness and redissolved in 100 μL double distilled water. Separation and quantitation of amino acids was done by HPLC and fluorescence detection after pre-column derivatization with o-phthaldialdehyde (Morland et al., 2004). Radiolabeling of amino acids was determined by scintillation counting of 1-minute fractions of the HPLC eluate (Morland et al., 2004).
Expression of genes associated with transport and oxidative metabolism of fructose and glyceraldehyde in rat brain
Expression of genes related to transport and oxidative metabolism of fructose and glyceraldehyde was investigated in 6 animals that served as controls in a study on the effects of long-term antiepileptic drug treatment on gene expression in the brain (Hassel et al., 2010). Animals had free access to standard chow and tap water. For 90 consecutive days, twice daily, they received a solution of sucrose, 2 mol/L, and parabenes, 0.1% (weight/volume; as preservative), 3.33 mL/kg bodyweight, through a gastric tube. Four hours after the last dose the animals were deeply anesthetized with phenobarbital and decapitated, and the frontal cortical poles were harvested, as were the hippocampi and samples that comprised pons and medulla oblongata. Results for these latter structures were similar to those for frontal cortex, and most will not be reported here. Total RNA was extracted with Trizol (Invitrogen) and subjected to DNAse digestion with Qiagen RNeasy columns. RNA samples (10 μg) were sent to the National Institute of Neurological Disorders and Stroke-National Institute of Mental Health Affymetrix Microarray Consortium (TGEN, Phoenix, AZ, USA) for analysis with rat GeneChips (genome RA230.2 from Affymetrix, Santa Clara, CA, USA), and GeneChip scanning. 16 000 genes were analyzed for each brain on each chip. The mean value for all the fluorescence intensities of each chip was scaled to 190. The full data set is available on the Gene Expression Omnibus, GEO (http://www.ncbi.nlm.nih.gov/geo/), dataset GSE2880.
Quantitative real-time polymerase chain reaction
Expression of ketohexokinase, hexokinase 1, Glut5, Glut1 and Glut3 was investigated in neocortex and, for comparison, in liver in five male Wistar rats by quantitative real-time polymerase chain reaction (qRT-PCR). RNA isolation (including on-column DNase digestion) and cDNA synthesis were done using Purelink RNA Mini Kit and Superscript Reverse Transcriptase II from Invitrogen (Grand Island, NY, USA). A simplex qRT-PCR was performed with the iQ™5 Multicolor real-time PCR System and iQ™ SYBR Green SuperMix Kit (BIO-RAD, Hercules, CA, USA) to amplify transcripts of interest and endogenous housekeeping controls: hypoxanthine phosphoribosyltransferase 1 (HPRT1), β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), each in duplicate. Fluorescent data were acquired at the 60°C step. Melting curve analysis was used to verify single species PCR products. Cycle thresholds were normalized by subtracting the measured cycle threshold of each gene of interest from the geometric mean of the cycle threshold of the three internal control genes. All experiments had a “no template” negative control. The primers used are summarized in Supplementary Table 1. Data were analyzed by a relative quantification method. For comparison with microarray data qRT-PCR data for brain were normalized to a value 1060 for hexokinase 1.
Hexokinase activity with fructose or glucose
Hexokinase activity with fructose or glucose as substrate was measured at saturating conditions as described (Lowry and Passoneau, 1972). The reaction mixture was (in mmol/L) Tris buffer, 25 (pH 8.1), NADP, 0.5, MgCl2, 2, fructose or glucose, 10, glucose-6-phosphate dehydrogenase, 0.2 U/mL, and phosphoglucoisomerase 0.4 U/mL (Sigma, St Louis, MO, USA). Homogenates, 5% (weight/volume) in sucrose, 0.32 mol/L, were made from frontal cortex (without the underlying white matter). Triton X-100 was added to a final concentration of 0.3 % (vol/vol); the samples were left on ice for 10 minutes and centrifuged at 5 000 × g. Two μL of supernatant were added to 100 μL of reaction mixture. The reaction was started by adding 5 μL ATP, 10 mmol/L. Formation of NADPH was measured spectrophotometrically at 340 nm and 37°C. The reaction was linear with time and added homogenate.
13C Nuclear magnetic resonance spectroscopy
Inverse-gated 13C NMR spectroscopy was performed on a Bruker Avance 600 (Billerica, MA, USA) with 10 240 scans per sample and a pulse angle of 30° (Hassel and Bråthe, 2000). The spectral width was 39.6 kHz, and 64 000 data points were used. Formation of [13C]lactate was calculated from the integrals of the lactate C3 peaks at 20 ppm (Hassel and Bråthe, 2000).
Data presentation and statistics
14C Labeling data are presented as specific activity (dpm/nmol), 13C NMR spectroscopy data are presented as μmol [13C]lactate/L. Gene expression data, whether by microarray or qRT-PCR, are relative values (see above). Groups were compared with Student’s t-test, and a p<0.05 was considered significant. The investigators were not blinded to the identity of the samples.
Results
Radiolabeling of amino acids after intracerebral injection of [U-14C]fructose or [U-14C]glyceraldehyde
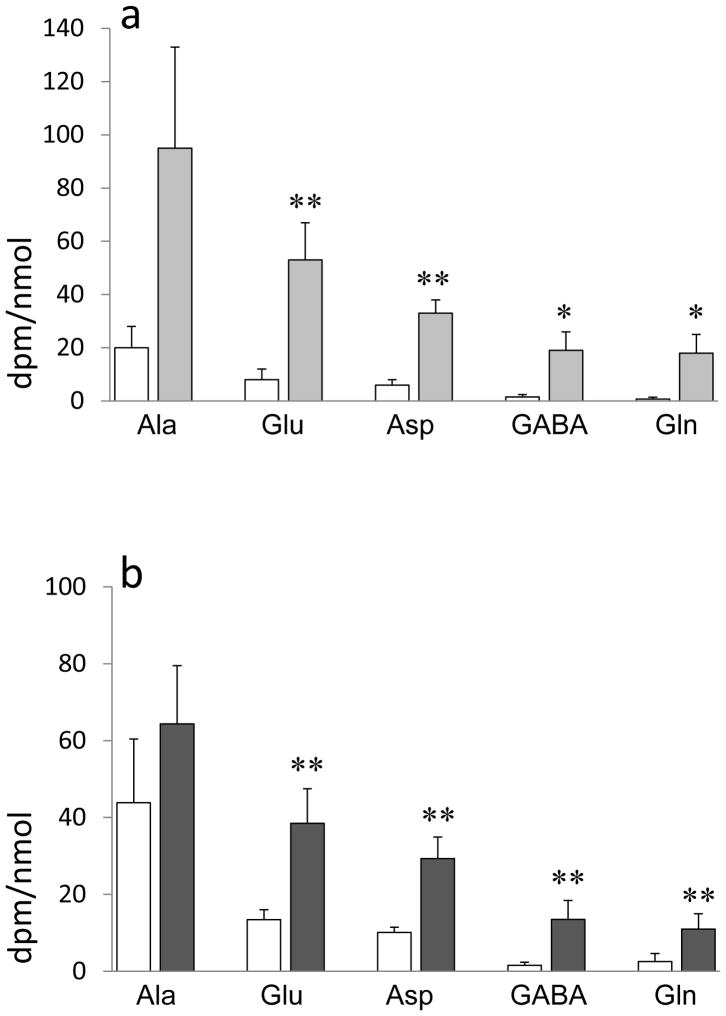
Infusion of [U-14C]fructose into rat neocortex led to 14C-labeling primarily of alanine, glutamate, and aspartate (Fig. 1a). Alanine achieved a higher specific activity than glutamate, presumably reflecting the entry of fructose or its metabolites into glycolysis with subsequent labeling of glycolytic end product pyruvate and hence alanine, before metabolism through the TCA cycle, causing labeling of glutamate and aspartate. The labeling of glutamate and aspartate increased significantly when the survival time after completion of the infusion was increased from 1 to 10 minutes. With the longer survival time GABA and glutamine also became more strongly labeled.
Fig. 1. 14C-Labeling of amino acids from [U-14C]fructose and [U-14C]glyceraldehyde in neocortex in vivo.
Specific activity of amino acids related to glycolysis (alanine) and tricarboxylic acid cycle activity (glutamate, aspartate, glutamine, GABA). Anesthetized rats received an infusion into the frontal cortex of [U-14C]fructose, 0.75 μCi, or [U-14C]glyceraldehyde, 0.35 μCi, over 5 minutes. Animals were decapitated 1 minute (white columns) or 10 minutes (grey columns) after completion of the infusion. Data are dpm/nmol amino acid, means ± SD values; N = 4 per time point. Asterisks: difference from corresponding values obtained at 1 minute; *: p < 0.05; **: p<0.01; Student’s t-test.
Infusion of the fructose metabolite [U-14C]glyceraldehyde into rat neocortex led to a pattern of amino acid labeling that was similar to that obtained with [U-14C]fructose (Fig. 1b). Alanine achieved a higher specific activity than glutamate, reflecting entry of glyceraldehyde into glycolysis before metabolism through the TCA cycle.
13C-Labeling of lactate after intracerebral injection of [U-13C]fructose or [U-13C]glucose
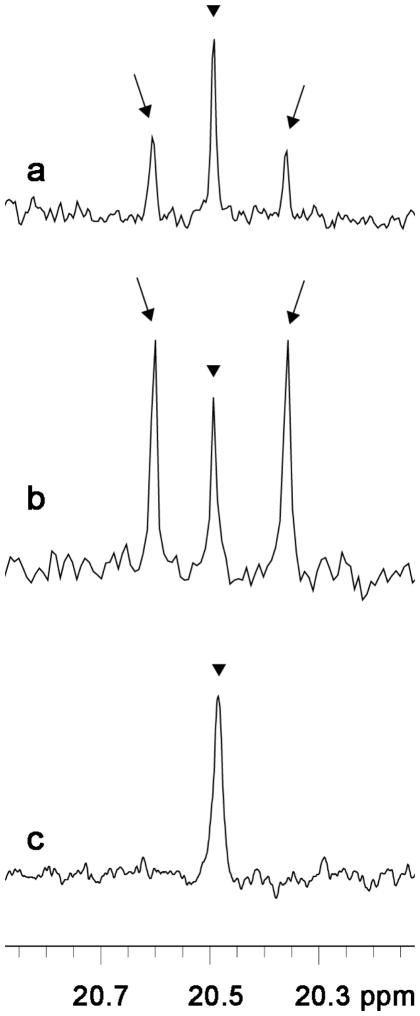
Infusion of [U-13C]fructose or [U-13C]glucose over 15 minutes into rat neocortex led to detectable 13C labeling only of lactate by 13C NMR spectroscopy (Fig. 2). It should be realized that this technique is far less sensitive than radiolabeling techniques (Morris and Bachelard, 2003), explaining why 13C-labeling of amino acids was not seen. 13C-Enrichment of lactate from [U-13C]fructose was 0.6 ± 0.3%, which was 18% of the enrichment achieved with [U-13C]glucose (3.3 ± 0.3%; p=8 ×10−5; N=4 in each group). Serum lactate was not detectably labeled after intracerebral infusion of [U-13C]fructose (Fig. 2) or [U-13C]glucose (not shown), nor was any other serum metabolite.
Fig. 2. 13C-Labeling of lactate from [U-13C]fructose or [U-13C]glucose in neocortex in vivo.
Typical 13C NMR spectra obtained ex vivo after infusion into the frontal cortex of [U-13C]fructose (a), or [U-13C]glucose (b), 50 mmol/L, over 15 minutes. 13C-Labeling of lactate is seen as double peaks (arrows) on each side of the naturally occurring [13C]lactate (arrowheads). Serum lactate (c) was not detectably labeled after intracerebral infusion of [U-13C]fructose. Rats were anesthetized. Ex vivo 13C NMR spectroscopy was performed on protein-free brain and serum extracts. The volume of serum that was extracted was 1 mL. The same absence of lactate labeling in serum was seen after intracerebral infusion of [U-13C]glucose (not shown).
Metabolism of isotopically labeled fructose and glucose by synaptosomes
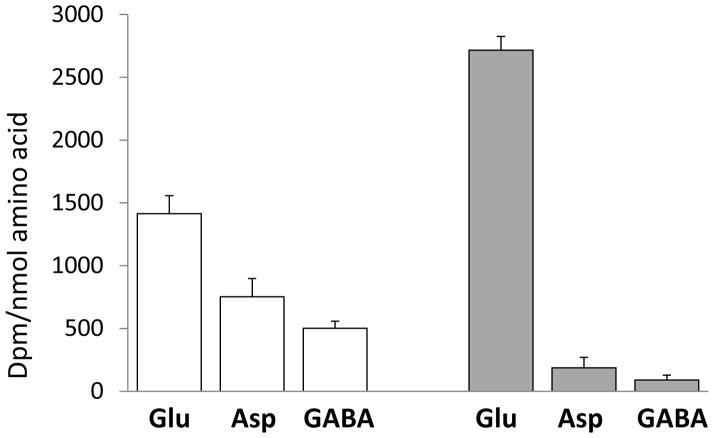
Exposure of purified synaptosomes to [U-14C]fructose or [U-14C]glucose for 10 minutes led to radiolabeling of glutamate and less labeling of aspartate, GABA, and alanine (Fig. 3). [U-14C]Fructose labeled glutamate more strongly, and aspartate and GABA less strongly, than did [U-14C]glucose. Thus, the distribution of label between glutamate, aspartate, and GABA was significantly different in synaptosomes exposed to [U-14C]fructose or [U-14C]glucose, as reflected in an aspartate/glutamate labeling ratio of 0.07±0.03 and 0.53±0.05 and a GABA/glutamate labeling ratio of 0.03±0.01 and 0.36±0.01 for [U-14C]fructose- and [U-14C]glucose-exposed synaptosomes, respectively (p<10−5). None of the substrates labeled glutamine, as would be expected in this preparation of nerve terminals, because glutamine synthetase is expressed in glia and not in neurons (Martinez-Hernandez et al., 1977). Exposure of synaptosomes to [U-14C]fructose for 20 minutes did not significantly change the degree of radiolabeling of glutamate (3 300 ± 900 dpm/nmol), but the labeling of aspartate increased ten-fold (to 1 630 ± 10 dpm/nmol), a sign that more radiolabel had traversed the TCA cycle to the oxaloacetate step where oxaloacetate equilibrates with aspartate.
Fig. 3. Metabolism of [14C]glucose and [14C]fructose by isolated nerve endings.
Isolated nerve terminals (synaptosomes) were prepared from neocortex of Wistar rats and incubated in a Krebs solution containing 1 μCi [U-14C]glucose (white bars) or [U-14C]fructose (grey bars). The concentration of [U-14C]glucose and [U-14C]fructose in the incubation media was 7 and 13 μmol/L, respectively. Radiolabeling of amino acids is expressed as specific activity (dpm/nmol), mean+SD values; N=4 per value. Note the difference in label distribution in glutamate, aspartate, and GABA obtained with [U-14C]glucose and [U-14C]fructose.
To assess the ability of synaptosomes to clear extracellular fructose at higher concentrations than those obtained with radiolabeled fructose (13 μmol/L), we incubated synaptosomes with [U-13C]fructose or [U-13C]glucose at 0.1, 1, 3, or 10 mmol/L for 1 or 3 hours. Metabolism was assessed from the formation of 13C-labeled lactate in the medium by 13C NMR spectroscopy. Lactate formation was not detected from [U-13C]fructose at any concentration or time point, but it was readily detected from [U-13C]glucose at ≥1 mmol/L after 1 and 3 hours, [13C]lactate being 41 ± 14 and 199 ± 8 μmol/L, respectively, after 1 and 3 hours of incubation with [U-13C]glucose, 3 mmol/L (N=3 per time point).
Expression of genes associated with oxidative fructose metabolism in rat neocortex
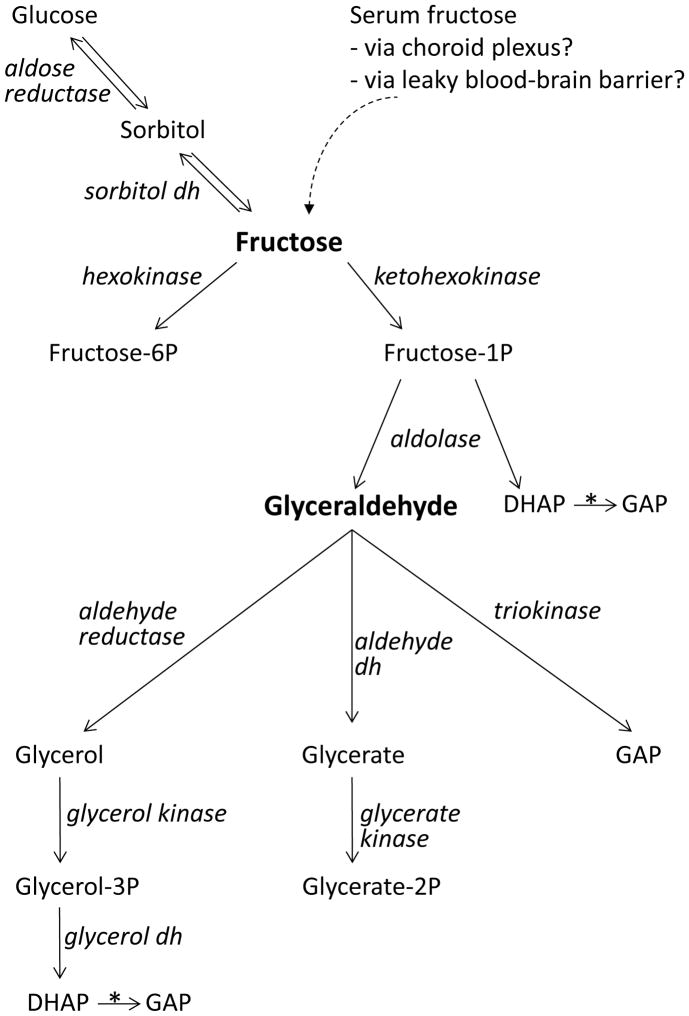
Fructose may be formed in the brain from glucose through the polyol pathway (Stewart et al., 1967; Fig. 4). The first and rate-limiting enzyme of the polyol pathway, aldose reductase, which reduces glucose to sorbitol, was expressed in rat neocortex (Table 1). Sorbitol may be oxidized to fructose by sorbitol dehydrogenase, which was also expressed (Table 1).
Fig. 4. Pathways for oxidative metabolism of fructose and glyceraldehyde.
Scheme showing the origin of fructose in the brain and the enzymatic metabolism of fructose along three alternative pathways: via hexokinase to fructose-6-phosphate, an intermediate of glycolysis; via sorbitol dehydrogenase to sorbitol and hence via aldose reductase to glucose (the polyol pathway); via ketohexokinase to fructose-1-phosphate, which is cleaved by an aldolase into dihydroxyacetone-phosphate (DHAP) and glyceraldehyde. While DHAP is a glycolytic intermediate that is converted into glyceraldehyde-3-phosphate (GAP) by triose phosphate isomerase (*), glyceraldehyde may be metabolized along three different pathways: via aldehyde reductase to glycerol, which is phosphorylated by glycerol kinase to glycerol-3-phosphate, which in turn is converted into glycolytic intermediate DHAP by glycerol-3-phosphate dehydrogenase; via aldehyde dehydrogenase to glycerate, which is converted into glycolytic intermediate 2-phosphoglycerate; via triokinase to glycolytic intermediate glyceraldehyde-3-phosphate. Influx of fructose into the brain via the choroid plexus or openings in the blood brain barrier may be inferred from previous studies (see Introduction), but has not been directly demonstrated. dh: dehydrogenase.
Table 1.
Expression of genes related to fructose metabolism in rat neocortex
| Gene | Function | Neocortex mRNA by microarray | Neocortex mRNA by PCR | Liver mRNA by PCR | |
|---|---|---|---|---|---|
| Aldose reductase | Glucose → Sorbitol | 730 ± 90 | |||
| Sorbitol dehydrogenase | Sorbitol → Fructose | 170 ± 10 | |||
| Ketohexokinase | Fructose → Fructose-1P | n.s.* | 0.042 ± 0.016 | 638 ± 36 | |
| Hexokinase 1 | Fructose → Fructose-6P | 1060 ± 70 | 1060 ± 80** | 13 ± 2 | |
| Aldolase A |
|
Fructose-1,6P → DHAP+GA3P or Fructose-1P → DHAP + GA |
8920 ± 330 | ||
| Aldolase B | n.d. | ||||
| Aldolase C | 5080 ± 870 | ||||
| Glut5 | Fructose transporter | n.r.e. | 43 ± 4 | 27 ± 5 | |
| Glut1 | Glucose transporter | 420 ± 50 | 793 ± 63 | 18 ± 3 | |
| Glut3 | Glucose transporter | 440 ± 50 | 1150 ± 23 | 1.9 ± 0.3 |
mRNA from frontal cortex (neocortex) of six male Wistar rats was subjected to microarray analysis (rat GeneChips, genome RA230.2, Affymetrix). The values are fluorescence intensities in arbitrary units; the mean expression value for all 16 000 detected genes was 190. Brain and liver total RNA was prepared from each of five Wistar male rats and subject to qRT-PCR. Values are normalized to the mean of the expression level for three housekeeping genes (HPRT1, β-actin and GAPDH). Data are mean ± SD values. n.s.: not significantly different from zero; n.d.: not detectable; n.r.e. not reliably expressed at 37 ± 9. DHAP: dihydroxyacetone phosphate, GA3P: glyceraldehyde-3 phosphate: GA: glyceraldehyde.
reliably expressed only in 3 out of 6 samples, but reliably expressed in all 7 brain stem samples at 63 ± 17.
For comparison with the microarray data, qRT-PCR data for the brain samples were normalized to a value of 1060 for hexokinase 1.
Hexokinase 1, which converts fructose into fructose-6-phosphate, was expressed in neocortex (Table 1). In a hexokinase enzyme assay, the maximal activity in frontal neocortex with fructose as substrate was 31 ± 2 nmol/mg tissue/min. (mean ± SD values; N = 3). The activity with glucose as substrate was 45 ± 4 nmol/mg tissue/min. These results show that hexokinase may metabolize fructose efficiently; they are similar to previously published in vitro values (Meakin et al., 2007), and much higher than glycolytic activity in vivo, which is approximately 0.5 nmol/mg tissue/min in rat brain (Duarte and Gruetter, 2013).
The fructose-specific hexokinase, ketohexokinase, which converts fructose into fructose-1-phosphate, was reliably expressed only in 3 out of 6 neocortical samples at a low fluorescence level of 42 ± 5, as could be detected in the microarray analysis. But not being reliably detected in three samples, it was not significantly expressed in the group as a whole; however, ketohexokinase was reliably expressed in all seven samples of pons + medulla oblongata at a fluorescence level of 63 ± 17. qRT-PCR confirmed the expression of ketohexokinase in neocortex (in 5 out of 5 samples), but at a very low level (Table 1). In liver, in contrast, ketohexokinase was expressed much more strongly than hexokinase 1 (Table 1). Expression of glucokinase, a main hexokinase in liver, was not measured.
Cleavage of fructose-1-phosphate into dihydroxyacetone phosphate and glyceraldehyde may occur with any aldolase (Araki et al., 2004). Aldolases A and C were highly expressed, whereas aldolase B, which has a relative preference for fructose-1-phosphate (Pezza et al., 2007; see Discussion), was not detectably expressed in rat neocortex (Table 1).
Expression of genes associated with fructose transport
Class II hexose transporters (Glut 5, 7, 9, and 11) recognize fructose as substrate (Augustin, 2010). The detected fluorescence value for the fructose transporter Glut5 (Slc2a5) was 37 ± 9, but it was flagged ‘absent’ by the microarray analysis program. However, qRT-PCR confirmed the expression of Glut5 in rat neocortex at approximately 4% of the level of the (neuronal) glucose transporter Glut3 (Slc2a3; Table 1). Glut9 (Slc2a9) was not expressed (fluorescence values 4 ± 5, not different from zero), whereas Glut7 (Slc2a7) and Glut11 (Slc2a11) are lacking in rat (Li et al., 2004; Scheepers et al. 2005). The hexose transporter Glut8 (Slc2a8), which recognizes fructose, was expressed in neocortex at a fluorescence level of 205 ± 39. However, a class III hexose transporter, Glut8 is located intracellularly, and it is not known whether it may translocate to the plasma membrane (Augustin, 2010). In addition, Glut2 (Slc2a2) and the sodium-dependent Naglt1 may transport fructose (Augustin, 2010), but neither was expressed in rat neocortex. In contrast, glucose transporters Glut1 and Glut3 (Slc2a1 and 3, respectively), which do not transport fructose (Takakura et al., 1991; Seatter et al., 1998), were highly expressed (Table 1).
In liver, qRT-PCR showed that, unlike the situation in neocortex, Glut5 was expressed at a level similar to Glut1 and more strongly than Glut3. Expression of Glut2, the main glucose transporter in the liver, was not measured.
Expression of genes associated with oxidative glyceraldehyde metabolism in neocortex
The expression of ketohexokinase, although low, in neocortex may allow formation of glyceraldehyde, which is highly prone to AGE formation with proteins (Suárez et al., 1988), from fructose. Three different pathways may be active in the metabolism of glyceraldehyde into glycolytic intermediates (Fig. 4). First, triokinase, which catalyzes phosphorylation of glyceraldehyde to glyceraldehyde-3-phosphate was expressed at low, but detectable levels (Table 2), whereas glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase was highly expressed. Second, three isoforms of aldehyde dehydrogenase, which may convert glyceraldehyde into glycerate (Leicht et al., 1978), were expressed in neocortex (Table 2). Glycerate may be phosphorylated by glycerate kinase, which was expressed at low levels. The product, 2-phosphoglycerate, is the substrate of the glycolytic enzyme enolase. Enolase 1 and 2 were highly expressed (Table 2). Third, aldehyde reductase, which reduces glyceraldehyde to glycerol (Vander Jagt et al., 1992); was highly expressed, whereas glycerol kinase, which catalyzes conversion of glycerol into glycerol-3-phosphate was expressed at low levels (Table 2). Glycerol-3-phosphate is oxidized to dihydroxyacetone phosphate by glycerol-3-phosphate dehydrogenase. The NAD+-dependent and the FAD-dependent isoforms were both expressed in rat neocortex. Triose phosphate isomerase, which catalyzes conversion of dihydroxyacetone phosphate into glyceraldehyde-3-phosphate, was highly expressed (Table 2).
Table 2.
Expression levels of genes associated with metabolism of D-glyceraldehyde in rat hippocampus.
| Gene | mRNA levels |
|---|---|
| Triokinase → glyceraldehyde-3P | 34 ± 8 |
| Glyceraldehyde-3P dh* | 8400 ± 630 |
| Aldehyde dh 1 → glycerate | 205 ± 60 |
| Aldehyde dh 2 → glycerate | 290 ± 30 |
| Aldehyde dh 3 → glycerate | 240 ± 40 |
| Glycerate kinase → glycerate-2P | 23 ± 9 |
| Enolase 1* | 3180 ± 200 |
| Enolase 2* | 2500 ± 480 |
| Aldehyde reductase → glycerol | 3610 ± 600 |
| Glycerol kinase → glycerol-3P | 88 ± 14 |
| Glycerol-3P dh NAD+ → dihydroxyacetone-P | 550 ± 25 |
| Glycerol-3P dh FAD → dihydroxyacetone-P | 110 ± 20 |
| Triosephosphate isomerase* | 4680 ± 720 |
mRNA from frontal cortex (neocortex) was subjected to microarray analysis (rat GeneChips, genome RA230.2, Affymetrix). Data are fluorescence intensities; mean ± SD values; N = 7. Abbreviation: dh: dehydrogenase. In the left column the D-glyceraldehyde-derived products of the various enzyme-mediated reactions are indicated.
These enzymes belong to the glycolytic pathway, therefore their products are not shown.
Thus, glyceraldehyde-reducing or -oxidizing enzymes were expressed at intermediate to high levels, whereas the kinases that would phosphorylate glyceraldehyde or its metabolites glycerate or glycerol to allow entry into the glycolytic pathway, were expressed at low, but detectable levels.
Discussion
Fructose is metabolized in neocortex primarily through hexokinase activity
This study shows that neocortical cells are able to remove extracellular fructose by uptake across the plasma membrane and that intracellular fructose undergoes oxidative metabolism, as could be seen from the 14C labeling of amino acids after intracerebral injection of [14C]fructose. 14C-Labeling of alanine reflects entry of [14C]fructose into some stage of the glycolytic pathway with labeling of glycolytic end product pyruvate and hence alanine. 14C Labeling of glutamate and aspartate reflects subsequent oxidative metabolism through the TCA cycle with formation of α-ketoglutarate and oxaloacetate, from which glutamate and aspartate are formed, respectively. Active metabolism of fructose in nerve terminals was evident from the 14C labeling of glutamate, GABA, and aspartate in synaptosomes exposed to [14C]fructose.
Several pathways may be operative in the metabolism of fructose. First, phosphorylation of fructose to fructose-6-phosphate by hexokinase would channel fructose into glycolysis (Fig. 4). Hexokinase activity with fructose as substrate was similar to that with glucose and probably accounted for a major part of the metabolism of [14C]fructose in the present study. Second, reversal of the polyol pathway, the genes of which were expressed in rat neocortex, could result in formation of glucose from fructose (Fig. 4). However, the high Km for sorbitol in the sorbitol dehydrogenase reaction (480 mmol/L; O’Brien et al., 1983) could mean that this is a little travelled pathway with sorbitol at 20 μmol/L, which is the concentration found in rat brain (Stewart et al., 1967). Third, fructose may be phosphorylated to fructose-1-phosphate by ketohexokinase (Fig. 4). Ketohexokinase activity has been found in whole human brain homogenates at ~0.2 nmol/mg tissue wet weight × min−1, or <10% of the activity in liver (Bais et al., 1985). Previous studies showed expression of ketohexokinase in rat cerebellar Purkinje cells (Funari et al., 2005) and in mouse optic nerve, but not in mouse neocortex (Meakin et al., 2007). We found that the enzyme was expressed at a very low level in rat neocortex. Thus, the ketohexokinase pathway is probably active, but of minor quantitative importance in rat neocortex. Aldolases A and C were highly expressed in rat neocortex. These isozymes may cleave both fructose-1-phosphate and fructose-1,6-bisphosphate, but have a preference for the latter substrate (Km for fructose-1,6-bisphosphate ≤10 μmol/L; Km for fructose-1-phosphate >10 mmol/L; Araki et al., 2004). Aldolase B, which has a relative preference for fructose-1-phosphate (Km 0.7 mmol/L; Pezza et al., 2007), was not expressed in rat brain, which agrees with the notion that the ketohexokinase pathway is of minor quantitative importance in rat neocortex. However, fructose-1-phosphate cleavage (aldolase) activity has been detected in human brain, although at low level (Bais et al., 1985). From the above it is clear that some formation of glyceraldehyde from fructose may occur in neocortex. As shown in this study, however, neocortex expresses several genes encoding enzymes that would channel glyceraldehyde into the glycolytic pathway. Moreover, neocortical cells are able to eliminate glyceraldehyde by uptake and oxidative metabolism, as could be seen from the conversion of [14C]glyceraldehyde into alanine and glutamate after intracerebral injection in the present study.
Fructose is transported by Glut5 in the brain
The finding that [14C]fructose is metabolized in neocortex points to the presence of a fructose transporter in neocortical cells. Glut5 (Slc2a5), which is the prototype fructose transporter (Augustin, 2010), was expressed at approximately 4% of the level of the neuronal glucose transporter Glut3 and probably accounted for the cellular uptake of fructose in the present study, because other cell membrane-associated transporters that recognize fructose were not expressed (Glut2, Glut7, Glut9, Glut11, and Naglt1). Whereas Glut5 was only weakly expressed in brain relative to Glut1 and Glut3, hexokinase metabolized fructose and glucose at similar rates. Therefore, transport across the plasma membrane was probably rate-limiting for neocortical fructose clearance, a conclusion that is consistent with the finding that lactate formation from [13C]fructose was much lower than that from [13C]glucose, both after intracerebral infusion in vivo and in isolated nerve terminals; the latter formed lactate from [13C]glucose, but not from [13C]fructose present at millimolar concentrations. However, the lack of lactate formation from [13C]fructose in nerve terminals may have been influenced by lower levels of glycolytic intermediates that act as positive modulators of glycolytic enzymes, e.g. fructose-1,6-bisphosphate, which activates pyruvate kinase (Imamura et al., 1972).
Expression of Glut5 has previously been reported in rat hippocampus, cerebellum, hypothalamus, choroid plexus, and whole brain preparations (Rand et al., 1993; Funari et al., 2005; Shu et al., 2006; Page et al., 2013; Ueno et al., 2014); in some studies expression was seen in microglia (Payne et al., 1997; Shu et al., 2006). The finding that isolated nerve terminals metabolized [14C]fructose points to the presence of Glut5 in these structures. Experiments with brain slices suggested that fructose is taken up by glial cells and not by neurons (Izumi and Zorumski, 2009). This suggestion seemed to agree with the finding that fructose to some degree supported the growth of cultured astrocytes, but not of neurons (Wiesinger et al., 1990). However, expression of Glut5 by Purkinje cells (Funari et al., 2005) and oxidative metabolism of [14C]fructose by cultured cerebellar granule cells (Maher et al., 1996) and isolated neocortical nerve terminals (this study) point to metabolism of fructose at least by some neuronal populations or cellular compartments. This notion is strengthened by the finding that the pattern of amino acid radiolabeling from [14C]fructose, with a specific activity of alanine > glutamate > glutamine, the same pattern as that obtained with [14C]glucose (Gaitonde et al., 1974), the main neuronal energy substrate.
A role for nerve terminals in clearing fructose from the extracellular space
Isolated nerve terminals metabolized extracellular [14C]fructose at a concentration (13 μmol/L) that is similar to concentrations measured extracellularly in the hypothalamic area in the rat (~7 μmol/L; Page et al., 2013). This finding suggests a physiological role for nerve terminals in clearing fructose from the extracellular space in neocortex. The greater labeling of glutamate relative to GABA in nerve terminals exposed to [14C]fructose rather than [14C]glucose suggests a greater capacity for fructose removal by nerve terminals of glutamatergic than GABAergic neurons. The greater labeling of aspartate from [14C]glucose than from [14C]fructose agrees with this interpretation, since aspartate is concentrated in GABAergic neurons (Ottersen and Storm-Mathisen, 1985; Hassel et al., 1995). In this context it should be pointed out that the transport activities of the fructose transporter Glut5 and the neuronal glucose transporter Glut3 cannot be directly compared from the data obtained with [14C]fructose and [14C]glucose (see Fig. 3), because we lack kinetic data, specifically kcat values, for the two transporters. Although earlier studies suggested a lack of fructose transport across the blood-brain barrier (Oldendorf, 1971; Thurston et al.,1972), new studies suggest that fructose does enter the brain, e.g. at sites with a less restrictive blood-brain barrier, such as the hypothalamus (Page et al., 2013), through the choroid plexus (Ueno et al., 2014), and maybe more so in older than in younger individuals (Shu et al., 2006). In addition, neocortex may be exposed to fructose formed from glucose through the polyol pathway (Stewart et al., 1967). Removal of extracellular fructose may be important in the cerebral cortex, as fructose may cause AGE formation. Extracellular AGE formation with β-amyloid as the protein substrate may have a role in the development of amyloid plaques in the brain (Fawyer et al., 2012). Clearance of extracellular fructose by nerve terminals may therefore be a detoxification mechanism to reduce AGE formation and protect neocortex against the development of Alzheimer type pathology.
Supplementary Material
Abbreviations used
- AGE
advanced glycation end products
- GAPDH
glycerol 3-phosphate dehydrogenase
- HPRT1
hypoxanthine phosphoribosyltransferase 1
- NMR
nuclear magnetic resonance
- qRT-PCR
quantitative real-time polymerase chain reaction
- TCA cycle
tricarboxylic acid cycle
Footnotes
Disclosure
None of the authors has any conflict of interest relevant to this manuscript.
Supplementary information on primers used for qRT-PCR is available at the Journal’s homepage.
References
- Aragno M, Mastrocola R, Medana C, Restivo F, Catalano MG, Pons N, Danni O, Boccuzzi G. Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology. 2005;146:5561–5567. doi: 10.1210/en.2005-0712. [DOI] [PubMed] [Google Scholar]
- Arakaki TL, Pezza JA, Cronin MA, Hopkins CE, Zimmer DB, Tolan DR, Allen KN. Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function. Protein Sci. 2004;13:3077–3084. doi: 10.1110/ps.04915904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- Bais R, James HM, Rofe AM, Conyers RA. The purification and properties of human liver ketohexokinase. A role for ketohexokinase and fructose-bisphosphate aldolase in the metabolic production of oxalate from xylitol. Biochem J. 1985;230:53–60. doi: 10.1042/bj2300053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, Kikuchi S, Saito T. Glyceraldehyde-derived advanced glycation end products in Alzheimer’s disease. Acta Neuropathol. 2004;108:189–193. doi: 10.1007/s00401-004-0871-x. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Gruetter R. Glutamatergic and GABAergic energy metabolism measured in the rat brain by 13C NMR spectroscopy at 14.1 T. J Neurochem. 2013;126:579–590. doi: 10.1111/jnc.12333. [DOI] [PubMed] [Google Scholar]
- Fawver JN, Schall HE, Petrofes-Chapa RD, Zhu X, Murray IV. Amyloid-β metabolite sensing: biochemical linking of glycation modification and misfolding. J Alzheimers Dis. 2012;30:63–73. doi: 10.3233/JAD-2012-112114. [DOI] [PubMed] [Google Scholar]
- Funari VA, Herrera VL, Freeman D, Tolan DR. Genes required for fructose metabolism are expressed in Purkinje cells in the cerebellum. Brain Res Mol Brain Res. 2005;142:115–122. doi: 10.1016/j.molbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: An electron microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Gaitonde MK, Nixey RW, Sharman IM. The effect of deficiency of thiamine on the metabolism of [U-14C]glucose and [U-14C]ribose and the levels of amino acids in rat brain. J Neurochem. 1974;22:53–61. doi: 10.1111/j.1471-4159.1974.tb12178.x. [DOI] [PubMed] [Google Scholar]
- Hassel B, Westergaard N, Schousboe A, Fonnum F. Metabolic differences between primary cultures of astrocytes and neurons from cerebellum and cerebral cortex. Effects of fluorocitrate. Neurochem Res. 1995;20:413–420. doi: 10.1007/BF00973096. [DOI] [PubMed] [Google Scholar]
- Hassel B, Bråthe A. Cerebral metabolism of lactate in vivo: evidence for neuronal pyruvate carboxylation. J Cereb Blood Flow Metab. 2000;20:327–336. doi: 10.1097/00004647-200002000-00014. [DOI] [PubMed] [Google Scholar]
- Hassel B, Shaw R, Taubøll E, Gjerstad L, Dingledine R. Region-specific changes in gene expression in rat brain after chronic treatment with levetiracetam or phenytoin. Epilepsia. 2010;51:1714–1720. doi: 10.1111/j.1528-1167.2010.02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Taniuchi K, Tanaka T. Multimolecular forms of pyruvate kinase. II Purification of M2 -type pyruvate kinase from Yoshida ascites hepatoma 130 cells and comparative studies on the enzymological and immunological properties of the three types of pyruvate kinases, L, M1, and M2. J Biochem (Tokyo) 1972;72:1001–1015. doi: 10.1093/oxfordjournals.jbchem.a129962. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Glial-neuronal interactions underlying fructose utilization in rat hippocampal slices. Neuroscience. 2009;161:847–854. doi: 10.1016/j.neuroscience.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht W, Heinz F, Freimüller B. Purification and characterization of aldehyde dehydrogenase from bovine liver. Eur J Biochem. 1978;83:189–196. doi: 10.1111/j.1432-1033.1978.tb12083.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236–G242. doi: 10.1152/ajpgi.00396.2003. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passoneau JV. A Flexible System of Enzymatic Analysis. Academic Press; New York: 1972. pp. 174–177. [Google Scholar]
- Lustig RH, Schmidt LA, Brindis CD. Public health: The toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- Lüth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Webster J, Arendt T, Münch G. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb Cortex. 2005;15:211–220. doi: 10.1093/cercor/bhh123. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Cantley LC. Metabolic syndrome: F stands for fructose and fat. Nature. 2013;502:181–182. doi: 10.1038/502181a. [DOI] [PubMed] [Google Scholar]
- Maher F, Davies-Hill TM, Simpson IA. Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J. 1996;315:827–831. doi: 10.1042/bj3150827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Meakin PJ, Fowler MJ, Rathbone AJ, Allen LM, Ransom BR, Ray DE, Brown AM. Fructose metabolism in the adult mouse optic nerve, a central white matter tract. J Cereb Blood Flow Metab. 2007;27:86–99. doi: 10.1038/sj.jcbfm.9600322. [DOI] [PubMed] [Google Scholar]
- Morland C, Boldingh KA, Iversen EG, Hassel B. Valproate is neuroprotective against malonate toxicity in rat striatum: an association with augmentation of high-affinity glutamate uptake. J Cereb Blood Flow Metab. 2004;24:1226–1234. doi: 10.1097/01.WCB.0000138666.25305.A7. [DOI] [PubMed] [Google Scholar]
- Morris P, Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR Biomed. 2003;16:303–312. doi: 10.1002/nbm.844. [DOI] [PubMed] [Google Scholar]
- Mortensen OH, Larsen LH, Orstrup LK, Hansen LH, Grunnet N, Quistorff B. Developmental programming by high fructose decreases phosphorylation efficiency in aging offspring brain mitochondria, correlating with enhanced UCP5 expression. J Cereb Blood Flow Metab. 2014;34:1205–1211. doi: 10.1038/jcbfm.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MM, Schofield PJ, Edwards MR. Polyol-pathway enzymes of human brain. Partial purification and properties of sorbitol dehydrogenase. Biochem J. 1983;211:81–90. doi: 10.1042/bj2110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson I, Mattice L, Davies P. Glucose transporter Glut 5 expression in microglial cells. Glia. 1997;21:327–331. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pezza JA, Stopa JD, Brunyak EM, Allen KN, Tolan DR. Thermodynamic analysis shows conformational coupling and dynamics confer substrate specificity in fructose-1,6-bisphosphate aldolase. Biochemistry. 2007;46:13010–13018. doi: 10.1021/bi700713s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand EB, Depaoli AM, Davidson NO, Bell GI, Burant CF. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993;264:G1169–G1176. doi: 10.1152/ajpgi.1993.264.6.G1169. [DOI] [PubMed] [Google Scholar]
- Ronne-Engström E, Carlson H, Liu Y, Ungerstedt U, Hillered L. Influence of perfusate glucose concentration on dialysate lactate, pyruvate, aspartate, and glutamate levels under basal and hypoxic conditions: a microdialysis study in rat brain. J Neurochem. 1995;65:257–262. doi: 10.1046/j.1471-4159.1995.65010257.x. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Okada Y, Nabetani M. Effect of mannose, fructose and lactate on the preservation of synaptic potentials in hippocampal slices. Neurosci Lett. 1994;171:125–128. doi: 10.1016/0304-3940(94)90621-1. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Stehouwer CD, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20:369–382. doi: 10.1002/dmrr.488. [DOI] [PubMed] [Google Scholar]
- Scheepers A, Schmidt S, Manolescu A, Cheeseman CI, Bell A, Zahn C, Joost HG, Schürmann A. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol. 2005;22:339–351. doi: 10.1080/09687860500166143. [DOI] [PubMed] [Google Scholar]
- Seatter MJ, De la Rue SA, Porter LM, Gould GW. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry. 1998;37:1322–1326. doi: 10.1021/bi972322u. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience. 2006;140:889–895. doi: 10.1016/j.neuroscience.2006.02.071. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Suárez G, Rajaram R, Bhuyan KC, Oronsky AL, Goidl JA. Administration of an aldose reductase inhibitor induces a decrease of collagen fluorescence in diabetic rats. J Clin Invest. 1988;82:624–627. doi: 10.1172/JCI113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MA, Sherman WR, Kurien MM, Moonsammy GI, Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967;14:1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Kuentzel SL, Raub TJ, Davies A, Baldwin SA, Borchardt RT. Hexose uptake in primary cultures of bovine brain microvessel endothelial cells. I Basic characteristics and effects of D-glucose and insulin. Biochim Biophys Acta. 1991;1070:1–10. doi: 10.1016/0005-2736(91)90139-y. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Levy CA, Warren SK, Jones EM. Permeability of the blood-brain barrier to fructose and the anaerobic use of fructose in the brains of young mice. J Neurochem. 1972;19:1685–1696. doi: 10.1111/j.1471-4159.1972.tb06213.x. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nishi N, Nakagawa T, Chiba Y, Tsukamoto I, Kusaka T, Miki T, Sakamoto H, Yamaguchi F, Tokuda M. Immunoreactivity of glucose transporter 5 is located in epithelial cells of the choroid plexus and ependymal cells. Neuroscience. 2014;260:149–157. doi: 10.1016/j.neuroscience.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J Biol Chem. 1992;267:4364–4369. [PubMed] [Google Scholar]
- Wada H, Okada Y, Uzuo T, Nakamura H. The effects of glucose, mannose, fructose and lactate on the preservation of neural activity in the hippocampal slices from the guinea pig. Brain Res. 1998;788:144–1450. doi: 10.1016/s0006-8993(97)01532-1. [DOI] [PubMed] [Google Scholar]
- Wiesinger H, Thiess U, Hamprecht B. Sorbitol pathway activity and utilization of polyols in astroglia-rich primary cultures. Glia. 1990;3:277–282. doi: 10.1002/glia.440030407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.