Abstract
New treatment strategies for inflammatory bowel disease are needed and parasitic nematode infections or application of helminth components improve clinical and experimental gut inflammation. We genetically modified the probiotic bacterium Escherichia coli Nissle 1917 to secrete the powerful nematode immunomodulator cystatin in the gut. This treatment was tested in a murine colitis model and on post-weaning intestinal inflammation in pigs, an outbred model with a gastrointestinal system similar to humans. Application of the transgenic probiotic significantly decreased intestinal inflammation in murine acute colitis, associated with increased frequencies of Foxp3+ Tregs, suppressed local interleukin (IL)-6 and IL-17A production, decreased macrophage inflammatory protein-1α/β, monocyte chemoattractant protein -1/3, and regulated upon activation, normal T-cell expressed, and secreted expression and fewer inflammatory macrophages in the colon. High dosages of the transgenic probiotic were well tolerated by post-weaning piglets. Despite being recognized by T cells, secreted cystatin did not lead to changes in cytokine expression or macrophage activation in the colon. However, colon transepithelial resistance and barrier function were significantly improved in pigs receiving the transgenic probotic and post-weaning colon inflammation was reduced. Thus, the anti-inflammatory efficiency of a probiotic can be improved by a nematode-derived immunoregulatory transgene. This treatment regimen should be further investigated as a potential therapeutic option for inflammatory bowel disease.
Introduction
Parasitic worms modulate host immune responses in order to induce an anti-inflammatory environment that favors their persistence and reproduction for many years in immunocompetent hosts. Parasite-driven immunoregulation has been exploited to ameliorate inflammatory disorders in mice1 and humans.2 In particular, immunoregulatory excretory/secretory components of parasitic nematodes offer potential biologics for future treatment of inflammatory disorders.3 Protease inhibitors from the cystatin family are important in host immunomodulation by parasitic worms, in particular shown for nematodes.4 In previous studies, we demonstrated that cystatin from the rodent nematode Acanthocheilonema viteae (AvCys) has strong anti-inflammatory properties when in contact with immune cells.5,6 Intraperitoneal application of the recombinant protein in murine disease models, namely dextran sodium sulfate-induced colitis and ovalbumin- or birch pollen-induced allergic airway hyperreactivity led to a significant reduction of inflammatory processes and pathology.6,7 Macrophages are the main target of nematode cystatin and the ameliorating effect in airway hyperreactivity could be reversed by macrophage depletion.6 Immunoregulation by nematode cystatin was associated with induction of interleukin (IL)-10 via exploitation of the host cell mitogen-activated protein kinase (MAPK) pathway.6,8 Together these results provide evidence for the potential of AvCys as a candidate to ameliorate inflammatory bowel disease (IBD).
IBD is an auto-inflammatory disorder characterized by phases of unregulated intestinal inflammation separated by intermittent phases of asymptomatic remission. The induction and propagation of IBD is multifactorial, involving both genetic and environmental risk factors. Over 163 gene loci and numerous lifestyle and environmental factors such as diet, smoking, and gastrointestinal microbial composition have been associated with the onset of IBD.9 To date no therapy is able to efficiently treat all patients afflicted with the condition. As a result, most IBD patients are unable to maintain lifelong remission and therefore endure recurrent symptomatic relapse.
In the current study, we aimed to develop a treatment strategy focusing on a site-directed and prolonged release of the nematode immunomodulator, AvCys, in the gut. We chose Escherichia coli Nissle 1917 (EcN) as a carrier. This probiotic is successfully used to maintain remission in IBD patients.10 In clinical trials involving patients with both active and inactive colitis, EcN was comparable to the standard non-steroidal anti-inflammatory treatment in reducing disease activity indices and maintaining remission phases.11 In experimental murine colitis models, EcN led to a marked decrease in gut inflammation and reduced expression of pro-inflammatory cytokines.12 EcN has been shown to suppress the adhesion and invasion of pathogenic bacteria,13,14 support intestinal barrier function,15 and prevent epithelial damage induced by dextran sodium sulfate.16 EcN has also been reported to reduce T cell expression of pro-inflammatory cytokines and up-regulate the expression of anti-inflammatory IL-10.17 Additionally, EcN colonizes the human intestine, while lacking virulence factors, making it a safe and effective probiotic for use in colitis patients.18 Hence, we chose EcN as an ideal carrier organism for the site-directed delivery of AvCys to the gut and tested whether we can enhance the probiotics' anti-inflammatory activity by a nematode immunomodulatory component.
In this study, we describe for the first time a transgenic probiotic generated to express a parasite-derived immunoregulatory protein (PCT 12183268.7). Applying the transgenic EcN secreting A. viteae cystatin (EcN-AvCys) in vivo we show the following: (i) the secretion of AvCys by the transgenic probiotic enhanced EcN-mediated amelioration of gut inflammation in a murine model of colitis, (ii) the transgene was safe when applied in high doses to pigs as an outbred model organism providing an intestinal tract highly similar to the human gut, and (iii) the transgene significantly ameliorated post-weaning gut inflammation in pigs and increased intestinal epithelial barrier function.
Results
Transgenic EcN secretes nematode cystatin and suppresses murine acute colitis
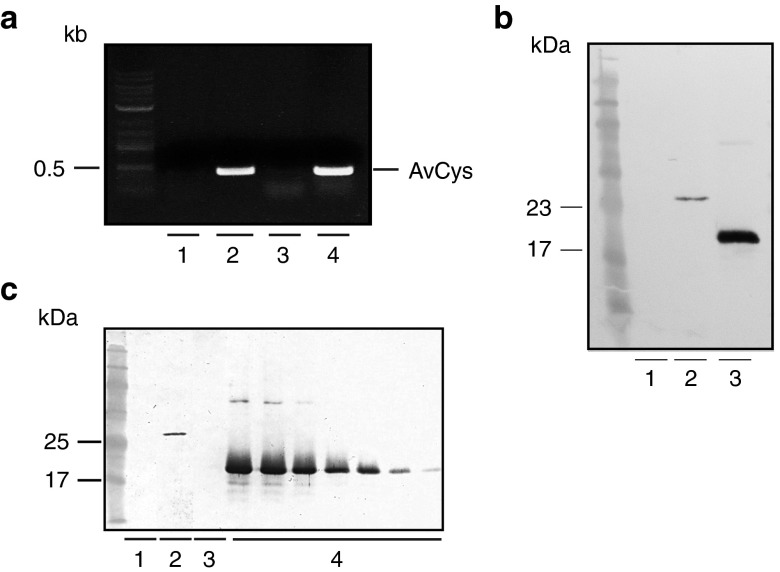
To generate transgenic EcN-AvCys, we ligated the functional AvCys gene into pMu13, a modified version of the native EcN plasmid pMut13, and transformed the construct into EcN. Polymerase chain recation (PCR) analysis of plasmid DNA from EcN-AvCys with AvCys specific primers confirmed the insertion of the gene in the transgenic probiotic (Figure 1a). The expression and secretion of AvCys by transgenic EcN-AvCys was confirmed through Western blot analysis of EcN-AvCys culture supernatant (EcN-AvCysSN) with a monoclonal anti-AvCys antibody. EcN-AvCys secretes significant amounts of recombinant AvCys in cell culture media when cultures were grown to a concentration of 3 × 108 CFU EcN/ml (Figure 1b,c). The slightly increased size of AvCys secreted by the transgenic EcN compared to AvCys derived from a standard expression vector is due to the presence of hlyA secretion sequence (see Material and Methods). AvCys (18 ± 6 ng/ml) were determined by ELISA in multiple batches of EcN-AvCysSN (data not shown).
Figure 1.
Generation of transgenic EcN expressing AvCys. (a) Polymerase chain reaction with AvCys specific primers amplified the AvCys gene in EcN-AvCys (2) and positive control (4), but not in EcN (1) or the negative water control (3). (b) Western blot analyses using an AvCys-specific antibody on supernatants of EcN (1) and EcN-AvCys (2) grown in Luria broth medium and recombinant AvCys as positive control (3). (c) Western blot of 1 ml of an Iscove's modified Dulbecco medium (IMDM) control (1), supernatant from EcN-AvCys grown in IMDM (2), supernatant from EcN grown in IMDM (3), and 7 lanes loaded with recombinant AvCys as a standard ranging from 200–5 ng (4).
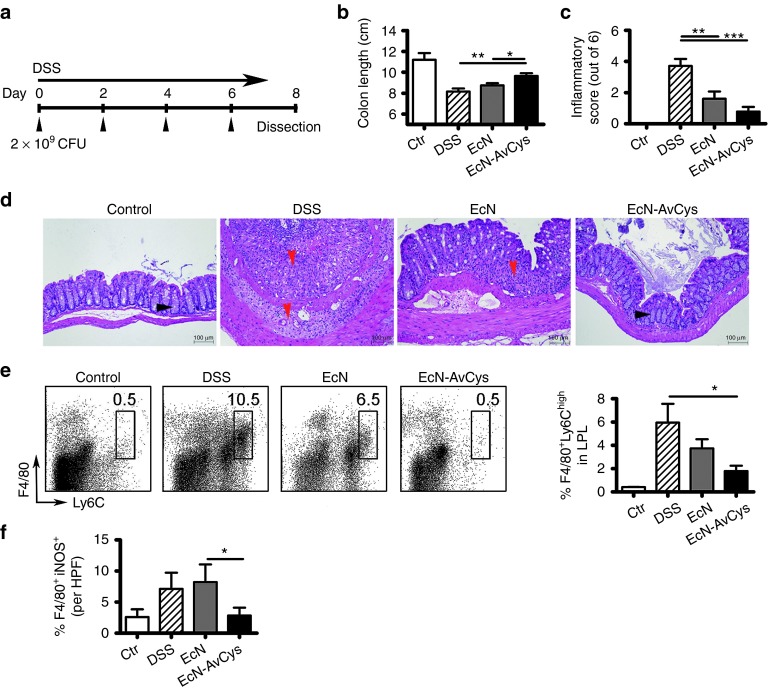
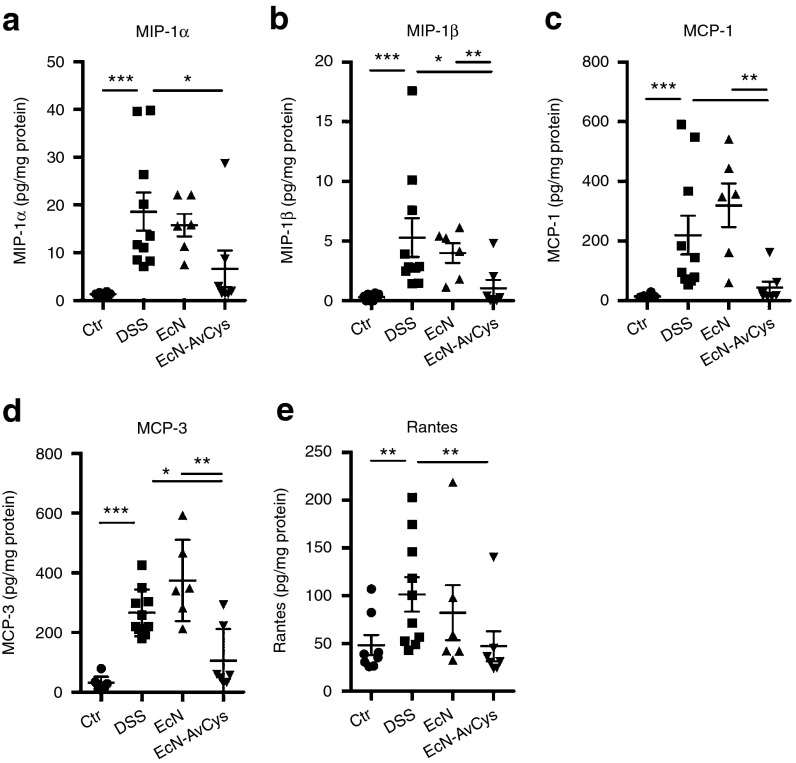
In a preceding study we found that recombinantly expressed AvCys applied intraperitoneally reduced colon inflammation in acute dextran sodium sulfate (DSS) colitis.6 Thus, we aimed to test for anti-colitic effects of site directed prolonged delivery of AcCys in the gut via application of the generated transgenic EcN-AvCys. EcN-AvCys and EcN were fed to mice receiving 3% DSS in drinking water to induce acute inflammation of the colon. Preliminary results suggested that EcN did not naturally colonize the gastrointestinal tract of mice (data not shown); therefore, 2 × 109 transgenic or control bacteria were applied by oral gavage in 100 µl of saline every 2nd day, while control mice received 100 µl of saline alone (Figure 2a). While body weight development in response to treatment was relatively heterogenous (not shown), treatment with EcN-AvCys significantly inhibited inflammation-associated colon shortening compared to DSS and EcN controls (Figure 2b). Histological cross sections of the distal colon showed that the application of EcN diminished DSS-induced inflammation as described previously.12,16 Compared to DSS controls, this was even more apparent for EcN-AvCys which significantly reduced damage to the colon with reduced cellular infiltration, preserved tissue architecture, and reduced thickening of the colon wall (Figure 2c,d). Macrophages are essentially involved in the inflammatory process in the acute DSS colitis model,19 and we detected significantly lower numbers of F4/80+Ly6Chi expressing monocytes/macrophages in lamina propria (LP) leukocyte isolates from EcN-AvCys recipients compared to DSS treated controls, while EcN control treatment had no such effect (Figure 2e). Similarly, only low numbers of iNOS+F4/80+ M1-type macrophages were detectable in colon cross sections of mice treated with EcN-AvCys and this was significantly reduced compared to mice treated with the control EcN (Figure 2f). To determine whether EcN-AvCys affected the expression of chemoattractants involved in monocyte migration to sites of tissue damage and inflammation, we analyzed the expression of macrophage inflammatory protein (MIP)-1α/β (Figure 3a,b), monocyte chemoattractant protein (MCP)-1/3 (Figure 3c,d), and regulated upon activation, normal T-cell expressed, and secreted (RANTES) (Figure 3e) in colon tissue. Compared to DSS-treated controls exhibiting strong protein expression of all chemokines, this was significantly suppressed in mice treated with EcN-AvCys, but not in EcN-treated controls (Figure 3). These effects were also apparent comparing the EcN-AvCys group to EcN controls for MIP-1β, MCP-1, and MCP-3 (Figure 3b–d). Thus, the secretion of AvCys by the transgenic probiotic reduced the DSS-induced expression of leukocyte chemotactic factors, which possibly led to the decreased migration of monocytes to the colon and lowered gut inflammation.
Figure 2.
Effects of EcN-AvCys on acute murine DSS-colitis. (a) Experimental setup. EcN-AvCys and EcN treated mice were orally administered with 2 × 109 CFU of the respective bacteria every second day while DSS was applied via drinking water for seven days. A DSS treated control group (DSS) and healthy controls (ctr) were orally gavaged with saline every second day. (b) Colon lengths of mice upon dissection are represented in centimeter. (c) Compiled histopathological colitis scores. (d) Representative pictures as used for histopathological scoring of distal colon sections. Red arrowheads depict areas of strong immune cell infiltration and loss of normal tissue architecture, black arrowheads mark normal crypt architecture. Also note the reduced colon wall thickening in response to EcN-AvCys treatment compared to DSS and EcN controls. (e) Representative flow cytometry plots showing the detection of F4/80+ Ly6Chigh expressing monocytes/macrophages in LPL. Bar graph shows percentages of F4/80+ Ly6Chigh cells detected in groups of mice. (f) Numbers of F4/80+iNOS+ M1 macrophages detected via immunohistochemistry in colon cross sections. Mean + SEM is shown. *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U-test). Data are pooled from 3 individual experiments with 2–3 (naïve controls)—5 mice (DSS, DSS + probiotics) per group. LPL, lamina proria leukocytes; HPF, high power field (400× magnification).
Figure 3.
Effects of EcN-AvCys on colon chemokine expression in DSS-colitis. Protein levels of MIP-1α (a), MIP-1β (b), MCP-1 (c), MCP-3 (d), and RANTES (e) detected in distal colon tissue of naïve control mice (n = 4), DSS controls (n = 5), EcN controls (n = 3) and mice fed EcN-AvCys (n = 4). Chemokine levels are expressed in relation to total protein content. Mean ± SEM for two colon explants derived from individual mice is shown. Data derive from one of two individual experiments with similar results. *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U-test).
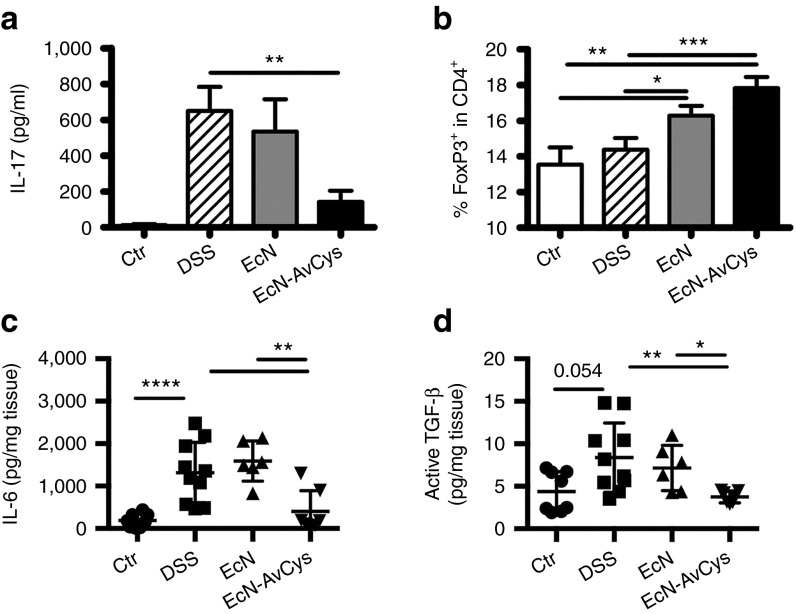
Reduced pathology after treatment with the transgenic probiotic was accompanied by the reduced production of pro-inflammatory IL-17A in gut-draining lymph nodes of mice treated with EcN-AvCys compared to DSS controls (mLN, Figure 4a). As Tregs were positively affected by the application of recombinant AvCys in disease models in our previous studies,6,7 we analyzed the frequencies of Foxp3+ cells in mLN. EcN-AvCys treatment led to a highly significant increase in frequencies of CD4+Foxp3+ Tregs compared to both healthy and DSS controls (Figure 4b). To gain the insight in a possible modulation of cytokines differentially instructing/supporting Th17 cells and Tregs, we assessed the local levels of IL-6, IL-23, and transforming growth factor (TGF)-β. Interestingly, IL-6 production was significantly suppressed comparing DSS controls and mice treated with EcN-AvCys, but not in EcN controls (Figure 4c). TGF-β production, however, was also significantly suppressed in mice fed the transgenic probiotic compared to DSS controls (Figure 4d). IL-23 supporting Th17 maintenance while restraining Treg activity,20,21 was detectable in similarly low amounts in all groups (data not shown). Taken together these data indicate that the expression of AvCys by transgenic EcN-AvCys has an additive effect on the amelioration of colitis compared to treatment with EcN in an experimental acute colitis model, presumably by affecting local chemokine and cytokine production restraining monocyte migration and Th17 differentiation.
Figure 4.
Effects of EcN-AvCys on local cytokine production and Tregs in DSS colitis. (a) Concentration of IL-17A in supernatants of mLN cells stimulated with concanavalin A for 48 hours as determined by ELISA. (b) Frequencies of Foxp3+ Tregs in the CD4+ population in mLN as detected by flow cytometry. (c,d) Levels of IL-6 (c) and active TGF-β in supernatants of distal colon explants cultured for 24 hours expressed in relation to weight of cultured tissue. Data in (a) and (b) are pooled from three individual experiments with 2–3 (naïve controls)—5 mice (DSS, DSS + probiotics) per group. Mean + SEM is shown. Data in (c) and (d) depict analyses of two colon explants derived from individual mice (n = 3–5 per group) and mean ± SEM is shown. Data derive from one of two individual experiments with similar results. *P < 0.05, **P < 0.01, ***P < 0.001 (Mann–Whitney U-test).
Transgenic probiotic is safe in pigs and ameliorates post-weaning gut inflammation
The pig has a digestive tract highly similar to that of humans,22 and post-weaning piglets develop a moderate, non-contagious inflammatory reaction in the gut as a consequence of stress, and the diet change.23,24,25 We aimed to determine whether the application of EcN-AvCys was safe when applied repeatedly in high dosages to piglets in the sensitive post-weaning phase. Therefore, we performed a feeding trial with 30 post-weaning piglets that were orally inoculated with 1010 EcN or EcN-AvCys every 48 hours for 2 weeks (Figure 5a). During the first week of the study trial, one and two piglets succumbed to post-weaning morbidity in the saline and EcN control groups, respectively, while all recipients of EcN-AvCys survived. No differences in body weight were detected between the groups at day 7 or 14 of the trial (Figure 5b) and blood smears performed on the day of dissection showed that all groups had similar proportions of monocytes, granulocytes, or lymphocytes in the peripheral blood (data not shown). In order to ascertain that AvCys was produced by EcN-AvCys in pigs, mLN cells were stimulated with recombinant AvCys or a control protein and analyzed for their proliferative responses. As expected, only mLN cells from EcN-AvCys treated pigs proliferated specifically in response to recombinant AvCys compared to the control protein (Figure 5c), showing that AvCys was produced in vivo and recognized by the immune system. To assess whether the post-weaning associated intestinal inflammatory response was affected by probiotic treatment, we scored distal colon cross sections histopathologically. Expectedly, pigs from the saline control group exhibited moderate signs of inflammation which was not altered by EcN feeding (Figure 5d). Recipients of EcN-AvCys, however, exhibited a reduced infiltration of immune cells and significantly reduced inflammatory scores compared to the saline control group (Figure 5d).
Figure 5.
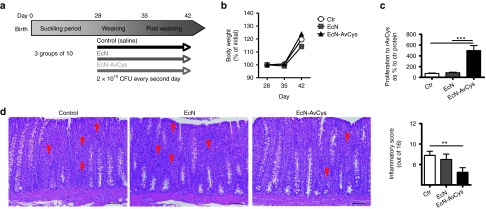
Safety and immune recognition of EcN-AvCys in pigs and effect on post-weaning gut inflammation. (a) Three groups of four week old weaned piglets were inoculated with either saline (ctr, n = 9), 2 × 1010 CFU EcN (EcN, n = 8) or 2 × 1010 EcN-AvCys (EcN-AvCys, n = 10) every 48 hours over 14 days. (b) Piglets were weighed at day (D) 28, 35, and 42 and body weight as a percentage of D28 weight is reported. (c) Proliferative response of mLN leukocytes after 48 hours incubation with a recombinant control protein or recombinant AvCys as determined by 3H-thymidine incorporation. Values are expressed as proliferation indices and mean + SEM is shown. (d) Representative pictures used for histopathological scoring of distal colon sections (left) and compiled inflammatory scores for all pigs (right). Red arrowheads depict areas of extensive immune cell infiltration. **P < 0.05, ***P < 0.001 (Mann–Whitney U-test).
Since we identified macrophages as target of AvCys in our previous studies,6,8 we analyzed their frequencies and swine leukocyte antigen (SLA) II expression in the colon LP by flow cytometry. Neither the frequencies of CD163+ macrophages nor their expression of SLA II was altered in response to EcN-AvCys application (Supplementary Figure S1a). As Treg frequencies were positively affected by EcN-AvCys feeding in the murine colitis model, we analyzed mLN and colon LP CD4+ cells for frequencies of Foxp3+ Tregs and expression of CD25; however, frequencies of CD4+CD25+Foxp3+ Tregs were similar in gut-draining lymph nodes and colon of all groups (Supplementary Figure S1b).
To determine if the decrease in colon inflammation in the EcN-AvCys treated group was associated with local changes in cytokine production, the colonic expression of inflammatory cytokines IL-6, IL-8, IL-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α as well as the regulatory cytokines IL-10 and TGF-β was analyzed by real-time PCR, however no significant differences were observed between groups (Supplementary Figure S1c). In addition, LP cells isolated from the colon were stimulated with phytohemagglutinin and cytokine levels quantified by ELISA. We detected trends for a decreased production of IL-6, IFN-γ, and TNF-α not reaching statistical significance comparing the EcN-AvCys and saline control group, while protein levels of IL-10 were similar when comparing saline controls and EcN-AvCys-treated piglets (Supplementary Figure S1d).
Collectively these data show that the application of EcN-AvCys to piglets was well tolerated with no loss of piglets in the sensitive post-weaning phase. Colon inflammation was significantly suppressed in recipients of EcN-AvCys compared to saline controls, while EcN control feeding had no such effect. EcN-AvCys feeding did not lead to significant changes in local immune parameters. We thus asked whether EcN-AvCys might differentially affect epithelial barrier functions potentially involved in reducing post-weaning gut inflammation.
Transgenic probiotic improves intestinal barrier function
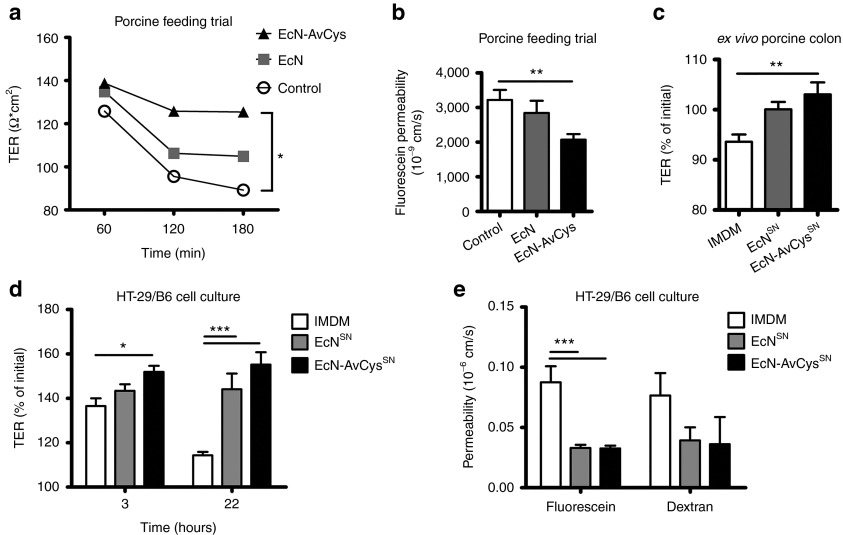
To assess whether the secretion of AvCys affected the documented support of EcN for intestinal barrier function,16,26,27 proximal colon tissue explants from pigs were analyzed in Ussing chambers after the feeding trial to determine the transepithelial resistance (TER) and paracellular passage of the small tracer molecule fluorescein (332 Da). Interestingly, while TER of explants from the EcN and especially the saline control fed group successively declined over time, colon explants from the EcN-AvCys group showed relatively stable TER values leading to a significantly higher resistance at 180 minutes compared to tissue from saline-treated controls (Figure 6a). Furthermore, a significant decrease in paracellular passage of the organic solute fluorescein was observed with colon tissue from EcN-AvCys treated piglets, but not in EcN inoculated controls, when compared to the saline control group (Figure 6b). These data suggest that EcN-AvCys improved epithelial barrier function in vivo.
Figure 6.
Effects of EcN-AvCys on epithelial barrier functions. (a) Transepithelial resistance (TER) of excised colon tissue from pigs treated with EcN (n = 8), EcN-AvCys (n = 10), and controls (n = 9) was determined in Ussing chambers at 60, 120, and 180 minutes after tissue stabilization. (b) Colon tissue as in a was excised from euthanized piglets and permeability to the 332Da tracer molecule fluorescein was determined in Ussing chambers. (c) TER across colon tissues excised from untreated, healthy piglets after exposure to either IMDM (IMDM, n = 7), EcN conditioned IMDM media (EcNSN, n = 7) or EcN-AvCys conditioned IMDM media (EcN-AvCysSN, n = 7) for 6 hours. Values are expressed as % of the initial value after tissue stabilization. (d) TER of HT-29/B6 cell monolayers exposed to either IMDM (n = 6), EcNSN(n = 6) or EcN-AvCysSN (n = 6) for 3 and 22 hours. Values are expressed as percent of the initial value after tissue stabilization. (e) Paracellular flux of fluorescein and 4 kDA dextran measured with HT-29/B6 cell monolayers in Ussing chambers after preincubation with EcNSN (n = 8), EcNAvCysSN (n = 7), or unconditioned media as a control (n = 8) for 22 hours. Mean + SEM is shown. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way or two-way ANOVA).
Next we aimed to ascertain if prolonged contact of intestinal tissue with EcN-AvCys in vivo was necessary for the supportive effects on epithelial barrier functions or whether exposure to EcN-AvCys culture supernatant (EcN-AvCysSN) could induce similar effects. Thus, we determined TER of colon tissue from untreated, weaned piglets before and after exposure to either EcN-AvCysSN or EcNSN. The relatively brief contact (6 hours) of colon tissue to EcN-AvCysSN also significantly increased TER compared to the control group, while EcNSN had an intermediate effect not reaching statistical significance (Figure 6c).
We next asked if the effects of EcN-AvCysSN on epithelial barrier functions were restricted to porcine gut tissue or also detectable in cultures of epithelial cells devoid of macrophages and other immune cells. We thus incubated monolayers of the human colon epithelial cell line HT-29/B6 with EcN-AvCysSN or EcNSN. Compared to the control media, a significant increase in TER was observed when cells were incubated for 3 hours with EcN-AvCysSN, but not with EcNSN, while exposure for 22 hours to either EcN-AvCysSN or EcNSN led to highly significant increases in resistance (Figure 6d). To determine whether the increased TER of HT-29/B6 monolayers in response to EcN-AvCysSN and EcNSN could be ascribed to the differential modulation of small and large pores permitting the paracellular passage of anorganic ions we measured the flux of fluorescein passing through small pores (approx. 4.5Å) and labeled 4 kDa dextran restricted to passage through larger pores (> 7Å). While contact to EcN-AvCysSN and EcNSN significantly decreased the passage of fluorescein, the passage of dextran was not affected significantly (Figure 6e). Hence, EcN and EcN-AvCys predominantly affect the availability of small pores for the passage of small anorganic solutes leading to an increase in TER in monocultures of human epithelial cells.
Taken together, these data show that both EcN and EcN-AvCys affect the TER of epithelial monocultures in vitro; however, EcN-AvCys has a strong potential to enhance intestinal epithelial barrier functions in vivo. We show that the released protein in the bacterial culture supernatant also enhanced barrier function of porcine colon explants after relatively short contact. It awaits further investigation whether immune cells such as macrophages are mediating these effects on gut tissue.
Discussion
IBD affects approximately 3.6 million North Americans and Europeans as an incurable disease that often requires lifelong treatment. There is an apparent and urgent need to develop new IBD therapies that are safe, effective, cost efficient, and have the potential to treat a broad spectrum of patients suffering from IBD of varying etiologies. Cystatin from a parasitic worm (AvCys) is an interesting candidate for the treatment of IBD as the recombinant protein efficiently inhibits inflammatory responses in murine models of allergic airway hyperreactivity and colon inflammation.6,7,8 Here we aimed to generate and test a system allowing for the site directed, prolonged release of the potent nematode immunomodulator in the gut. As vehicle we chose E. coli Nissle 1917, as this probiotic, marketed as Mutaflor (Ardeypharm, Herdecke, Germany), has been shown to efficiently suppress infant diarrhea and IBD in clinical trials,11,28 and to ameliorate colitis in animal models.12,16,29 Additionally, EcN readily colonizes the human gut and is suggested as a safe carrier for therapeutic proteins.18 Thus, we tested for its effects on intestinal inflammation in a mouse model of IBD and spontaneously occurring inflammation in post-weaning pigs, the latter serving as a genetically diverse model with a gastrointestinal system close to that of humans.
We confirm that EcN inhibits murine intestinal inflammation as shown in previous studies.12,16,29 Importantly, the high efficiency of EcN in ameliorating murine colitis could be further improved by the secretion of the nematode AvCys. This beneficial effect was associated with significantly reduced frequencies of inflammatory F4/80+Ly6Chigh monocytes/macrophages and a decrease in iNOS+ M1 macrophage numbers in the colon. Our previous studies show that macrophages are the dominant target of AvCys, that they transiently produce high amounts of IL-10 after contact with AvCys and subsequently suppress inflammatory T cells.6,8 The current study shows that the application of EcN-AvCys significantly suppressed the production of chemokines in the colon. MIP-1α/β, MCP-1/3, and RANTES efficiently recruit monocytes/macrophages, T cells, and granulocytes to sites of tissue damage and inflammation, activate monocytes/macrophages, and lead to degranulation of attracted granulocytes.30 The increased expression of these chemokines has been associated with active disease in IBD patients.31,32 As activated monocytes and macrophages are important chemokine sources, it is tempting to speculate that AvCys produced by the transgenic probiotic interferes with local chemokine production by tissue resident macrophages and subsequent leukocyte migration to the gut. Future studies will further address these newly detected effects of AvCys to unravel whether the drastically reduced colon inflammation in mice fed EcN-AvCys primarily reflects an effect on macrophage-mediated recruitment of inflammatory monocytes.
Production of IL-17A, a cytokine essentially involved in DSS-induced pathology,33 was significantly suppressed, while Treg frequencies in the gut draining lymph nodes were increased in recipients of EcN-AvCys. Although we did not formally test the contribution of Tregs to the suppressed colitic response in recipients of the transgenic probiotic in the present study, their increased frequencies correlating with a reduced inflammatory cytokine expression and lower tissue damage are in line with the established view of the central importance of Tregs in controlling intestinal inflammatory processes.34 Furthermore, in previous studies, Foxp3+ Tregs were also positively affected when recombinant AvCys was applied intraperitoneally in disease models of experimental airway hyperreactivity and colitis and their depletion partially abolished the protective effects of rAvCys.6,7 The reciprocity between Th17 and Treg developmental programs35 make it tempting to speculate that AvCys secreted by EcN tips the balance between Tregs and Th17 cells. Our data show that local levels of IL-6, a cytokine driving Th17 differentiation in presence of TGF-β,35 sharply decline in response to EcN-AvCys treatment, which might be involved in the significantly lower IL-17 levels detected after feeding of the transgenic probiotic. Furthermore, levels of active TGF-β were also significantly lower in mice after EcN-AvCys treatment, a factor possibly further restricting the differentiation of Th17 cells. It remains to be investigated whether IL-21 and IL-1β supporting Th17 differentiation,36,37 are also controlled by EcN-AvCys feeding. IL-23 supporting Th17 maintenance while restraining Treg activity20,21 was not affected by EcN-AvCys feeding in our study (data not shown) and thus is unlikely to be responsible for the observed changes.
Retinoic acid produced by intestinal CD103+ dendritic cells (DC) supports Treg induction and function by enhancing TGF-β and restricting IL-6 signaling.38 Thus future trials will have to elucidate whether changes in intestinal DC producing retinoic acid are associated with the preferential support for Tregs and suppression of IL-17 responses by EcN-AvCys.
Our previous studies show that AvCys utilizes MAPK signaling resulting in IL-10 expression in macrophages.8 Recently it has been shown that ERK signaling is involved in the Treg/Th17 dichotomy. Blockade of ERK activation results in suppression of Th17 development by interfering with IL-6 mediated RORγt expression, while supporting TFG-β driven upregulation of Tregs.39 Thus AvCys might interfere with the Treg/Th17 balance in vivo by affecting MAPK pathways in T cells.
We did not find increased IL-10 production by T cells in response to EcN-AvCys treatment in our study. It thus is unlikely that Foxp3+ or Foxp3- T cells, by producing IL-10, restrained Th17 responses, a mechanism previously shown by Huber et al.40
Taken together the data provided in this study suggest that a reduced chemokine expression in the gut, low numbers of inflammatory monocytes/macrophages, support for Tregs by a yet unknown mechanism and suppression of IL-6 and Th17 responses resulted in the highly significant suppression of inflammation by EcN-AvCys in a murine model of acute colitis.
For further verification of the anti-inflammatory effects of the transgenic probiotic, we chose to investigate pigs as outbred individuals with a gastrointestinal tract serving as an excellent model for the human digestive system.22 Pigs are susceptible to colonization with EcN,41 and, importantly, transiently suffer from a failure to thrive after weaning. This sensitive post-weaning phase leads to considerable loss in commercial pig farming and is associated with spontaneous gut inflammation that is not caused by any particular pathogen, but rather induced by stress and the enforced diet change, leading to microbiota changes and gut malfunction.23,24 We show that feeding high doses of transgenic probiotic bacteria over two weeks was well tolerated and did not lead to adverse effects. EcN-AvCys fed pigs showed significantly reduced signs of post-weaning inflammation in the distal colon. At the dissection time point chosen for our pig trial, reduced colon inflammation scores in piglets fed EcN-AvCys were not associated with changes in the mRNA expression of pro- and anti-inflammatory cytokines in the colon. It has previously been reported that initial inflammatory cytokine responses in the porcine gut decline within the second week post-weaning.42 Thus, possible initial differences in gut cytokine expression patterns induced by EcN-AvCys-feeding involved in the reduction of gut inflammation may have been missed at the endpoint of our trial, while differences in immune cell infiltrates and thus inflammation scores were still apparent. In line with the colon mRNA data, LP cells isolated from the colon of all treatment groups responded with similar cytokine secretion patterns to polyclonal stimulation in vitro. Thus future trials will include earlier sampling time points to assess possible local immune changes in more detail.
As neither local frequencies of Tregs nor colon macrophage numbers and activation status assessed via SLA II expression were altered in pigs fed EcN-AvCys it seemed unlikely that these players were directly responsible for the detected difference in colon inflammation. Future studies will have to assess possible effects of EcN-AvCys on chemokine expression in the porcine gut. However, our data show that EcN-AvCys positively affected epithelial barrier functions in vivo and in vitro. Others have shown that EcN supports the intestinal epithelial barrier and restrains both chemical and pathogen-induced destruction of barrier function.15,16,27 In our studies, TER of colon explants was most markedly improved after feeding of transgenic EcN-AvCys. A similar effect was detected when colon tissue from untreated pigs was subjected to culture supernatant of the transgenic probiotic. These data indicate that AvCys released by the probiotic bacteria was sufficient to support TER and, in some conditions, surpassed the effects of EcN alone. This previously unrecognized effect of nematode cystatin was most consistently seen with porcine gut tissue, while prolonged in vitro exposure to EcNSN and EcN-AvCysSN similarly affected TER in human epithelial cell monocultures, most likely via the availability of small pores for paracellular ion passage.43 However, our data show that EcN-AvCysSN increased TER of human colon epithelial cells more readily after short-term exposure than EcNSN. More work is needed to clarify whether the transgenic probiotic may also increase the barrier function of human gut explants and whether immune cells such as macrophages are needed for the physiological changes detected in this study.
To our knowledge we are the first to design and successfully apply a probiotic carrier for a characterized parasitic immunoregulatory protein, but probiotics have been used previously as vehicles for immunomodulatory factors.44,45 The fact that the etiology of IBD is associated with over 100 different gene polymorphisms,9 and insufficiently understood distinct causes for microbiome distruptions,46 underlines the importance of treatment options for this multifaceted disease. It is not surprising that currently there is a lack of therapies that reliably work in a broad spectrum of patients, unless accompanied by sometimes heavy side effects as seen with corticosteroid treatment. Combining several probiotic strains may increase the likelihood to significantly ameliorate IBD. EcN-AvCys may be a new therapeutic option for IBD, as parameters of experimental and spontaneous inflammation and tissue integrity could be further improved by the transgenic probiotic compared to “standard” EcN. In clinical trials between 16% and 36% of IBD patients responded to EcN treatment alone.11 For patients unresponsive to EcN or other probiotic therapeutics, the application of a transgenic probiotic secreting a potent parasite immunomodulator could improve the chances of induction and maintenance of remission.
In conclusion, we show that a transgenic probiotic bacterium expressing and secreting a potent helminth immunoregulator is able to improve intestinal epithelial barrier function in porcine tissue and human cell cultures and significantly inhibits inflammation in a murine colitis model as well as in post-weaning piglets. Hence, the transgenic EcN-AvCys represents a promising therapeutic approach, taking advantage of an evolutionary designed immunoregulator for the treatment of colitis and warrants further investigation as a potential therapy for a wide range of human IBD patients.
Materials and Methods
Ethics statement. All animal experiments were approved by and conducted in accordance with guidelines of the appropriate committee (Landesamt fuer Gesundheit und Soziales, Berlin, Germany) under license numbers LAGeSo Reg. Nr. G0144/10 and G0350/09.
Construction of the transgenic probiotic. EcN was a kind gift by Dr. Tobias Oelschläger from the University of Würzburg. The strain carries a kanamycin resistance cassette on the EcN specific plasmid pMUT2 and a genetically modified version of the native EcN specific plasmid pMUT1. The modified pMUT1 plasmid (pMU13) was designed for functionality as a cloning vector through the addition of a tetracycline resistance cassette, a multiple cloning site and a hemolysin A secretion system. All NsiI cut sites outside of the hlyA gene were deleted to allow for insertion of genes within the hlyA component and to ensure that protein expressed from the inserted gene is secreted.
For insertion of AvCys, primers were designed to add NsiI restriction enzyme cut sites to both the 3′ and 5′ ends of the AvCys sequence when amplified (forward 5′-ACGTATGCATTGGTGCGCTGTGAAGA-3′, reverse 5′-ACGTATGCATTCACTGATGAGAGTACT-3′). The amplified AvCys gene was then inserted into the pMU13 vector and transformed into chemically competent EcN. Positive clones were confirmed with primers specific to the AvCys insert with flanking regions in the plasmid (forward 5′-TCGTGTCGACGGTTTTGGTGCGCTGTGAAGAAC-3′, reverse 5′-ACATGCGGCCGCTCACACTGATGAGAGTA-3′) with an expected amplicon size of 431bp via PCR. EcN-AvCys supernatants were analyzed to ensure secretion. Proteins were precipitated from 5 ml of supernatant from cultures of EcN or EcN-AvCys grown to OD600 = 1, which corresponded to a concentration of 3 × 108 CFU EcN/ml, and analyzed via Western blot with a monoclonal AvCys-specific antibody.
Production of EcN and EcN-AvCys supernatant. Supernatants of EcN or EcN-AvCys were produced from cultures in Luria broth media containing 20 µg/ml tetracycline, 50 µg/ml kanamycin, and 50 µg/ml streptomycin incubated overnight at 37 °C with shaking at 200 rpm. Cultures were diluted 1:1,000 in fresh Iscoves modified Dulbeccos media (IMDM) with 4 mmol/l L-glutamine (PAA, Austria) and further incubated at 37 °C with shaking at 200 rpm until reaching an OD600 = 1.00. Cultures were centrifuged and supernatant filtered through 0.2 µm filters, before being stored at −20 °C. Concentration of AvCys was determined using a direct coating ELISA using a recombinant AvCys standard curve and an anti-rAvCys monoclonal antibody developed in house.
Animal experimentation
Murine dextran sodium sulfate colitis. Male, 9–11 week old C57BL/6 mice (Charles River, Sulzfeld, Germany) were randomly assigned to one of four groups kept separately under specific pathogen free conditions in filter topped cages with standard bedding, environmental enrichment, and 12 hours day/night cycles in three independent replication experiments: untreated controls (n = 2–3, 5 in total), DSS controls (n = 5, 14 in total), EcN-treated DSS controls (n = 5, 12 in total), and an EcN-AvCys treated DSS group (n = 5, 14 in total). All animals except untreated controls received 3.0% DSS in the drinking water for seven days. Immediately after the introduction to DSS and every 48 hours thereafter the EcN-AvCys group and EcN control group were treated via oral gavage with 100 μl saline containing 2 × 109 CFU EcN-AvCys or EcN, respectively. Untreated and DSS control groups received saline alone. Animals were sedated by isofluran inhalation (Foren, Abbott) and euthanized by cervical dislocation on day eight after start of DSS feeding. Mice experiencing a body weight loss of 20% before day weight were euthanized and excluded from further examination. The study was approved by the local state office for occupational health and technical safety (LAGeSo Reg. Nr. G0144/10).
Swine study. Thirty male Pietran x Landrace cross piglets from a local commercial breeding facility were delivered and subsequently weaned at age of 28 days and randomly assigned to one of three groups; a transgenic EcN-AvCys group (n = 10), an E. coli Nissle treated control group (n = 8 due to losses during first week of trial), and a saline-treated control group (n = 9 due to loss during first week of trial). They were allocated in a level-2 biosecured, environmentally controlled experimental facility (Federal Institute for Risk Assessment, Berlin, Germany), 4–5 pigs per pen of 8.5 m2. Pigs in two pens formed one group. The pens were in separated rooms to prevent cross contamination. No signs of infections were visible during the trial or at dissection and pigs were free of concurrent worm infections. Rooms were constantly ventilated and kept at temperature of 26 °C. Every 48 hours for a total of 14 days pigs were orally inoculated with 1 ml of saline containing 2 × 1010 CFU/ml EcN-AvCys or EcN, respectively, or 1 ml of 0.9% saline alone. Animals were weighed at day 0, 7, and 14. On day 14, animals were sedated by intramuscular injection of azaperone (2 mg/kg, Stresnil) before being deeply sedated with ketamine (25 mg/kg, Ursotamin, Serumwerke Bernburg, Bernburg, Germany), and finally euthanized with a lethal intravenous dose of pentobarbital (200 mg/kg, Narcoren, Merial, Hallbogmoos, Germany). Just before the euthanasia full blood was collected from the heart. The study was approved by the local state office for occupational health and technical safety (LAGeSo Reg. Nr. G0350/09).
Histopathological analysis and immunohistochemistry. Porcine and murine distal colon sections were fixed in 3.7% formalin, dehydrated using ethanol concentrations increasing from 70% to 96%, cleared with xylene, embedded in paraffin, mounted, and stained with hematoxilin/eosin (H&E) for histopathological scoring (blinded) according to two systems. The scoring parameters for the DSS-induced colitis model were as follows: inflammation (0: no inflammation; 1: increased number of inflammatory cells in LP; 2: inflammatory cells extending into the submucosa; 3: transmural inflammatory infiltrates), and tissue damage: (0: no mucosal damage; 1: discrete epithelial lesion; 2: erosion or focal ulceration; 3: severe mucosal damage with extended ulcerations extending into bowel wall). Porcine colon cross sections were scored according to the following parameters: infiltration (1: minimal; 2: mild; 3: moderate; 4: severe), degree of infiltration (1: mucosal; 2: mucosal and focal submucosal; 3: mucosal and submucosal; 4: transmural), epithelial surface damage (1: focal denudation; 2: extensive denudation; 3: erosion; 4: ulceration), crypt epithelial damage (1: sporadic crypt abscesses; 2: multiple crypt abscesses), and hyperplasia (1: minimal; 2: mild; 3: moderate; 4: severe).
For detection of M1 macrophages, 1–2 µm sections of formalin-fixed, paraffin-embedded tissue were cut, deparaffinized, and subjected to a protein-induced epitope retrieval step. Slides were rinsed in Tris-buffered saline (pH 7.4) prior to incubation with monoclonal rat anti-mouse F4/80 antibody (clone BM8, Invitrogen; dilution 1:100) for 30 minutes at room temperature followed by incubation for 30 minutes with biotinylated secondary antibody donkey anti-rat (Invitrogen; dilution 1:200). For detection, DAKO REALTM Detection System, Alkaline Phosphatase/RED was used. The stained sections were then subjected to a heat-induced epitope retrieval step. Slides were rinsed in cool running water, washed in Tris-buffered saline (pH 7.4) and treated with Peroxidase Blocking Solution (Dako) prior to incubation with polyclonal rabbit anti mouse-iNOS antibody (Abcam; 1:100). For detection, EnVision+ System-HRP (DAB) kit (Dako) was used. Alkaline phosphatase was revealed by Fast Red as chromogen for 30 minutes, and horse radish peroxidase was developed with a highly sensitive diaminobenzidine (DAB) chromogenic substrate for 5 minutes. For negative controls, primary antibodies were omitted. Nuclei were counterstained with hematoxylin and slides mounted with gelatine (Merck). Images were acquired using a AxioImager Z1 microscope (Carl Zeiss MicroImaging). Positive cells were quantified in 10 high power fields (hpf = 0.237 mm2). All immunohistochemical evaluations were performed in a blinded manner.
Cell isolation and culture. For the isolation of leukocytes from spleen and gut draining mLNs minced tissue was passed through 70 µm cell strainers. For isolation of gut LP leukocytes colon tissue was washed twice in cold Hanks' balance salt solution followed by two washes for 20 minutes at 37 ° and 180 rpm in HBSS/5mmol/l EDTA. Remaining tissue was minced and stirred in 5% FCS RPMI medium containing 200 U/ml of collagenase VIII (Sigma-Aldrich, Munich, Germany) and 1 U/ml collagenase D (Roche, Mannheim, Germany) at 37 °C for 1 hours. Samples were then passed over 70 µm cell strainers, washed, put on a 40/70% Percoll (GE Healthcare, Munich, Germany) gradient, spun at room temperature (800×g for 20 minutes), and lymphocytes recovered from the interphase. After erythrocyte lyses, spleen, mLN and LP cells were washed and resuspended in complete RPMI 1640 (PAA, Austria) containing 10% fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 20 mmol/l L-glutamine (Biochrom, Germany) and counted with a CASY Cell Counter (Innovatis, Germany). Cells were plated at a concentration of 3.5 × 105 cells/well on 96-well plates in 200 µl and kept for 48 hours with media, concanavalin A (conA, 2 µg/ml) or recombinant AvCys (9.6 μg/ml), or phytohemagglutinin (PHA, 2 µg/ml).
Cytokine analysis. Murine specific ELISA antibody pairs for IL-17A (eBioscience) and porcine specific ELISA antibody pairs for IL-6, IFN-γ, TNF-α, and IL-10 (R&D Systems) were used according to manufacturer recommendations to determine cytokine levels in cell culture supernatants. Cytokines in murine colon tissue were analyzed applying the ProcartaPlex system (eBioscience). In brief, 0.5 cm tissue samples were excised from the distal colon, washed in cRPMI, weighed and homogenized in 250 µl ProcartaPlex lysis buffer containing a protease inhibitor cocktail (Roche, Germany) using FastPrep-24 Lysing Matrix Tubes D (MP Biomedical, Germany). After spinning (10,000×g, 10 minutes, 4 °C), supernatants were collected and protein content measured by a bicinchoninic acid assay (Thermo Scientific Pierce, USA). MIP1-α, MIP-1β, MCP-1, MCP-3, and RANTES were quantified according to the manufacturer's instructions using the BioPlex Multiplex system (BioRad, USA) and expressed in relation to the total protein content. IL-6 and IL-23 were measured accordingly in culture supernatants of colon explants kept in RPMI 1640 (PAA, Austria) containing 1% fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin and 20 mmol/l L-glutamine (Biochrom, Germany) at 37 °C for 24 hours and expressed in relation to mg colon tissue in cultures. Active TGF-β in colon explant supernatants was measured with the MFB-F11 reporter cell line.47 Cells were adhered for 4 hours to 96-well flat-bottom plates at 4 × 104 cells/well in DME containing 4.5% glucose 10% FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin followed by 2 wash steps with PBS and a 2 hours starvation period in 50 µl serum-free medium. Then 50 µl of test samples was applied. Doubling dilutions of rhTGF-β1 (R&D Systems) starting with 1 ng/ml were used as a standard. After 24 hours, samples were measured on a Synergy H1 microplate reader (BioTek, USA) using a chemiluminescent SEAP Reporter Gene Assay (Roche, Germany) kit as per the manufacturer's instructions.
Flow cytometry. Murine mLN and colon LP leukocyte preparations were stained with the following antibodies purchased from eBioscience: αCD4-Percp-eFluor710 (clone RM4-5), αCD25-APC (clone PC61.5), αFoxp3-eFluor450 (clone FJK-16S), αF4/80-biotin (clone BM8), αCD11b-FITC (clone M1/70), αGR-1-APC (clone RB6-8C5), αLy6C-eFluor450 (clone HK1.4). αSiglec-F-PE (clone E50-2440) for the exclusion of eosinophils was purchased from (BD Biosciences). Porcine leukocytes were stained with the following antibodies: unconjugated αCD25 (clone K231.3B2), unconjugated αSLA class II DR (cone 2E9/13), αCD163-PE (clone 2A10/11) and biotinylated sheep αmouse IgG (all AbD Serotec, Germany). αCD4a-PE (clone 74-12-4) was purchased from Southern Biotech (USA). αFoxP3-eFluor450 staining kit (clone FJK-16S), streptavidin-PE-Cy7 and eFluor 780 fixable viability dye were purchased from eBioscience (USA). Samples were acquired using an LSR II flow cytometer (BD Biosciences). Analysis was performed using FloJo software (Treestar, USA).
Quantitative real-time PCR. Porcine distal colon segments were snap frozen in liquid nitrogen. As per manufacturers protocols, 200 mg of distal colon tissue was homogenized using the FastPrep-24 Lysing Matrix Tubes D (MP Biomedical, Germany), RNA was extracted using innuPREP RNA kit (Analytikjena, Germany) and finally cDNA transcribed with the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Germany). Real-time PCR reactions were conducted in a Mastercycler ep gradient S thermocycler with a Realplex2 detection system (Eppendorf, Germany) using FastStart Universal SYBR Green Master Mix (Roche). Specific primers for the swine housekeeping gene RPL19 as well as the cytokines TGF-β, IL-6 and IL-10 were adapted from Pieper et al. 2012.48 Specific primers for swine IL-8, IL-12, IFN-γ, and TNFα were developed based on the NCBI reference gene sequences. IL-8: forward-GGTCTGCCTGGACCCCAAGGAA, reverse-TGGGAGCCACGGAGAATGGGTT, NCBI ref. seq. NM_213867.1; IL-12: forward-AGCCCGGGACCGCTACTACAG, reverse-GGGGGAGGGGTCTGCTCCATC, NCBI ref. seq. NM_214013.1; IFN-γ: forward-TCCAGCGCAAAGCCATCAGTG, reverse-ATGCTCTCTGGCCTTGGAACATAGT, NCBI ref. seq. NM_213948.1; TNF-α: forward-CAAGCCACTCCAGGACCCCCT, reverse-AGAGTCGTCCGGCTGCCTGT, NCBI ref. seq. NM_214022.1. The ΔΔCT method was used to determine the fold increase of these cytokines in treatment groups compared to the control group using CT values normalized to a house keeping gene (RPL19).
Thymidine proliferation assay. Porcine mLN cells were incubated with rAvCys or a recombinant control protein for 72 hours and then pulsed with 1 µCi of 3H-thymidine (MP Biomedicals, Germany) for 20 hours. The proliferative response was analyzed using a beta-counter (PerkinElmer).
Electrophysiological studies. Stripped mucosae were mounted into Ussing chambers driven by a multi-channel computer-controlled voltage clamp device as described previously.49 The bath solution contained (in mmol/l): 113.6 NaCl, 2.4 Na2HPO4, 0.6 NaH2PO4, 21 NaHCO3, 5.4 KCl, 1.2 CaCl2, 1 MgSO4, 10 D(+)-glucose, 0.5 β-OH-butyrate and 2.5 glutamine. The solution was gassed and mixed using a bubble lift (95% O2 and 5% CO2, pH 7.4). The temperature was kept constant at 37 °C. Antibiotics (Azlocillin 50 mg/l and Tobramycin 4 mg/l served to prevent bacterial growth and had no effect on short circuit current (ISC in µA/cm2). Each side of the tissue was perfused with 5 ml of bathing solution and BSA (final concentration of 0.002%) was routinely added and short-circuit current (ISC in µA/cm2) and transmucosal resistance (TER in Ω∙cm2) were continuously recorded. Resistance of the bathing solution and electrode offsets were determined and subtracted from raw data before each experiment. Permeability to fluorescein (Sigma, Taufenkirchen, Germany) from mucosal to serosal compartment was determined during a 2 hours period (1–3 hours total time) in Ussing chambers in voltage-clamp mode. Briefly, 100 µmol/l of fluorescein was added to the mucosal compartment and serosal concentration was measured as fluorescence using a plate reader (TECAN 200M, Groeding, Austria). Permeability was calculated from: (dQ/dt)/(A×Co), where dQ/dt is the cumulative amount of tracer compound appearing in the receiver compartment vs. time, A is the area tissue, and Co is the initial concentration of the tracer in the donor compartment. HT-29/B6 cells, a subclone of the human colon carcinoma cell line HT-29, were cultured in RPMI 1640 (PAA Laboratories GmbH, Pasching, Austria) supplemented with 10% fetal calf serum, and 1% penicillin/streptomycin 37 °C in an air atmosphere containing 5% CO2. For experiments, cells were seeded on Millicell PCF filter supports (effective area 0.6 cm2, 3 µm pores, Millicell PCF, Millipore, Schwalbach, Germany). Confluent monolayers were incubated in IMDM, EcN or EcN-AvCys IMDM supernatant from both sides. TER (Ω∙cm2) was determined with an ohmmeter (D. Sorgenfrei, Institute of Clinical Physiology). For the differential assessment of fluorescein and dextran fluxes, HT-29/B6 cell layers were preincubated with IMDM, EcN- or EcN-AvCyst-conditioned medium for 22 hours. Permeability of preincubated HT-29/B6 cell layers to fluorescein (Sigma, Taufenkirchen, Germany) and TRITC-labelled 4 kDa dextran (TdB Consultancy AB, Uppsala, Sweden) from mucosal to serosal compartment was determined during a 3 hours period in Ussing chambers in voltage-clamp mode. To this end, both fluorescein (final concentration 100 µmol/l) and TRITC-labelled 4 kDa dextran (final concentration 200 µmol/l) were added to the mucosal compartment and serosal concentration was measured as fluorescence using a plate reader (TECAN 200M, Groeding, Austria).
Statistics. Groups were compared with one-way and two-way ANOVA or Mann–Whitney U-test using GraphPad Prism software (San Diego, USA). Data is reported as means + SEM and differences were deemed significant if P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Immune parameters in response to EcN-AvCys in swine feeding trial.
Acknowledgments
We thank Tobias Oelschläger of the Universität Würzburg who gifted us the pMUT13 cloning plasmid and B Sonnenburg, M Müller, Y Weber, and A Fromm for excellent technical assistance. This research was supported by German Research Foundation, grant 852 (to S.H., J.D.S., K.N., and L.H.W.) and the Broad Medical Research Program (to S.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that no competing interests exist.
Supplementary Material
Immune parameters in response to EcN-AvCys in swine feeding trial.
References
- Whelan RA, Hartmann S, Rausch S. Nematode modulation of inflammatory bowel disease. Protoplasma. 2012;249:871–886. doi: 10.1007/s00709-011-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Klotz C, Ziegler T, Daniłowicz-Luebert E, Hartmann S. Cystatins of parasitic organisms. Adv Exp Med Biol. 2011;712:208–221. doi: 10.1007/978-1-4419-8414-2_13. [DOI] [PubMed] [Google Scholar]
- Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun. 2003;71:2422–2429. doi: 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- Daniłowicz-Luebert E, Steinfelder S, Kühl AA, Drozdenko G, Lucius R, Worm M, et al. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol. 2013;43:201–210. doi: 10.1016/j.ijpara.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011;7:e1001248. doi: 10.1371/journal.ppat.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi CA, Malfertheiner P. Escherichia coli Nissle 1917 (Mutaflor): new insights into an old probiotic bacterium. Dig Dis. 2011;29:600–607. doi: 10.1159/000333307. [DOI] [PubMed] [Google Scholar]
- Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, et al. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem Pharmacol. 2011;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- Schierack P, Kleta S, Tedin K, Babila JT, Oswald S, Oelschlaeger TA, et al. E. coli Nissle 1917 Affects Salmonella adhesion to porcine intestinal epithelial cells. PLoS One. 2011;6:e14712. doi: 10.1371/journal.pone.0014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Günzel D, et al. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014;7:369–378. doi: 10.1038/mi.2013.55. [DOI] [PubMed] [Google Scholar]
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazalé T, Raupach B, et al. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun. 2005;73:1452–1465. doi: 10.1128/IAI.73.3.1452-1465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf AM, Gunzer F, Deppenmeier S, Tapadar D, Hunger JK, Schmidt MA, et al. Intestinal immunity of Escherichia coli NISSLE 1917: a safe carrier for therapeutic molecules. FEMS Immunol Med Microbiol. 2005;43:373–384. doi: 10.1016/j.femsim.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, et al. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993–6003. doi: 10.4049/jimmunol.1003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Gauvreau H, Harding J. Diagnostic investigation of porcine periweaning failure-to-thrive syndrome: lack of compelling evidence linking to common porcine pathogens. J Vet Diagn Invest. 2012;24:96–106. doi: 10.1177/1040638711425939. [DOI] [PubMed] [Google Scholar]
- Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Pedersen KS, Kristensen CS, Nielsen JP. Demonstration of non-specific colitis and increased crypt depth in colon of weaned pigs with diarrhea. Vet Q. 2012;32:45–49. doi: 10.1080/01652176.2012.675091. [DOI] [PubMed] [Google Scholar]
- Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. Int J Biochem Cell Biol. 2012;44:341–349. doi: 10.1016/j.biocel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement Altern Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Göttl C, et al. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11:372–378. doi: 10.1128/CDLI.11.2.372-378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, et al. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–206. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12–16. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, Pulendran B. The gut feeling of Treg cells: IL-10 is the silver lining during colitis. Nat Immunol. 2009;10:1141–1143. doi: 10.1038/ni1109-1141. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim YG, Núñez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Liu H, Yao S, Dann SM, Qin H, Elson CO, Cong Y. ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur J Immunol. 2013;43:1716–1726. doi: 10.1002/eji.201242889. [DOI] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleta S, Steinrück H, Breves G, Duncker S, Laturnus C, Wieler LH, et al. Detection and distribution of probiotic Escherichia coli Nissle 1917 clones in swine herds in Germany. J Appl Microbiol. 2006;101:1357–1366. doi: 10.1111/j.1365-2672.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- Hu C, Song J, Li Y, Luan Z, Zhu K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br J Nutr. 2013;110:681–688. doi: 10.1017/S0007114512005508. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118 Pt 22:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Ahn JH, Park SH, Do KH, Kim J, Moon Y. Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics. Infect Immun. 2012;80:1079–1087. doi: 10.1128/IAI.05820-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuvaraj S, Peppelenbosch MP, Bos NA. Transgenic probiotica as drug delivery systems: the golden bullet. Expert Opin Drug Deliv. 2007;4:1–3. doi: 10.1517/17425247.4.1.1. [DOI] [PubMed] [Google Scholar]
- Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years. World J Gastroenterol. 2014;20:1192–1210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Berber E, Zhang H, Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC Cell Biol. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R, Kröger S, Richter JF, Wang J, Martin L, Bindelle J, et al. Fermentable fiber ameliorates fermentable protein-induced changes in microbial ecology, but not the mucosal response, in the colon of piglets. J Nutr. 2012;142:661–667. doi: 10.3945/jn.111.156190. [DOI] [PubMed] [Google Scholar]
- Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6) Am J Physiol. 1991;261 4 Pt 1:C574–C582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immune parameters in response to EcN-AvCys in swine feeding trial.