Abstract
Implementation of the lung allocation score (LAS) in 2005 led to transplantation of older and sicker patients without altering 1-year survival. However, long-term survival has not been assessed and emphasizing the 1-year survival metric may actually sustain 1-year survival while not reflecting worsening longer-term survival. Therefore, we assessed overall and conditional 1-year survival; and the effect of crossing the 1-year threshold on hazard of death in three temporal cohorts: historical (1995–2000), pre-LAS (2001–2005) and post-LAS (2005–2010). One-year survival post-LAS remained similar to pre-LAS (83.1% vs. 82.1%) and better than historical controls (75%). Overall survival in the pre-and post-LAS cohorts was also similar. However, long-term survival among patients surviving beyond 1 year was worse than pre-LAS and similar to historical controls. Also, the hazard of death increased significantly in months 13 (1.44, 95% CI 1.10–1.87) and 14 (1.43, 95% CI 1.09–1.87) post-LAS but not in the other cohorts. While implementation of the LAS has not reduced overall survival, decreased survival among patients surviving beyond 1 year in the post-LAS cohort and the increased mortality occurring immediately after 1 year suggest a potential negative long-term effect of the LAS and an unintended consequence of increased emphasis on the 1-year survival metric.
Introduction
Lung transplantation can improve quality of life and survival in patients with end-stage lung disease (1). Prior to 2005, lungs were allocated based on length of time on the waitlist. However, this system likely contributed to higher waitlist mortality for patients with diagnoses associated with rapid decline in lung function, particularly idiopathic pulmonary fibrosis (IPF) (2,3). In May of 2005, the lung allocation score (LAS)—a composite score incorporating physiological and comorbid variables that predict waitlist mortality and 1-year posttransplant survival—was implemented in an effort to reduce waitlist mortality and increase lung utilization in order to maximize benefit to the recipient population (4).
Since implementation, the LAS has successfully reduced waitlist time (5). Multiple before-and-after analyses have reported no change in posttransplant survival (6–11). However, 1-year survival was the primary or sole metric used in all of these analyses. Short-term survival gains in other solid organ transplants have not consistently been associated with improved long-term survival (12). We previously reported that a higher LAS was independently predictive of worse posttransplant survival (13), as have others (14), suggesting that over time, prioritizing patients with the highest waiting list mortality may jeopardize long-term posttransplant survival. To date, longer-term outcomes (i.e. beyond 1 year) in the LAS era have not been sufficiently evaluated.
Also, concurrent to the implementation of the LAS, there has been increased scrutiny of transplant program performance by governmental agencies, private payers and the United Network for Organ Sharing. One-year survival is the core metric provided by the Scientific Registry of Transplant Recipients (SRTR) to examine transplant program quality (15). Both private and public entities, as well as patients and referring physicians, look to the SRTR 1-year survival figure as an important, and often only, metric to evaluate individual transplant programs (16). Simply having a 1-year survival percentage below expected can jeopardize a program’s ability to continue performing transplants (17–19).
Given the uncertain association between short- and long-term survival and the increased emphasis on maintaining adequate 1-year survival statistics, we hypothesized that this metric may not adequately assess the impact of the LAS on long-term survival and might report an artificially suppressed mortality prior to 1 year. We therefore performed an analysis of long-term survival after lung transplantation including a specific comparison of the impact of crossing the 1-year survival threshold on the hazard of death in three distinct temporal cohorts.
Materials and Methods
This analysis was exempted from review by the Institutional Review Board as only publicly available, de-identified data were used. This study used data from the SRTR. The SRTR data system includes data on all donor, waitlisted candidates and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Cohorts
All recipients of lung transplantation between January 1, 1995 and November 30, 2011 were available for analysis. Heart–lung transplants and patients less than 18 years of age were excluded. To remove the impact of administrative censoring on the ability to analyze post 1-year survival, the cohort was restricted to patients transplanted at least 18 months prior to the administrative censoring date of December 1, 2011. To isolate the effect of implementation of the LAS from background trends of improving survival, we compared three distinct temporal cohorts: May 1, 2005–June 1, 2010 (post-LAS); January 1, 2001–April 30, 2005 (pre-LAS); and January 1, 1995–December 31, 2000 (historical control). Transplant data were extracted for recipient age, donor age, gender, medical condition, ventilation and extracorporeal membrane oxygenation (ECMO) status, diagnosis, ischemic time and time on waitlist and compared across the cohorts.
Statistical analysis
Overall posttransplant survival rates and survival rates conditioned on surviving to 1 year posttransplantation (conditional survival) for the three cohorts were calculated by Kaplan–Meier statistics and assessed via marginal hazard ratios (MHR) for death. Post-LAS conditional survival was also analyzed by recipient diagnosis and age (greater or less than 50 years old). p-Values were not included for 1-year survival because data represent the entire at risk population and not a sample of a larger whole. Individuals were censored as re-transplanted or lost to follow-up and were otherwise administratively censored at December 1, 2011.
To formally test whether the hazard for death changed at 1 year post-transplantation, a piecewise exponential model was fit to the three cohorts. This model allowed for direct estimation of the hazard and assumed that the hazard (risk of death) was constant in a specified time period and then shifted at the designated time points. The null hypothesis assumed no difference between the hazards at two time intervals. We allowed the hazard to be constant in the 90 days prior to 1 year and then constant within the six 30-day intervals (180 days) following the 1-year mark. To examine whether the observed change in hazard was different between the three cohorts, an interaction term between cohort and time period was estimated. Models were adjusted for donor and recipient age, recipient gender, ventilator and ECMO status, waitlist time, ischemic time and diagnosis. Missing values were imputed using regression-based single imputation. For all parameter estimates, 95% confidence intervals (CIs) were calculated with associated p-values. Sensitivity analyses were performed by varying the baseline hazard from 1 to 6 months prior to the 1-year time point. Models were also adjusted for donor and recipient characteristics (see Table 1) though the inference was unchanged and were fit with interactions between hazard change and center size (greater or less than 50 transplants per year) and LAS score. Additional sensitivity analyses were performed analyzing re-transplants as deaths, varying the time cutoffs between temporal cohorts and analyzing by recipient diagnosis in the post-LAS cohort. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R 2.14 (R Foundation for Statistical Computing, Vienna, Austria) statistical software packages.
Table 1.
Baseline characteristics by temporal cohort
| 1995–2000 Historical control |
2001–04/2005 Pre-LAS cohort |
5/2005–6/2010 Post-LAS cohort |
p-Value1 | |
|---|---|---|---|---|
| Total transplants | 5081 | 4628 | 7437 | |
| Days of follow-up | 1429 (337, 3306) | 1817 (567, 2593) | 714 (345, 1170) | |
| Demographics | ||||
| Recipient age | 51.0 (40, 58) | 55.0 (44, 60) | 57.0 (47.0, 63) | <0.001 |
| Donor age | 29.0 (19, 42) | 30.0 (20, 44) | 31.0 (21, 46) | <0.001 |
| Gender (male) | 2542 (50.0%) | 2329 (50.3%) | 4337 (58.3%) | <0.001 |
| Time on waitlist (days) | 301 (123, 578) | 298 (106, 680) | 76 (23, 241) | <0.001 |
| Clinical status | ||||
| Medical condition | <0.001 | |||
| ICU | 192 (3.8%) | 163 (3.5%) | 603 (8.2%) | |
| Hospitalized | 300 (6.0%) | 207 (4.5%) | 576 (7.7%) | |
| Not hospitalized | 4543 (90.2%) | 4257 (92.0%) | 6258 (84.1%) | |
| On ventilator | 153 (3.0%) | 122 (2.6%) | 449 (6.0%) | <0.001 |
| On ECMO | 8 (0.2%) | 28 (0.6%) | 79 (1.1%) | <0.001 |
| Candidate diagnosis | <0.001 | |||
| A | 2607 (51%) | 2311 (50%) | 2341 (33%) | |
| B | 290 (6%) | 182 (4%) | 229 (3%) | |
| C | 861 (17%) | 674 (15%) | 935 (13%) | |
| D | 1133 (22%) | 1411 (30%) | 3778 (51%) | |
| Other | 190 (4%) | 50 (1%) | 2 (0.03%) | |
| Ischemic time (min) | 255 (193, 329) | 271 (207, 340) | 398 (236, 363) | <0.001 |
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit. Diagnosis groups are: A, obstructive lung disease; B, pulmonary vascular disease; C, cystic fibrosis or immunodeficiency disorder and D, restrictive lung disease.
Median and interquartile range for continuous variables; counts and percentages for categorical.
Kruskal–Wallis and chi-square tests for continuous and categorical variables, respectively.
Results
Between January 1, 1995 and June 1, 2010, 17 146 adults received lung transplants. Median lengths of follow-up for the historical, pre-LAS and post-LAS cohorts were 1429, 1817 and 714 days, respectively. There were differences among patients transplanted in the three time periods: patients in the post-LAS era were older, received lungs from older donors, were more likely to be male, more likely to have IPF and less likely to have chronic obstructive pulmonary disease. They were also more likely to be in a hospital or an intensive care unit (ICU), more likely to be mechanically ventilated or receiving ECMO, and spent less time on the waitlist (Table 1).
Kaplan–Meier curves of overall posttransplant survival are shown in Figure 1. One-year survival was slightly better in the post-LAS (83.1%) and pre-LAS (82.1%) cohorts than in the historical control (74.9%). Long-term survival post-LAS was also similar to the pre-LAS cohort (MHR 0.99, interquartile range [IQR] 0.93–1.06) and better than the historical control (MHR 0.75, IQR 0.70–0.79). However, survival post-LAS among patients who survived beyond 1 year was worse than pre-LAS (MHR 1.12, IQR 1.02–1.23) and similar to the historical control (MHR 0.98, IQR 0.90–1.08) (Figure 2). Figure 2B also reveals a transient worsening of survival that occurs shortly after the 1-year threshold and lasts approximately 100 days in the post-LAS cohort. This period is associated with an 8% absolute increase in mortality and translates to approximately 17 excess deaths. There was no difference in the conditional survival by recipient diagnosis or age (Figure S1).
Figure 1. Overall survival by cohort.
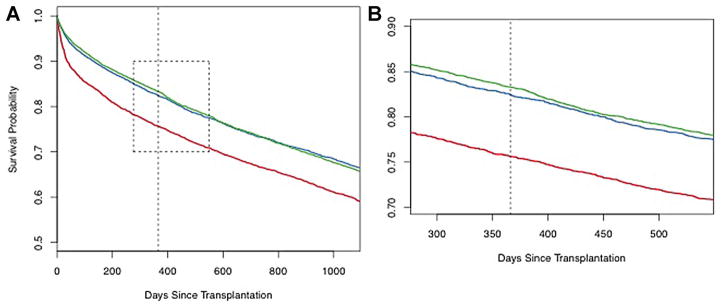
Kaplan–Meier survival curves for the three cohorts: historical control (1995–2000) (red line); pre-LAS (2001–4/2005) (blue line); and post-LAS (5/2005–6/2010) (green line). Panel A—full curves out to 1095 days; Panel B—expanded curves (area outlined in Panel A) centered on the 1-year time point (dashed black line) out to 1.5 years.
Figure 2. Conditional survival by cohort.
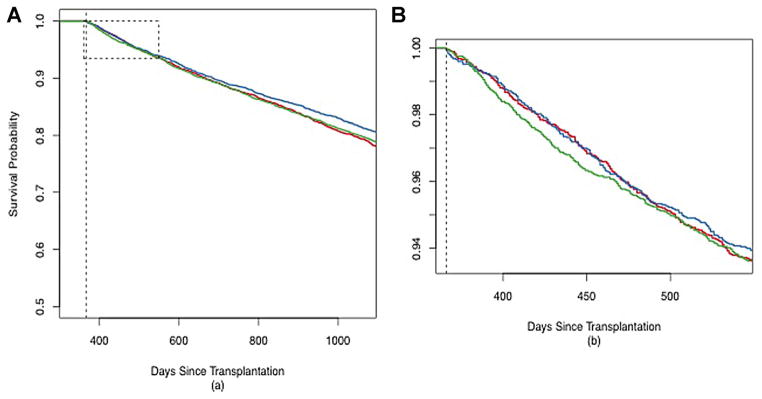
Kaplan–Meier survival curves conditioned on survival to 1-year posttransplantation for the three cohorts: historical control (1995–2000) (red line); pre-LAS (2001–4/2005) (blue line); and post-LAS (5/2005–6/2010) (green line). Panel A—full curves out to 1095 days; Panel B—expanded curves (area outlined in Panel A) starting at the 1-year time point (dashed black line) out to 1.5 years.
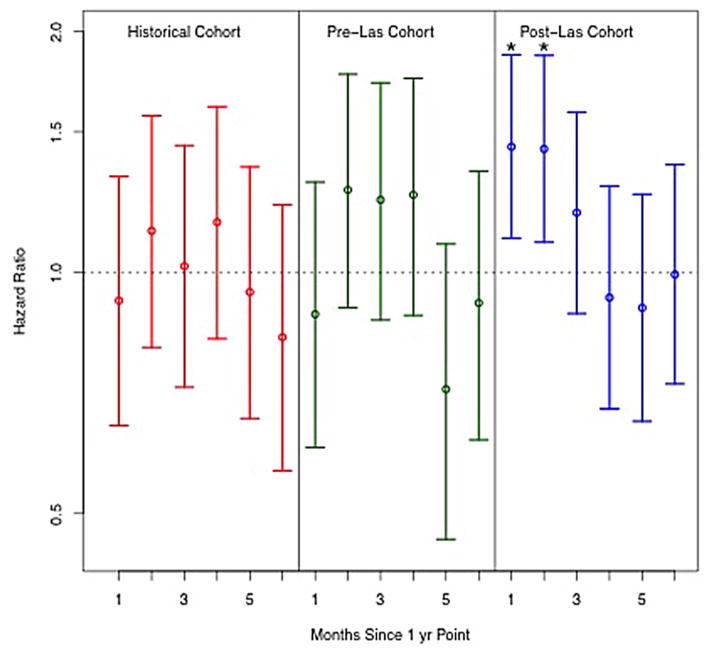
Interval-specific hazard ratios for death in 30-day intervals after 1 year (relative to 90 days prior to 1 year) are shown in Figure 3. The hazard for death was significantly increased in months 13 (1.44, 95% CI 1.10–1.87) and 14 (1.43, 95% CI 1.09–1.87) in the post-LAS cohort but not for any other time period. Weekly mortality rates for the three cohorts are shown in Table S1 and suggest an increasing mortality beginning at week 54 in the post-LAS cohort. The increased hazard in month 13 in the post-LAS cohort was significant relative to the pre-LAS cohort (interaction for time period and cohort 1.61, 95% CI 1.02–2.55) and nearly significant relative to the historical control (interaction 1.51, 95% CI 0.97–2.36). There were no other significant differences in hazard ratios for any other time period between 12 and 18 months between the three cohorts (Table S2).
Figure 3. Hazard ratio for mortality after 1 year.
Point estimates (circles) with 95% confidence intervals (error bars) for the hazard ratio of mortality in each month after 1 year compared to a baseline hazard calculated for the 90 days period prior to 1 year for the three cohorts: historical control (1995–2000) (red line); pre-LAS (2001–4/2005) (green line); and post-LAS (5/2005–6/2010) (blue line). *denotes significance at p <0.05.
Sensitivity analysis varying the baseline hazard rate from 1 to 6 months prior to 1 year provided similar results. Models adjusted for donor and recipient characteristics produced similar estimates and interactions for transplant center size and LAS score were nonsignificant suggesting little confounding or effect modification of the increase in hazard in the post-LAS cohort by donor or recipient characteristics, LAS or center size. Analyzing the outcome of death or re-transplant also yielded similar results (Table S3).
Results from sensitivity analyses using different temporal cohorts and analysis by recipient diagnosis are shown in the online supplement (Tables S4 and S5, respectively). The hazard of death was increased in months 13 and 14 for the 2004 through 2007 cohort and in months 13, 14 and 15 in 2008 through 2011 cohort (although statistical significance was lost for some months likely due to smaller sample size). Similarly, the hazard ratios remained elevated post-LAS for both the obstructive (Group A) and restrictive lung disease (Group D) groups (the two largest categories). No increased hazard occurred among patients with cystic fibrosis or immunodeficiency (Group C). Results for the pulmonary vascular disease group (Group B) were highly unstable due to small sample size and rare events.
Discussion
Our analysis confirms that despite transplanting older patients with more acute illness since adoption of the LAS, 1-year survival remains similar to the pre-LAS era and better than that from 1995 to 2000. In addition, overall survival was also stable compared to the pre-LAS cohort. However, mortality increased significantly in the 2 months immediately after the 1-year time point in the post-LAS cohort and survival among patients who survived to 1 year declined relative to pre-LAS and was similar to historical controls.
Since higher LAS scores are associated with worse posttransplant survival (13,14), it is notable that implementation of an allocation score that prioritizes sicker patients has not yet substantially reduced overall posttransplant survival. However, median follow-up in our post-LAS cohort was less than 2 years and the decline in survival occurring after the first year in the post-LAS cohort suggests that worse overall survival may be observed with longer follow-up. Similar to our findings, a recent analysis found that patients with an acute rise of >5 points in their LAS within 30 days of transplant had worse posttransplant survival suggesting that prioritizing acutely deteriorating patients may also adversely impact survival independent of absolute LAS (20).
A reduction in early mortality in the pre- and post-LAS cohorts remains the most dramatic change in mortality over the past 15 years. However, while survival after the first 100 days has remained relatively stable in the three cohorts, the mortality rate did increase after 1 year for the post-LAS cohort relative to pre-LAS. The LAS was not designed to predict mortality beyond 1 year, and it is not surprising that allocating lungs to older and sicker patients under this system would negatively impact long-term survival.
In addition to worse long-term survival among patients surviving to 1 year, we observed an abrupt increase in the hazard of mortality in the period immediately following the 1-year mark. This represents a transient 8% increase in mortality that is not easily explained by plausible biologic explanations. While protocolized changes in immune suppression, prophylactic regimens or relaxed surveillance practices could occur after 1 year, it is unlikely that any such clinical transition would transiently increase mortality for 2 months immediately after 1 year (Figures 2B and 3). Instead, we suspect the increased mortality in this period is due to a deferment of deaths until after the 1-year threshold and likely reflects an unintended consequence of the 1-year survival metric.
While implementation of the LAS occurred on a discreet date, increased regulatory oversight has been phased in over many years beginning with SRTR reporting of 1-year mortality followed by the Centers for Medicare and Medicaid Services issuing of proposed “conditions for participation” in 2005 (21) and a final rule in 2007 (22). Due to the overlapping effects of increasing regulatory oversight and implementation of the LAS, we cannot independently analyze the relative impact of these phenomena. However, as a sensitivity analysis, we analyzed different temporal cohorts using cutoffs that roughly correlate with periods of change in regulatory oversight in an attempt to decouple oversight from implementation of the LAS (Table S4). The hazard for death immediately after 1 year increased in both the 2004 through 2007 and 2008 through 2011 cohorts but to a greater degree in the latter cohort. We suspect that transplanting sicker patients with more complicated post-transplant courses combined with the increased scrutiny placed on the 1-year survival metric has exaggerated the impact of the 1-year threshold on physicians’ decisions to continue aggressive life sustaining care around this time period. However, we cannot rule out that this represents a delayed effect of an evolving change in physician behavior in response to increasing oversight. Analyzing the effect of crossing the 1-year threshold by indication for transplant showed a similar increase in the hazard of death among patients with obstructive (Group A) and restrictive (Group D) lung disease (Table S5), which were the groups most effected by implementation of the LAS (Table 1). There was no effect seen in Group C (mostly cystic fibrosis patients), which may indicate that the impact of increased regulatory oversight is at least in-part dependent on also transplanting older patients postimplementation of the LAS.
The most notable limitations of this analysis are those inherent to analyses of a national registry database. The available SRTR data do not contain sufficient patient-specific data to fully address whether simply crossing the 1-year threshold is causally associated with an increased hazard of death. Analysis of patient-specific data will be necessary to further assess the variables impacting mortality around the 1-year mark and to quantify the impact of the 1-year metric on physician decision making and the implications of these decisions on resource utilization. Also, we analyzed distinct temporal cohorts to isolate the effects of increased regulatory oversight and implementation of the LAS from background trends in improving clinical practice. While no retrospective analysis can completely control for the time bias between cohorts, we found that survival among patients surviving to 1 year was worse in the most recent cohort (i.e. post-LAS) and in the opposite direction of background trends further suggesting a negative effect of the LAS on long-term survival.
While 1-year survival has not changed post-LAS, further analysis of patient-specific data is required to quantify the cost and resources (e.g. hospital lengths of stay, ICU admissions and use of mechanical ventilation and renal replacement therapy) committed to preserving the 1-year survival of older and sicker patients transplanted in the post-LAS era. In a preliminary analysis of the Nationwide Inpatient Sample database, we showed that health-care resource utilization associated with incident hospitalization for lung transplant increased dramatically post-LAS and was out of proportion to increases associated with other solid organ transplantation (23).
Further analysis is required to establish causality between the 1-year survival metric and the effect of crossing the 1-year threshold on mortality immediately after 1 year; however, we think this is an important finding worthy of further investigation. An implication that physicians’ decisions regarding matters of life and death may not solely reflect patient-centric variables is a troubling but not entirely unexpected finding. Recent years have seen increased scrutiny on transplant programs to document acceptable outcomes with 1-year survival becoming the near sole metric by which they are evaluated. Survival information reported for each individual program are publicly available on the SRTR website with annotations labeling each program’s 1-year survival rates as “Below Expected,” “At Expected” or “Above Expected” in a format easily accessible and intended for a lay audience (15,16). Pressures to maintain acceptable 1-year survival can come in the form of corrective actions by governmental agencies, exclusion of coverage by private payers and avoidance of the program by patients and referring physicians. Designation as a low performing center can result in need to perform higher-risk transplants to sustain volume and a downward spiral of worst outcomes (17). Alternatively, low performing centers may become more risk averse thus rejecting appropriate organs or transplant candidates deemed to be an unacceptable risk (18,19).
Whether quality benchmarks in clinical medicine produce intended changes in provider behavior is debatable (24). With increased emphasis on quality and cost-effective analyses anticipated in an era of health-care reform, it is critical that we continue to assess the quality of our metrics and guard against negative unintended consequences. Inclusion of alternative metrics such as median survival post transplantation would be less susceptible to external pressure and may more reliably measure the performance of individual transplant centers.
Supplementary Material
Figure S1: Post-LAS conditional survival by recipient diagnosis and age. Kaplan–Meier survival curves conditioned on survival to 1 year posttransplantation for the post-LAS cohort. Panel A—survival by recipient diagnosis: Group A (obstructive) (red line); Group B (pulmonary vascular) (blue line); Group C (cystic fibrosis or immunodeficiency) (green line); Group D (restrictive). Panel B—survival by age ≥50 years (blue line) and <50 years (red line).
Table S1: Weekly mortality rates per 10 000 patients around the 1-year threshold by temporal cohort.
Table S2: Monthly hazard ratios for death after 1-year relative to 90 days prior to 1 year by temporal cohort with interaction terms between cohort and time period.
Table S3: Monthly hazard ratios for death or re-transplant relative to 90 days prior to 1 year by temporal cohort.
Table S4: Monthly hazard ratios for death relative to 90 days prior to 1 year using different temporal cohort.
Table S5: Monthly hazard ratios for death relative to 90 days by candidate diagnosis.
Acknowledgments
Supported in part by HL095686 (MRN) and the Ranzetta Family Fund.
Abbreviations
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- COPD
chronic obstructive pulmonary disease
- ECMO
extracorporeal membrane oxygenation
- HRSA
Health Resources and Services Administration
- ICU
intensive care unit
- IPF
idiopathic pulmonary fibrosis
- IQR
interquartile range
- LAS
lung allocation score
- MHR
marginal hazard ratios
- MMRF
Minneapolis Medical Research Foundation
- NIS
Nationwide Inpatient Sample
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Authors’ Contributions
BGM and JEL contributed equally to the design of the study, data analysis and interpretation, and drafting and revising of the article. BAG contributed to study design, performed the statistical analysis and contributed critically to data interpretation and revising the article. JJM, MRN, MZ, VV and DW made substantial contributions to interpretation of data and revising the article. GSD was responsible for conception and design of study, acquisition and interpretation of the data and contributed critically to drafting and revising the article. Drs. Maxwell, Dhillon, Goldstein and Levitt had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors provided final approval of the version to be published.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart, Lung Transplantation: 29th adult lung, heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 3.Pierson RN, 3rd, Barr ML, McCullough KP, et al. Thoracic organ transplantation. Am J Transplant. 2004;4(Suppl 9):93–105. doi: 10.1111/j.1600-6135.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 4.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 5.Lingaraju R, Blumenthal NP, Kotloff RM, et al. Effects of lung allocation score on waiting list rankings, transplant procedures. J Heart Lung Transplant. 2006;25:1167–1170. doi: 10.1016/j.healun.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Shiboski SC, Golden JA, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:468–474. doi: 10.1164/rccm.200810-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Oliveira NC, Osaki S, Maloney J, Cornwell RD, Meyer KC. Lung transplant for interstitial lung disease: Outcomes before and after implementation of the united network for organ sharing lung allocation scoring system. Eur J Cardiothorac Surg. 2012;41:680–685. doi: 10.1093/ejcts/ezr079. [DOI] [PubMed] [Google Scholar]
- 8.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: impact on disease severity and survival. Chest. 2007;132:1954–1961. doi: 10.1378/chest.07-1160. [DOI] [PubMed] [Google Scholar]
- 9.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: A multicenter study. J Thorac Cardiovasc Surg. 2008;135:166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 10.McCue JD, Mooney J, Quail J, Arrington A, Herrington C, Dahlberg PS. Ninety-day mortality and major complications are not affected by use of lung allocation score. J Heart Lung Transplant. 2008;27:192–196. doi: 10.1016/j.healun.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Osaki S, Maloney JD, Meyer KC, Cornwell RD, Edwards NM, De Oliveira NC. The impact of the lung allocation scoring system at the single national Veterans Affairs Hospital lung transplantation program. Eur J Cardiothorac Surg. 2009;36:497–501. doi: 10.1016/j.ejcts.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: The long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11:1226–1235. doi: 10.1111/j.1600-6143.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu V, Zamora MR, Dhillon GS, Weill D. Increasing lung allocation scores predict worsened survival among lung transplant recipients. Am J Transplant. 2010;10:915–920. doi: 10.1111/j.1600-6143.2009.03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity, mortality following transplantation. Chest. 2010;137:651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasiske BL, McBride MA, Cornell DL, et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant. 2012;12:1988–1996. doi: 10.1111/j.1600-6143.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson DM, Arrington CJ, Fant G, et al. SRTR program-specific reports on outcomes: A guide for the new reader. Am J Transplant. 2008;8:1012–1026. doi: 10.1111/j.1600-6143.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SR, Karp SJ, Curry MP, et al. Liver transplant center risk tolerance. Clin Transpl. 2012;26:E269–E276. doi: 10.1111/j.1399-0012.2012.01658.x. [DOI] [PubMed] [Google Scholar]
- 18.Howard RJ, Cornell DL, Schold JD. CMS oversight, OPOs and transplant centers and the law of unintended consequences. Clin Transpl. 2009;23:778–783. doi: 10.1111/j.1399-0012.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 19.Schold JD, Buccini LD, Srinivas TR, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013;13:67–75. doi: 10.1111/j.1600-6143.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsuang WM, Vock DM, Finlen Copeland CA, Lederer DJ, Palmer SM. An acute change in lung allocation score and survival after lung transplantation: A cohort study. Ann Intern Med. 2013;158:650–657. doi: 10.7326/0003-4819-158-9-201305070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid, Department of Health and Human Services. Medicare Program; Hospital Conditions of Participation: Requirements for Approval and Re-Approval of Transplant Centers To Perform Organ Transplants; Proposed Rule. In: Federal Register 42 CFR Parts 405, 482, and 488; February 4, 2005; 6140–6182.
- 22.Centers for Medicare and Medicaid, Department of Health and Human Services. Medicare Program; Hospital Conditions of Participation: Requirements for Approval and Re-Approval of Transplant Centers to Perform Organ Transplants; Final Rule. In: Federal Register 42 CFR Parts 405, 482, 488 and 498; March 30, 2007; 15198–15280.
- 23.Lee P, Maxwell BG, Levitt JE, et al. Lung allocation score increases health care resource utilization after lung transplantation [abstract] Chest. 2013;144(Suppl 4):110A. [Google Scholar]
- 24.Werner RM. Will using Medicare data to rate doctors benefit patients? Ann Intern Med. 2012;156:532–533. doi: 10.7326/0003-4819-156-7-201204030-00396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Post-LAS conditional survival by recipient diagnosis and age. Kaplan–Meier survival curves conditioned on survival to 1 year posttransplantation for the post-LAS cohort. Panel A—survival by recipient diagnosis: Group A (obstructive) (red line); Group B (pulmonary vascular) (blue line); Group C (cystic fibrosis or immunodeficiency) (green line); Group D (restrictive). Panel B—survival by age ≥50 years (blue line) and <50 years (red line).
Table S1: Weekly mortality rates per 10 000 patients around the 1-year threshold by temporal cohort.
Table S2: Monthly hazard ratios for death after 1-year relative to 90 days prior to 1 year by temporal cohort with interaction terms between cohort and time period.
Table S3: Monthly hazard ratios for death or re-transplant relative to 90 days prior to 1 year by temporal cohort.
Table S4: Monthly hazard ratios for death relative to 90 days prior to 1 year using different temporal cohort.
Table S5: Monthly hazard ratios for death relative to 90 days by candidate diagnosis.