Abstract
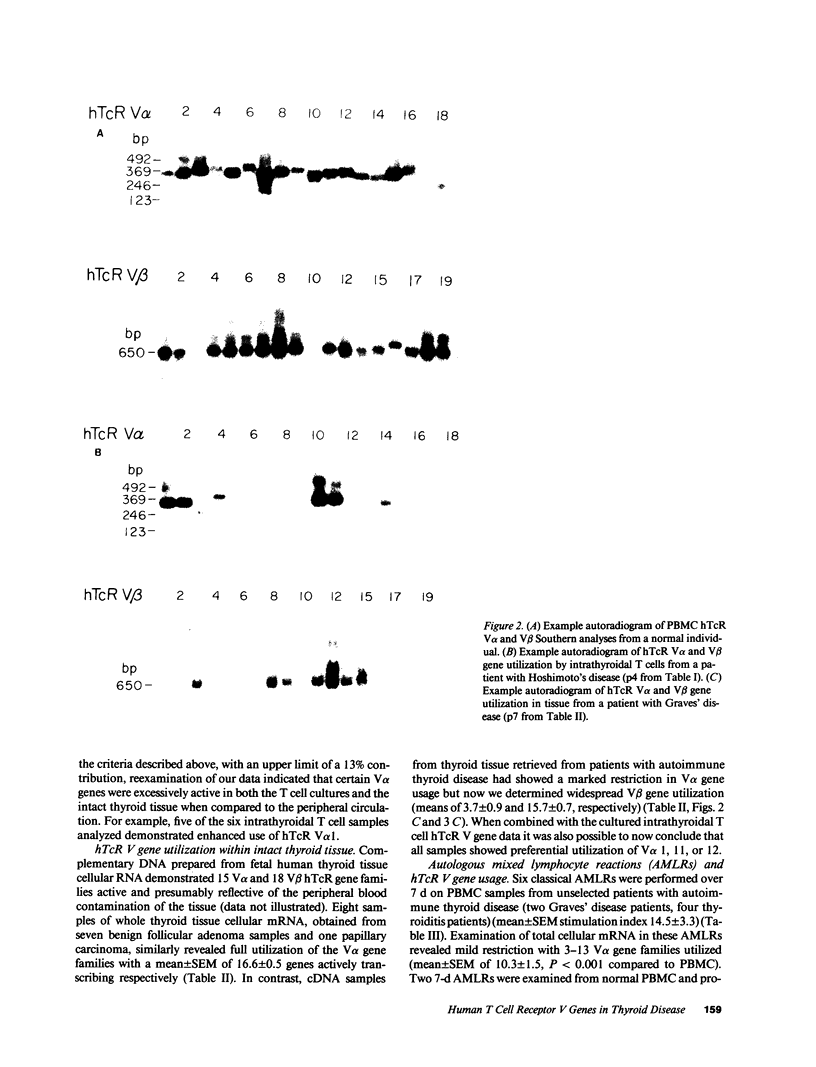
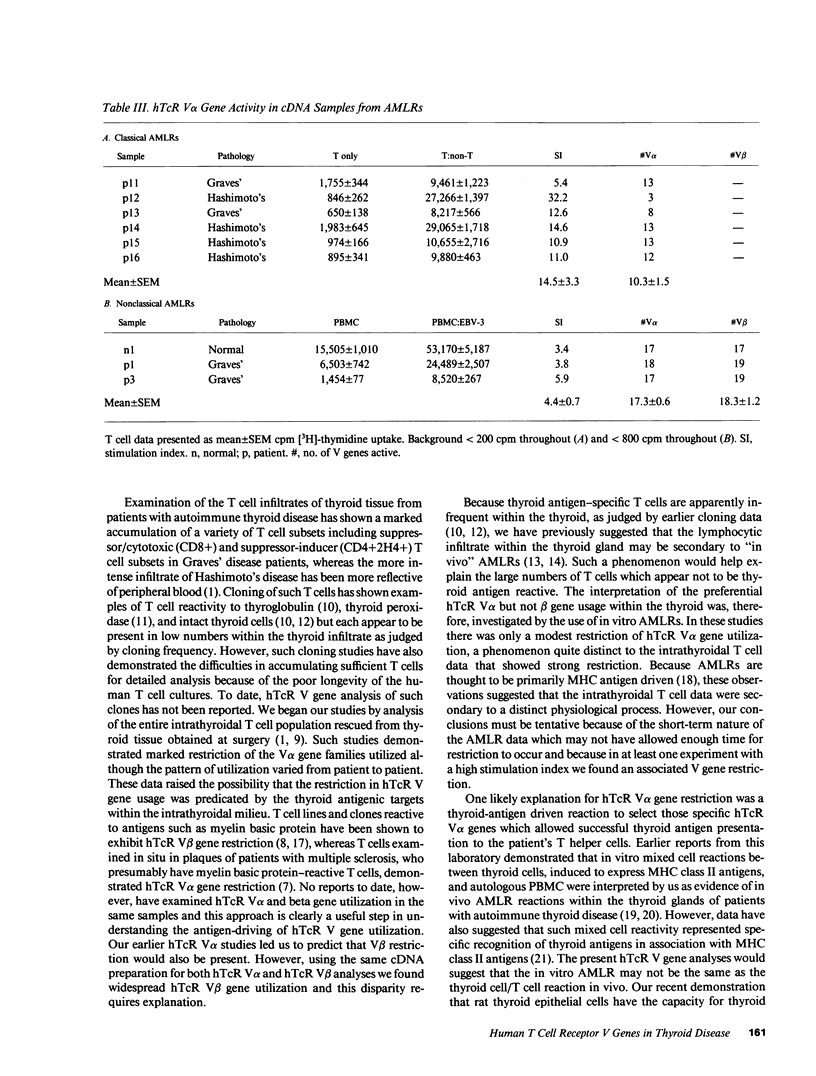
We have investigated the T cell receptor V alpha and V beta gene family usage by T lymphocytes infiltrating affected thyroids in patients with autoimmune thyroid disease. We show that the intrathyroidal T lymphocytes from patients (n = 6) with autoimmune thyroid disease display a widespread usage of V beta gene families with an average of 14.4/19 V beta gene families similar to the peripheral T lymphocytes of the same patients. Because we recently reported that the utilization of V alpha gene families is markedly reduced within these mitogen-stimulated intrathyroidal T cell populations, as well as within intact tissue from similar patients (n = 4) (overall mean of 4.0/18 families detected), these results indicate that in thyroids of patients with autoimmune thyroid disease the lymphocytes are selectively accumulating based on their V alpha rather than V beta elements. This preferential hTcR V alpha and widespread V beta gene usage was not mimicked in most 7-d autologous mixed lymphocyte reactions using non-T cell stimulators (n = 6) or EB-virus immortalized autologous B cell lines (n = 3). Hence, the selective V gene utilization by intrathyroidal T cells is likely to be secondary to multiepitopic thyroidal autoantigens activating thyroid infiltrating T cells or to the presence of a superantigenlike thyroidal self-antigen, capable of determining a selective infiltration or activation of a variety of T lymphocytes on the basis of their V alpha gene usage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Liblau R. S., Cohen L., Lehmann D., Tournier-Lasserve E., Rosenzweig A., Zhang J. W., Raus J. C., Bach M. A. Restricted T-cell receptor V beta gene usage by myelin basic protein-specific T-cell clones in multiple sclerosis: predominant genes vary in individuals. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2466–2470. doi: 10.1073/pnas.88.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Burns F. R., Li X. B., Shen N., Offner H., Chou Y. K., Vandenbark A. A., Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989 Jan 1;169(1):27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. F. Cocultures of human thyroid monolayer cells and autologous T cells: impact of HLA class II antigen expression. J Clin Endocrinol Metab. 1985 Sep;61(3):418–422. doi: 10.1210/jcem-61-3-418. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Cohen L., Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991 Jul 25;325(4):238–244. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Graves P. Human autoimmune thyroid disease: cellular and molecular aspects. Baillieres Clin Endocrinol Metab. 1988 Nov;2(4):911–939. doi: 10.1016/s0950-351x(88)80024-7. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Platzer M. The T cell suppressor defect in autoimmune thyroiditis: evidence for a high set 'autoimmunostat'. Clin Exp Immunol. 1986 Jan;63(1):73–79. [PMC free article] [PubMed] [Google Scholar]
- Fisfalen M. E., DeGroot L. J., Quintans J., Franklin W. A., Soltani K. Microsomal antigen-reactive lymphocyte lines and clones derived from thyroid tissue of patients with Graves' disease. J Clin Endocrinol Metab. 1988 Apr;66(4):776–784. doi: 10.1210/jcem-66-4-776. [DOI] [PubMed] [Google Scholar]
- Hirose W., Lahat N., Platzer M., Schmitt S., Davies T. F. Activation of MHC-restricted rat T cells by cloned syngeneic thyrocytes. J Immunol. 1988 Aug 15;141(4):1098–1102. [PubMed] [Google Scholar]
- Kimura H., Davies T. F. Thyroid-specific T cells in the normal Wistar rat. II. T cell clones interact with cloned wistar rat thyroid cells and provide direct evidence for autoantigen presentation by thyroid epithelial cells. Clin Immunol Immunopathol. 1991 Feb;58(2):195–206. doi: 10.1016/0090-1229(91)90136-x. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Lombardi G., Batchelor J. R., Reinsmoen N., Bach F. H. The molecular basis of alloreactivity. Immunol Today. 1990 Mar;11(3):83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- MacKenzie W. A., Schwartz A. E., Friedman E. W., Davies T. F. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987 Apr;64(4):818–824. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- Maron R., Zerubavel R., Friedman A., Cohen I. R. T lymphocyte line specific for thyroglobulin produces or vaccinates against autoimmune thyroiditis in mice. J Immunol. 1983 Nov;131(5):2316–2322. [PubMed] [Google Scholar]
- Martin A., Goldsmith N. K., Friedman E. W., Schwartz A. E., Davies T. F., Roman S. H. Intrathyroidal accumulation of T cell phenotypes in autoimmune thyroid disease. Autoimmunity. 1990;6(4):269–281. doi: 10.3109/08916939008998419. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Pette M., Fujita K., Wilkinson D., Altmann D. M., Trowsdale J., Giegerich G., Hinkkanen A., Epplen J. T., Kappos L., Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini L. A., Roman S. H., Davies T. F. Autoimmune thyroid disease and thyroid cell class II major histocompatibility complex antigens. Clin Endocrinol (Oxf) 1987 Feb;26(2):253–272. doi: 10.1111/j.1365-2265.1987.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. Transfer of experimental autoimmune thyroiditis with T cell clones. J Immunol. 1987 Feb 15;138(4):1092–1098. [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]








