Abstract
Alzheimer's dis ease (AD) is a leading cause of mortality in the developed world with 70% risk attributable to genetics. The remaining 30% of AD risk is hypothesized to include environmental factors and human lifestyle patterns. Environmental factors possibly include inorganic and organic hazards, exposure to toxic metals (aluminium, copper), pesticides (organochlorine and organophosphate insecticides), industrial chemicals (flame retardants) and air pollutants (particulate matter). Long term exposures to these environmental contaminants together with bioaccumulation over an individual's life-time are speculated to induce neuroinflammation and neuropathology paving the way for developing AD. Epidemiologic associations between environmental contaminant exposures and AD are still limited. However, many in vitro and animal studies have identified toxic effects of environmental contaminants at the cellular level, revealing alterations of pathways and metabolisms associated with AD that warrant further investigations. This review provides an overview of in vitro, animal and epidemiological studies on the etiology of AD, highlighting available data supportive of the long hypothesized link between toxic environmental exposures and development of AD pathology.
Keywords: Adult-onset disease, Alzheimer's disease, endocrine disruptors, environmental contaminants, metals, neuropathology, Parkinson's disease, pesticides, synergistic effects, toxins
BACKGROUND
Today, aging human populations around the globe are facing an epidemic of Alzheimer’s disease (AD), with the number of cases estimated to rise to nearly 106 million by 2050 [1]. Now representing the sixth leading cause of death in the United States (Fig. 1) [2, 3], AD accounts for 60 to 80 percent of reported cases of dementia [4] and 400,000 deaths in the U.S. alone in 2010 [4]. Human AD pathology is characterized by a progressive decline of cognitive function, memory, and intellectual ability [5] leading to irreversible neurodegenerative impairment. Although being diagnosed mostly as a late-onset disease [6], early onset (at age 40-50 years) AD has been observed in more than 200,000 people in the U.S. [7]. The key mediator of AD pathology is the brain amyloid-β protein that forms dimers and oligomers, leading to protein aggregation visible in the post-mortem brains as plaques. Plaques are accompanied by aggregates of phosphorylated tau protein called neurofibrillary tangles. Together these lesions are thought to cause synaptic loss and neuronal cell death, resulting in cognitive dysfunction [8, 9].
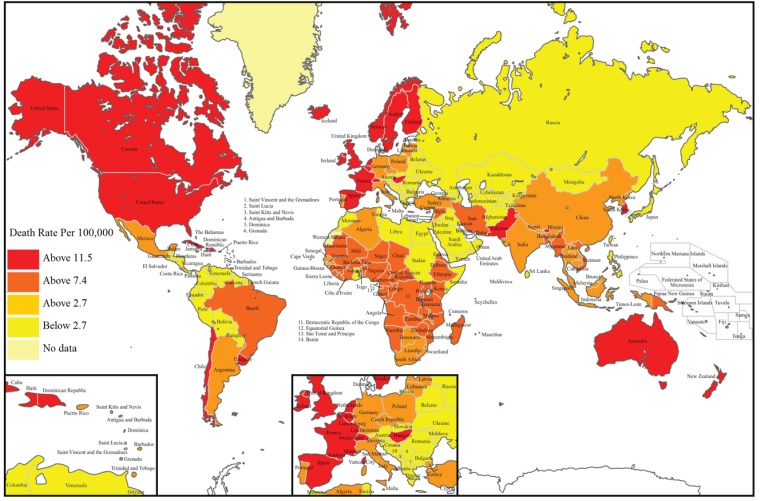
Fig. (1).
World map illustrating the global distribution of deaths caused due to Alzheimer’s Disease/Dementia using WHO data from 2011. Image courtesy: Image recreated from http://www.worldlifeexpectancy.com/cause-of-death/alzheimers-dementia/by-country/.
Multiple factors have been reported to contribute to the etiology of AD including, but not limited to, aging, genetics [10], head injury [11], and exposure to certain chemicals and compounds [12]. The genetic component of AD risk is well established as being associated mostly with the APOE-E4
allele [10] and with less common autosomal dominant forms of AD. In contrast, the role of environmental exposures and their mechanisms contributing to the pathogenesis of sporadic AD continues to be a subject of discussion. This is partly because of the presumably extended time lapse between exposure and onset of the disease. Scientists have proposed the LEARn (Latent Early–life Associated Regulation) model with an underlying “two-hit” theory, which combines genetic and environmental risk factors in an epigenetic pathway, suggesting that AD risk is established during early life [13, 14]. The progression of AD takes place over 1-3 decades and the estimated time between triggering events and onset of the disease ranges from several years to several decades, making it difficult to pinpoint particular causative factors. Of all risk factors associated with AD, genetic predisposition is believed to account for about 70% of the overall risk, with the remaining 30% thought to be due to obesity, smoking, lack of exercise, mid-life hypertension, diabetes and exposure during life to environmental agents [15, 16]. Recent research on AD has pinpointed the involvement of aggregated amyloid beta-protein and tau protein [17] while some studies emphasize the role of the LEARn pathway [18]. However, few studies have been directed towards the role of environmental toxins in the development of AD, and more laboratory and epidemiological studies are needed to identify possible associations. Importantly, while not all contaminants and toxins have been tested in research studies showing impact on the central nervous system (CNS), the risks of developing AD and Parkinson's disease (PD) in elderly persons as a result of neurologic impairments caused by environmental toxins is established [19].
In this article, we review the five categories of environmental agents, including (i) toxic metals, (ii) insecticides/pesticides, (iii) industrial/commercial pollutants, (iv) antimicrobials and (v) air pollutants, all known or hypothesized to induce or aggravate AD or AD-like progression in vitro, in animal models and in human research subjects (Figs. 2 and 3; Table 1). Toxic metals such as aluminium [20] and lead [21, 22] have been linked with numerous neurodegenerative diseases including AD, causing toxicity to multiple organs of the human body. Other elements such as copper and arsenic have been associated in experimental model systems with the disruption of homeostasis of brain amyloid-β protein [23, 24]. Chronic exposures to pesticides such as organophosphates [25], including occupational exposure especially in agriculture, have been shown to lead to cognitive and psychomotor impairment and possibly to the development of AD and Parkinson’s disease [19]. Murine neonates exposed to brominated flame retardants, which are readily absorbed by body fat, showed behavioural changes, while adult mice displayed impaired learning and memory [26]. Plasticizers (additives that soften plastic making it resilient and elastic) include bisphenol A and phthalates. These chemicals can cross the fetoplacental barrier, and were observed to result in growth retardation and neurological damage [27]. Broad spectrum antimicrobials, which are active ingredients of consumer products like soaps and toothpastes, are known to cause neurodevelopmental disturbances and behavioural changes; however, evidence directly linking these to AD is lacking [28, 29]. Studies utilizing animal models and epidemiological approaches have reported other evidence linking exposure to toxic metals [30, 31] and air pollutants [12] to neurological symptoms, including AD. Importantly, most of the implicated environmental toxins are endocrine disrupting chemicals featuring the potential to impair neurogenesis and cognitive function in the developing and aging brain, and affecting neurological function throughout the human lifespan [32].
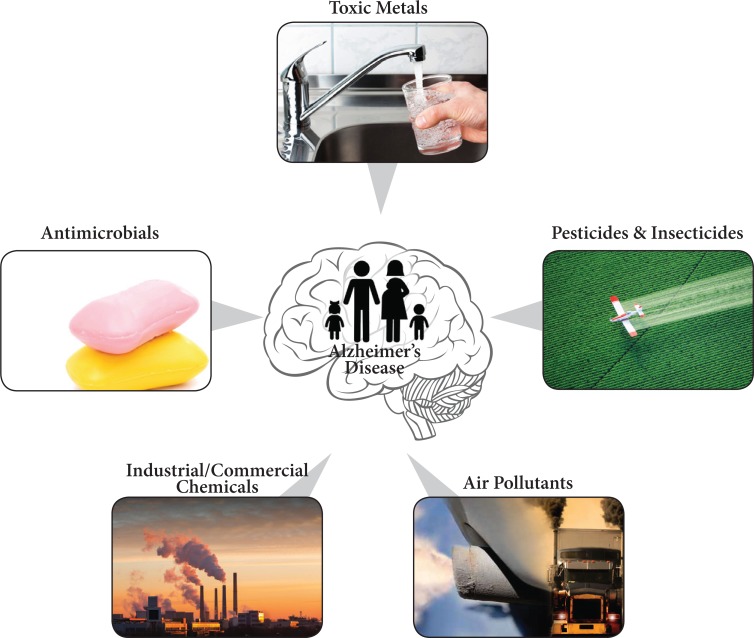
Fig. (2).
Environmental and man-made contaminants/toxins associated with AD include toxic metals, pesticides/insecticides, other industrial/ commerical chemicals, and air pollutants. Exposure occurring in utero, during child growth and development, in adult life causing an increased risk for AD and AD-like pathology later in life.
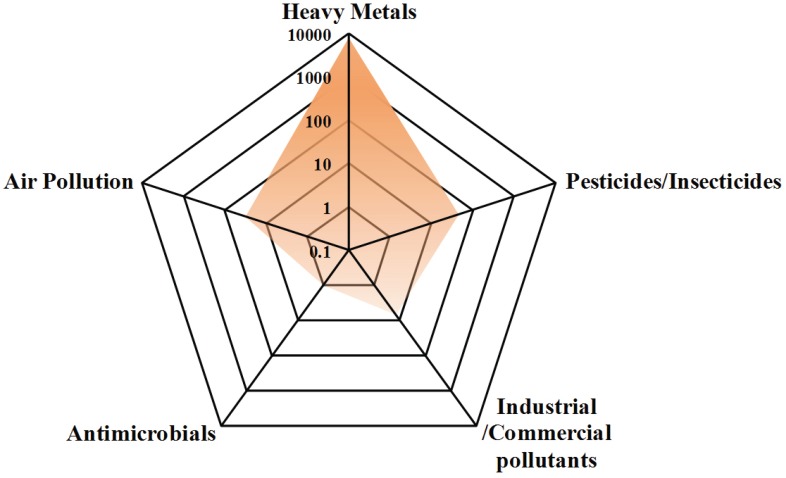
Fig. (3).
Radar graph representing studies published (8102 papers) on the five categories of environmental contaminants assocaited with AD or AD-like progression.
Table 1.
Research studies reporting environmental factors known or suspected to be directly or indirectly associated with patho-genesis of Alzheimer's disease.
| Environmental Factor | Dose Used in Experiment or Measured Levels | Reference | Human/ Animal/ In vitro Study | Summary |
|---|---|---|---|---|
| TOXIC METALS | ||||
| Aluminum | Above 1000 ng/L in drinking water may be a risk factor for dementia, especially, AD | [185, 186] | Human longitudinal epidemiological, PAQUID study 3,777 subjects yr. 2000 further yr. 2009 1,925 subjects | Membrane disruption or perturbation and increased deposition of senile plaques associated with spatial learning memory in AD |
| 13.2-14.4 µg/g dry weight (d.w.) Al brain compared to 0.8 µg/ g d.w. of normal brain in cats; 9-11 µg/ g d.w. of AD brain compared to 0.23- 2.7 µg/ g d.w. of normal brain in humans |
[37] | 18 cats injected with Aluminum; AD (n=5), Controls (n=3) |
Decreased cognitive function in cats; High Al levels associated with AD | |
| Control serum 580 ± 620 nmol/L; AD serum 905 ± 630 nmol/L | [23] | AD (n = 44) and Controls (n = 41) | increased uptake from dietary source | |
| 10 µM Al(III) | [43] | Amyloid-β protein experiments in presence of trace metals | Aggregation of Aβ42 to form amyloid fibrils, and later formation of plaque-like structures | |
| AD: 3.605-21.738 µg/g d.w.; Control 0.379 - 4.768, µg/g d.w. (post-mortem values) | [42] | AD (n=30), Control (n=30) | High Al levels associated with AD | |
| Arsenic | Long-term exposure to arsenic in groundwater of 240.15 ± 182.96 µg/L. On average, participants resided in their current residence for 34.12 years (sd = 20.01 years, range = 1–80 years) | [62] | Human longitudinal epidemiological Project FRONTIER Study (434 participants) | Long-term, low-level exposure to arsenic was significantly associated with poorer scores in global cognition |
| 10 µM sodium arsenite | [61] | Rat cerebellar granule neurons | Rat cerebellar granule neurons showed neurotoxicity, apoptosis and activation of p38 and JNK3 MAP kinases | |
| Concentrations of 13-15 mg/kg As in topsoil | [60] | Geological and epidemiological data: FOREGS Project, Delphi consensus study, mortality data by WHO | Slight changes in low-level environmental arsenic have the potential to change the prevalence and mortality of Alzheimer's disease and other dementias |
|
| Arsenic in drinking water at 10 µg/L | [187] | Accumulation in rat and human brain | Hyperphosphorylation of protein tau and overtranscription of the amyloid precursor protein | |
| Cadmium | AD hippocampal tissue: 0.547 g/g d.w.; AD cerebral cortex: 0.518 g/g d.w. Control hippocampal tissue: 0.472 g/g d.w. and cerebral cortex: 0.496 g/g d.w. (post-mortem values) | [42] | AD (n=30), Control (n=30) | High Cd levels associated with AD |
| CdCl2 concentration was 3.8 µM | [51] | Alzheimer's tau peptide R3 | Accelerate heparin-induced self-aggregation of tau peptide R3, or even independently induced aggregation of R3 | |
| Urinary cadmium levels (µg Cd/g creatinine) in company workers: 12.6 (0.4–38.4); in controls: 0.7 (0.1–2.0) (mean, range) | [50] | Cross-sectional, epidemiological study, total of 89 participants, 42 subjects exposed to Cd and 47 in control group | Neurobehavioral effects of occupational exposure to cadmium | |
| Increased concentration and accumulation associated with neurological symptoms | [49] | Astrocytes and neural cells | Astrocytic cytotoxicity, CNS neurological disorders like amyotrophic lateral sclerosis | |
| Cobalt | 100 or 200 µM CoCl2 | [188] | PC12 cell line (Pheochromocytoma neuronal cells) | Mitochondrial DNA damage due to reactive oxygen species (ROS) in neuronal cells |
| Nucleus basalis of Meynert AD 6.5 (1.13) and Control 3.5 (1.13) ng/g wet weight (post-mortem values) (mean, SEM) | [31] | AD (n=14) and control (n=15) | Imbalances of trace element in AD brain | |
| Occupational exposure limit in particulate matter 0.02 mg/m³ time-weighted average (TWA) | [48] | WHO report | Affect neuromuscular transmissions | |
| Increased concentration and accumulation associated with neurological symptoms | [49] | RGC-5 cells, an immortalized retinal ganglion cell line | Induced ROS may be associated with neuronal differentiation, AD and PD | |
| Copper | Cu (0.13 mg/L) as copper sulfate | [39] | C57BL6 mice dosed with copper sulfate | Cu could contribute to Aβ accumulation by altering its clearance and/or its production |
| Increased concentration and accumulation associated with neurological symptoms | [49] | Human neuroblastoma and astrocytoma cells | Effects on neurons and astrocytomas causing AD, ALS, PD | |
| The concentration of extracellular Cu2+ is typically 10 µM in blood plasma, with extracellular levels of Cu2+ reaching as high as 15 µM | [38] | Amyloid-β protein experiments in presence of copper | Structural changes in the amyloid-β protein with formation of plaques in senile brains | |
| Amygdala AD cases 13.0 ± 1.5; control 19.8 ± 1.5 µg/g, dry weight (significant 0.019 ) Hippocampus AD 12.6 ± 1.2; control 16.8 ± 0.9 µg/g, dry weight (significant 0.013 ) |
[40] | AD (n=10) controls (n=11) age-matched subjects | A significant decrease in Cu, and significant increases in Zn and Fe were found in AD hippocampus and amygdala, areas showing severe histopathologic alterations in AD | |
| Copper (µg/dL) (Mean ± SEM); control 94.53 ± 2.00 and AD 120.80 ± 4.23 | [85] | AD (n = 70), Controls (n = 75) | High serum levels of Cu in AD subjects compared to controls. | |
| Copper concentrations of 0.12 ppm (0.12 mg/L) | [30] | 68 male New Zealand White rabbits | Induces amyloid-b accumulation, formation of ‘senile plaque-like’ structures, reduction of glutathione peroxidase activity, increases in superoxide dismutase activity, and retardation of rabbits’ ability to learn a difficult task. | |
| Iron | Amygdala AD 60.6 ±4.9 and control 48.9 ±3.0; Hippocampus AD 48.7 ± 3.2 and control 42.1±1.9 ng/g wet weight (post-mortem values) (mean, SEM) | [31] | AD (n=14) and control (n=15) | Trace-element imbalances in AD brain |
| Hippocampus AD 288 ± 20; C 216 ± 16 (significant 0.036 *) | [40] | AD (n=10) controls (n=11) age-matched subjects | A significant decrease in Cu, and significant increases in Zn and Fe were found in AD hippocampus and amygdala, areas showing severe histopathologic alterations in AD | |
| Iron (µm/L) (Mean ± SEM): Control 12.45 ± 0.56 and AD 16.65 ± 0.61 | [85] | AD (n = 70), Controls (n = 75) | High Fe levels associated with AD | |
| 10 µM Fe (III) | [43] | Amyloid-β protein experiments in presence of trace metals | Aggregation of Aβ42 to form amyloid fibrils and later formed plaque-like structures | |
| AD hippocampal tissue and cerebral cortex: 204.7-810.4 g/g d.w.; control hippocampal tissue and cerebral cortex: 300.1-615.3 g/g d.w. (post-mortem values) | [42] | AD (n=30) and control (n=30) | High Fe levels associated with AD | |
| MRI to measure ferritin iron using field dependent relaxation rate increase (FDRI) method. | [44] | AD (n=31) and control (n=68) | In the hippocampus, higher levels of ferritin iron may be associated with more impaired tissue integrity in this region. | |
| Lead | 19−26 µg/dL in Pb exposed monkeys as compared to 3−6 µg/dL in the controls | [189] | 13 monkeys/group were dosed orally with vehicle or 1.5 mg/kg/day lead: Group 1, vehicle only; Group 2, lead continuously from birth; Group 3, lead from birth to 400 days of age and vehicle thereafter; Group 4, vehicle from birth to 300 days of age and lead thereafter. | AD pathogenesis is influenced by early life exposure |
| The mean (SD) blood lead level was 3.5 (2.2) µg/dL and tibia lead level was 18.7 (11.2) µg/g. | [22] | 991 sociodemographically diverse, community-dwelling adults | Age-related decrements in cognitive function may be associated with early lead exposure | |
| The median baseline blood, patella, and tibia lead concentrations were 5 µg/dL (Interquartile ranges 3–6), 25 µg/g bone mineral (17–37), and 20 µg/g bone mineral (13–28), respectively. | [46] | Human longitudinal epidemiological, 1089 participants in the Normative Aging Study | Cumulative exposure to lead can adversely affect performance on cognitive tests in the visuomotor domain. | |
| Mean patella lead was 25.0 µg/g bone (SD = 20.7), and mean tibia lead was 19.2 (SD = 14.6) | [190] | Human longitudinal epidemiological, VA Normative Aging Study (NAS); 362 participants | Lead exposure is associated with impaired motor function | |
| Manganese | Control animals 8.9 ± 1.1 µg/L and 109.9 ± 15.3 µg/L in Mn-exposed animals. | [55] | Seven adult male macaques, 5–6 years old received 330.28 ± 0.35 mg Mn/kg body weight (bw) | May initiate or accelerate a processes predisposing to AD like pathology and cognitive dysfunction |
| The mean ± SEM of frontal cortex Mn concentrations were: controls (n = 3) 0.207 ± 0.03 µg /g tissue and Mn-exposed (n = 4) 0.357 ± 0.06 µg /g tissue. | [54, 73] | Macaques receiving 3.3-5.0 mg Mn/kg weekly for 10 months showed that 61 genes were up-regulated and four genes were down-regulated in the frontal cortex relative to controls | Chronic manganese (Mn) exposure produces a neurological syndrome with psychiatric, cognitive and AD-like pathology, including up-regulation of amyloid-b precursor-like protein 1 (APLP1), a member of the amyloid precursor family | |
| Group 1 (Mn 10 µg and Mn 250 µg); Group 2 (NaCl and Mn 1000 µg) | [53] | Male Sprague-Dawley rats; Group 1 had 9 animals and Group 2 had 11 animals | Astrocytes are the initial targets of Mn toxicity in the CNS | |
| Mercury | 1x10-7 M Hg solution | [57] | Fresh water snail neurons exposed | Hg ions markedly disrupt the membrane structural integrity of neurites and the growth cones of neurons |
| 2-10 mM Hg2+ | [58] | Normal and AD brain homogenates treated | Hg inhibits GTP nucleotide binding to b-tubulin with diminished biological activity and abnormal partition | |
| Selenium | AD Se levels in plasma, erythrocytes and nails (32.59 µg/L, 43.74 µg/L and 0.302 µg/g) control (50.99 µg/L, 79.16 µg/L and 0.400 µg/g) | [65] | AD (n=28) (11 male and 17 female), Control (n=29) (10 male and 19 female) healthy volunteer elderly with normal cognitive function, mini-mental state examination (MMSE) | AD subjects showed lower Se levels |
| Se concentration was 5 mM Na2SeO3 | [66] | 15 Caenorhabditis elegans animals | High doses induce neurodegeneration of cholinergic neurons by depletion of glutathione, linked to the neuropathology of AD, amyotrophic lateral sclerosis, selenium damages cholinergic motor neurons and reduces their secretion of acetylcholine | |
| Zinc | Zinc ions | [70] | Molecular and kinetic modeling of zinc binding to the microtubule component protein tubulin and metallomic imaging mass spectrometry (MIMS) to show anatomically-localized and age-dependent zinc dyshomeostasis in specific brain regions of Tg2576 transgenic, mice, a model for AD | Sequestration of zinc by Aβ oligomers and plaques leads to reduce intra-neuronal zinc levels; low/moderate levels of zinc enhance tubulin polymerization, excessive zinc levels induce tubulin to form flat sheets rather than cylinders |
| AD hippocampal tissue 31.42-57.91 µg/g d.w. Controls 37.31-87.10 µg/g d.w. | [42] | AD (n=30), C (n=30) | Low Zn levels associated with AD | |
| High levels found in AD brain regions than controls | [71] | Cerebral zinc dyshomeostasis in AD | Abnormality in the uptake or distribution of zinc in AD brain causing aberrant extracellular and intracellular levels in several brain regions | |
| Serum C 12.3 and AD 10.9 µmol/L (means, p = 0.0007) | [23] | AD (n = 44) and C (n = 41) | Low Zn levels associated with AD | |
| Amygdala AD 89.9 ± 4.6 control 75.9 ± 2.7 (significant 0.027*), hippocampus AD 85.1 ± 4.7 control 72.0 ± 4.8 (significant 0.026*) inferior parietal AD 62.0 ± 2.0, control 56.7 ± 1.2 (significant 0.005**) | [40] | AD (n=10) controls (n=11) age-matched subjects | A significant decrease in Cu, and significant increases in Zn and Fe were found in AD hippocampus and amygdala, areas showing severe histopathologic alterations in AD | |
| PESTICIDES and INSECTICIDES | ||||
| Organochlorine pesticides (OCPs) include hexachlorocyclohexane (lindane, HCH), aldrin, dieldrin, endosulfan, p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE), o.p’-DDE, p.p’-dichlorodiphenyltrichloroethane (pp’-DDT), o,p’-DDT, p,p’-dichlorodiphenyldichloroethane (p,p’-DDD) and o,p’-DDD |
β-HCH (ng/ml) (mean ± SEM), C 0.24 ± 0.06 and AD 4.16 ± 0.55 Dieldrin (ng/ml) (mean ± SEM), C 0.16 ± 0.07 and AD 4.81 ± 0.79 pp’-DDE (ng/ml) (mean ± SEM), C 0.81 ± 0.22 and AD 2.52 ± 0.68 blood AD values significantly different from controls |
[24, 85] | AD (n = 70), C (n = 75) | High serum levels of HCH, dieldrin and p, p'-DDE in AD subjects compared to controls. β-HCH, dieldrin and p,p'-DDE levels are associated with risk of AD |
| Blood ranges for p,p’-DDT 1.55–33 174.0, o,p’-DDT 0.07–1878.1, p,p’-DDE 48.80–159 303.3 ng/g lipid | [191] | Center for the Health Assessment of Mothers and Children of Salinas study (CHAMACOS), a birth cohort study, n= 360 children | Prenatal exposure to DDT and DDE associated with neurodevelopmental delays during early childhood | |
| Median serum DDE levels from 7.6 ng/mL in the first trimester (1255.39 ng/g lipid) to 8.9 ng/mL in the third trimester (812.7 ng/g lipid). | [192] | 8.5 years follow-up, prospective perinatal cohort study in Morelos, Mexico, n=203 | Prenatal DDE impairs early child neurodevelopment at 3.5–5 years of age | |
| Residential concentrations of organochlorine pesticides and other pesticides in air range from 1-400 ng/m3 leading to average exposures among children as high as 4 ng/day | [193] | Pesticides and inner-city children | Neurodevelopmental toxicity caused by pesticides | |
| Organophosphate insecticides (OPIs) include methyl parathion, dimethyl parathion, trichlorfon (TCF), chlorpyrifos (CPF) | 0–30 mM methyl parathion and parathion | [62] | Human liver carcinoma (HepG2) cells | Oxidative stress induced by organophosphate insecticides causes toxicity by accumulation of acetylcholine, which inhibits acetylcholinesterase |
| Urine malathion 1.03 µg/L and chlorpyrifos 3.54 µg/L, concurrent exposure to OPs assessed by Urine dialkyl phosphate (DAP) metabolite levels 114.9 (105.7–125.0) nmol/L in mothers; DAP 45.5 (39.6–52.3) nmol/L in children | [194] | Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) of the Center for Children’s Environmental Health Research at the University of California, child and maternal, n = 356 | Adverse association of prenatal organophosphate pesticide exposure as measured by DAPs with mental development and pervasive developmental problems at 24 months of age (early neurodevelopment) | |
| Occupational exposure | [84] | Human longitudinal epidemiological, Cache County Memory Study with 5,092 participants | The risk of AD associated with organophosphate exposure (HR 1.53, 95%CI 1.05–2.23) was slightly higher than the risk associated with organochlorines (HR 1.49, 95% CI 0.99–2.24) | |
| ADI (Acceptable Daily Intake) reported by the WHO (Lu, 1995) for trichlorfon ADI = 0.011 mg/kg weight | [195] | Experimental groups T1, T2, T3, T4 (n = 16) with four male Wistar rats per group and a control group (n = 8); The rats of the groups T1 and T3 received a weekly dose of 11 µg/ kg of TCF for four or eight weeks, respectively; animals of groups T2 and T4, received a weekly dose of TCF (22 µg/kg) for four and eight weeks, respectively | Neuronal and astrocytic reactivity were significantly reduced in Trichlorfon-treated animals relative to controls, myelin reactivity significantly increased with abnormal distribution of myelin in white matter; neurotoxic damage on neuronal and astrocyte functional balance, abnormal myelin formation and cell damage | |
| Carbamates (carbofuran) | 0.016-4.0 X 10-6M carbofuran | [196] | Rodent (n=16) and human (n=68) erythrocyte | Inhibition of rodent and human erythrocyte acetylcholinesterase |
| 1 mg/kg orally for a period of 28 days | [197] | Mitochondria from male Wistar rat brains | Neurotoxicity by impairing mitochondrial impedes mitochondrial respiratory chain functions leading to oxidative stress and neurobehavioral deficits | |
| 1 mg/kg body weight carbofuran | [198] | Adult female albino Wistar rats | Early gestational carbofuran exposure diminishes neurogenesis, reduces the neural progenitor cells pool, produces neurodegeneration in the hippocampus, and causes cognitive impairments | |
| Carbofuran at 1 mg/kg/day in the study. | [99] | 10 Male Sprague-Dawley rats for control, carbofuran and deltamethrin treatment | Spatial learning, memory deficits and neuronal death with the mechanisms involving synapse damages; the pesticides also increase tau phosphorylation with inhibition of protein phosphatase 2A and activation of glycogen synthase kinase-3b | |
| Bipyridyles (paraquat) | Paraquat dose of 10 mg/kg | [93] | The littermate male APP/WT mice and APP/PRDX3 mice (n=6-10) were used for exposure study | Cognitive impairment and increased Aβ levels induced by paraquat exposure |
| Paraquat interacts with enzymatic targets in the CNS, such as AChE and butylcholinesterase | [92] | - | Neuropsychiatric complications, neurodevelopmental toxicity, induction of oxidative stress, inhibition of acetylcholinesterase and elicitation of cholinergic hyperstimulation | |
| Pyrethroids | Deltamethrin at 12.5 mg/kg/day | [99] | 10 Male Sprague-Dawley rats for control, carbofuran and deltamethrin treatment | Spatial learning and memory deficits and neuronal death in rats with the mechanisms involving synapse damages; the pesticides also increase tau phosphorylation with inhibition of protein phosphatase 2A and activation of glycogen synthase kinase-3b |
| Cypermethrin and permethrin doses were 1.49 and 34.05 mg/kg, respectively | [103] | Male and female Wistar rats 10 animals per group | Neonatal exposition to permethrin or cypermethrin induces long-lasting effects after developmental exposure causing behavioral changes, striatal monoamine level, and increased oxidative stress | |
| Neonicotinoids /Imidacloprid | Imidacloprid (337 mg/kg, 0.75 x LD50, in corn oil) | [199] | Pregnant Sprague-Dawley rats were treated with pesticide and effect observed from gestation to offspring birth | Gestational exposure to a single large, nonlethal, dose of imidacloprid produces significant neurobehavioral deficits and an increased expression of glial fibrillary acidic protein in several brain regions of the offspring on postnatal day 30, corresponding to the human early adolescent age. |
| Human peripheral blood lymphocytes (5 × 105 cells) with a viability >>92% were incubated with 9.5 × 10−6, 1.9 × 10−5, 2.8 × 10−5, 3.8 × 10−5 and 5.7 × 10−5 M imidacloprid; 2.8 × 10−4, 5.7 × 10−4, 8.3 × 10−4, 1.1 × 10−3 and 1.7 × 10−3 M imidacloprid in 1 mL of 1640 RPMI medium at 37◦C for 2 h | [176] | Human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides | Genotoxic and cytotoxic mechanism of neonicotinoid insecticides | |
| OTHER INDUSTRIAL AND COMMERCIAL CHEMICALS | ||||
| Alkylphenol-polyethoxylates (APEOs) including octylphenol (OP), nonylphenol (NP) | OP concentration 20 ng/g | [116] | Common snapping turtle injected subcutaneously with OP (n = 16) | OP exposure alters expression of members of the amyloid protein, disrupt hypothalamic development in young turtles |
| NP concentration 10 µM | [200] | Hippocampal and cortical neurons prepared from gestational day 18 Sprague–Dawley rat fetuses |
Impede normal brain development by inhibiting neuronal cell death | |
| OP concentration 0 to 1 mg/ml | [115] | >60, OP treated and 122 control cumulus oocyte complexes | Long-term harmful effects on reproductive and developmental physiology especially in vitro maturation and fertilization of bovine oocytes | |
|
Brominated flame retardants (BFRs) include hexabromocyclo-dodecane (HBCD), tetrabromobisphenol-A (TBBPA), decabromodiphenyl ether (DBDE), polybrominated diphenyl ethers (PBDEs) |
Hexabromocyclododecane (HBCD), tetrabromobisphenol-A (TBBPA) and decabromodiphenyl ether (DBPE), all are cytotoxic at low micromolar concentrations (LC50 being 2.7±0.7 µM, 15±4µM and 28±7µM, respectively) | [109] | SH-SY5Y neuroblastoma cells | Inhibition of Ca2+-ATPase, amyloid-b peptide release and apoptosis, neurotoxic and amyloidogenic in vitro |
| ΣPBDEs (congeners BDEs 47, 99, 100, 153) 0.3 to 2.6 ng/g lipids for maternal samples, and 0.4 to 0.8 ng/g lipids for child samples | [201] | The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) is a longitudinal birth cohort study of predominantly Mexican-American families in California’s Salinas Valley, n= 310 children | Prenatal and childhood PBDE exposures were associated with poorer attention, fine motor coordination, and cognition | |
| PBDE 47 and PBDE 99 at concentration of 10 mL/kg body weight comprising 2,2´,4,4´-tetrabromodiphenyl ether (PBDE 47), 0.7 mg (1.4 µmol), 10.5 mg (21.1 µmol)/kg bw; 2,2´,4,4´,5-pentabromodiphenyl ether (PBDE 99), 0.8 mg (1.4 µmol), 12.0 mg (21.1 µmol)/kg bw; tetrabromo-bis-phenol-A (TBBPA), 0.75 mg (1.4 µmol), 11.5 mg (21.1 µmol)/kg bw. | [110] | 3-4 litters from pregnant NMRI mice | Developmental neurotoxicants, potential neurotoxicant exposure through environment and human milk, given during a critical phase of neonatal development, when the maturation of the developing brain and CNS is at a stage of critical vulnerability, induce persistent neurotoxic effects | |
| 0.45, 0.9, or 9.0 mg 2,2 ,4,4 ,5,5 -hexaBDE/kg of body weight | [26] | 3-4 litters from pregnant NMRI mice | Human blood plasma total PBDE concentration 2.1 ng/g of lipids. PBDE disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice | |
| Dioxins (e.g.,s 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD)), polychlorinated biphenyls (PCBs), polychlorinated dibenzofurans (PCDFs) | TCDD 5 ppt or 25 ppt in diet | [202, 203] | Monkeys exposed perinatally (7 months before pregnancy to weaning) | Dioxins affect some specific functions in particular regions or cells of the brain at critical windows during the developmental period. Learning performance was decreased in offspring born to dams receiving lower doses of TCDD |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) 5 µg/kg bw | [221] | C57BL/6 backgrounds three pregnant females and five controls, three embryos per group | TCDD induced developmental neurotoxicity is modulated through an AhR dependent interaction with key regulatory neuronal differentiation pathways | |
| TCDD Single dose in corn oil (25 mg/kg body wt) by intraperitoneal injection. | [124] | Female Sprague-Dawley rats (n = 3 per time point) were sacrificed to extract protein for Western blot analysis at 1, 2, 3, 5 or 7 days after TCDD injection; rat pheochromocytoma (PC12) cells incubated with different concentrations (1, 10, 100, 300 or 1000 nM) of TCDD for 24 h at 370C. | Neurotoxicity and neuronal apoptosis in the rat brain cortex and PC12 cell line through the down-regulation of the Wnt/b-catenin signaling pathway |
|
| PCB Males 212 ng/L/g lipid | [129] | Longitudinal epidemiological study, n=303 | Levels showed low sperm motility in males | |
| Dioxin-like mono-ortho PCBs 0.032±0.047 (mean ± SD) | [127] | Slovakia Maternal blood n=760 and cord blood n=258 | Association of di-ortho-substituted PCBs with decreased motor development was found in cord but not maternal serum; decreased cognitive development and motor skills in children as well as their mothers | |
| Case 1: Serum PCB below 5 mcg/L; Case 2: PCB level of 250 mcg/L | [130] | Cases exposed to oil leaking from transformer at workplace containing PCBs | PCB-induced dementia may be characterized by impairments in confrontation naming and abnormally fast rates of forgetting on verbal and nonverbal memory tests | |
| Bisphenol A (BPA) | BPA at a rate of 50 g/kg/day | [140] | 3 control and 3 treated African green monkeys | Inhibition of estradiol-induced hippocampal and prefrontal cortex spine synapse formation by BPA; interferes with synaptic remodeling |
| BPA concentration 10 µM | [200] | Hippocampal and cortical neurons prepared from gestational day 18 Sprague–Dawley rat fetuses |
Impedes normal brain development by inhibiting neuronal cell death | |
| Dietary exposures below 5 mg/kg bw per day | [204] | Various studies | Changes in brain biochemical signaling, morphometric and cellular end-points within sexually dimorphic anatomical structures and neuroendocrine end-points were reported | |
| Phthalates (monoethyl phthalate (MEP), mono-2-ethylhexyl phthalate (MEHP), monobutyl phthalate (MBP), and monobenzyl phthalate (MBzP), diethylhexyl phthalate (DEHP)) | In blood, MEP (148 µg/L), MBP (15.1 µg/L), MBzP (7.0 µg/L), MEHP (5.4 µg/L), and MMP (4.5 µg/L) | [129] | Longitudinal epidemiological study, n=303 | Levels showed low sperm motility in males |
| Urine mono-ethyl phthalate (MEP) 138 ng/mL, Mono-n-butyl phthalate (MnBP) 85.61 ng/mL, mono-isobutyl phthalate (MiBP) 2.30 ng/mL, mono-benzyl phthalate (MBzP) 3.54 ng/mL, mono-3-carboxypropyl phthalate (MCPP) 1.75 ng/mL; four di-2-ethylhexyl phthalate (DEHP) metabolites [mono-2-ethylhexyl-phthalate (MEHP) 6.56 ng/mL, mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) 22.08 ng/mL, mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) 14.23 ng/mL, and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) 39.65 ng/mL; total DEHP 0.35 nmol/mL |
[144] | ELEMENT cohort studies conducted in Mexico City, 135 children (64 boys and 71 girls) | In girls, the Mental Development Index was negatively associated with urinary concentration of high molecular weight phthalates while boys’ Psychomotor Development Index was positively associated with urinary concentrations of low molecular weight phthalates | |
| LC50 of DEP was 48 ppm, 0 (solvent control), 1 (1/48th of LC50), 5 (1/9.6th of LC50) and 20 (1/2.5th of LC50) and 0.1 mg/L DEP concentration | [145] | Common carp with three treatment groups with three replicates in each treatment | Neurotoxicity, impaired neurodevelopment | |
| 5, 50 and 500 µg/L for DBP, and 5, 50 and 500 µg/L for DEP and 5:5 µg/L and 500:500 µg/L for the DBP and DEP mixture | [146] | Di-n-butyl phthalate (DBP), diethyl phthalate (DEP) and their mixture | Growth associated protein 43 (gap43), embryonic lethal abnormal vision-like 3 (elavl3), glial fibrillary acidic protein (gfap), myelin basic protein (mbp), α1-tubulin and neurogenin1 (ngn1) were significantly up-regulated after DBP, DEP and DBP–DEP mixture exposure in addition, acetylcholinesterase activity was significantly inhibited in the embryos | |
| HMW phthalates 120 (61–250) (DEHP metabolites) LMW phthalates 430 (175–1090) (MMP, monomethyl phthalate; MEP, monoethyl phthalate; MBP, monobutyl phthalate; and MiBP, mono-isobutyl phthalate.) |
[143] | Mount Sinai Children’s Environmental Health Study between 1998 and 2002 (n = 404) |
Atypical neonatal and early childhood behaviors, neurodevelopmental toxicity in utero may manifest as psychosocial deficits later in childhood | |
| ANTIMICROBIALS | ||||
| Parabens (ethyl-EP, benzyl-BzP, butyl-BuP, methyl-MP, propyl-PP) | Fish exposed to methyl paraben 1.68 mg/L in water | [28] | Common carp with three treatment groups with three replicates in each treatment | Neurotoxicity, neurodevelopmental disturbances and behavioral changes |
| Pregnant women: EP (60.6–451.5) MP (16.9–202.8) PP (0.94–65.4) Newborn infants: EP (39.9–272.3) MP (1.0–8.0) PP (0.84–15.2) (µg/L) | [167] | n=46 Korean women and their new born infants | Oxidative stress biomarkers which may contribute to child development | |
| 200 mg BuP/kg/day subcutaneously and orally | [150] | Total 60 rats; 12 albino in each group rats as follows: control 1, 0.25 ml/100 g bw /day subcutaneously; control 2, 0.25 ml/100 g bw /day orally; autistic-like group 3 treated as group 2 plus 800 mg valproic acid sodium salt/kg orally as one dose on the 12.5 gestational day; offspring group 4 butyl paraben subcutaneously and pregnant mothers 200 mg BP/kg/day; offspring group 5 butyl paraben orally and pregnant mothers 200 mg BP/kg/day | Neurodevelopmental disorders in offspring | |
| Triclosan (TCS) and Triclocarban (TCC) | Triclosan (ng/mL) 116.3 (range: 105.43-127.11) | [141] | 2003-2006 U.S. NHANES, n = 3,728 | Negatively affects human immune function |
| TCC and TCS at 10 µM | [148] | Recombinant rat hepatoma (H4L1.1c4) cells; wild-type myoblasts; recombinant human ovarian cancer cells; recombinant human cells [T47D-androgenresponsive element (ARE)] | TCC enhanced hormone-dependent induction of ER- and AR-dependent gene expression suggesting a new mechanism of action of endocrine-disrupting compounds; TCS structurally similar to noncoplanar ortho-substituted polychlorinated biphenyls, exhibited weak AhR activity but interacted with RyR1 and stimulated Ca2+ mobilization | |
| TCC supplemented chow (0.2% or 0.5% (w/w)) | [158] | Timed pregnant Sprague Dawley rats were fed control or TCC supplemented chow (0.2% or 0.5% (w/w)) ad lib from gestational day (GD) 5 until weaning/post natal day (PND) 21 | TCC exposure might influence maternal thyroid hormone homeostasis in vivo; only 13% of pups raised by 0.2% (w/w) TCC treated dams survived after weaning suggesting the critical exposure window affecting neonate survival is related to lactation because all neonates raised by control dams survived regardless of in utero exposure status | |
| AIR POLLUTANTS | ||||
| Particulate matter, Ozone | Ni NP inhalation (count median diameter 54 nm, at 1 mg/m3, which is the current Occupational Safety and Health Administration’s Permissible Exposure Limit for nickel hydroxide |
[174] | Male and female FVBN mice control = 5 and exposed mice = 11 (female = 5 and male = 6) | Inhalant exposures to a nickel nanoparticle model of air pollution caused rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in mice brains. |
| PM2.5, 35 µg/m3 (24 hours) | [175] | Air pollution in Mexico City metropolitan area children study subjects (Mexico City n=35 and Control n=8) | Impaired cognitive functions; altered immune responses include significant decreases in the numbers of natural killer cells and increased numbers of mCD14+ monocytes and CD8+ cells | |
| Criteria pollutants (O3, PM10, PM2.5, SO2, NO2, CO, and Pb) levels. PM2.5 comprises 50% of PM10 levels. Fine PM2.5 includes components emitted by motor vehicles like elemental carbon and particle-bound polycyclic aromatic hydrocarbons as well as endotoxins like lipopolysaccharides (LPS); ozone (O3) is formed by the combination of nitrogen oxides, volatile organic compounds and strong sunlight | [176] | Air pollution in Mexico City metropolitan area children study subjects (Mexico City n=35 and control n=8) | Presence of neocortical hyperphosphorylated tau with pretangle material and amyloid-β diffuse plaques in the frontal cortex of individuals exposed to urban air pollution suggests a link between oxidative stress, neuroinflammation, neurodegeneration, and chronic exposure to high concentrations of air pollution | |
| Ozone exposure was done daily for 4 h with a dose of 0.25 ppm | [177] | Male Wistar rats: group 1- exposed to an air stream free of ozone during 30 days, group 2-exposed for 15 days to ozone, group 3-exposed for 30 days to ozone, group 4-exposed for 60 days to ozone, and group 5-exposed for 90 days to ozone | Progressive neurodegeneration, impaired brain repair in the hippocampus similar to the aetiology seen in AD brains | |
| Volatile Organic Compounds (VOCs, e.g.. naphthalene, toluene, xylene) | Occupational exposure | [180] | AD (n=193) and control (n=243) | Exposure associated with onset of Alzheimer's disease due to neurotoxicity |
It remains presently unknown whether a single agent or mixtures of environmental factors or contaminants contribute to AD onset and disease progression. Further research is underway to provide new insights into potential mechanisms, with the goal of identifying environmental risk factors and developing strategies for reducing harmful exposures contributing to AD pathology. The Centers for Disease Control and Prevention in conjunction with the Agency for Toxic Substances and Disease Registry have developed Minimum Risk Levels for some hazardous substances, as tabulated in (Table 2). Having provided above a brief synopsis of the knowledge base of AD etiology, we discuss in the following in greater detail the five categories of environmental agents implicated with AD pathology.
Table 2.
Body burden (blood, urine, total body levels), minimum risk levels, and exposure limits for environmental contaminates in healthy individuals.
| Environmental Contaminants | Name | Levels in Blood, Urine (Mean/ Range) | Total Body Level | Minimal Risk Levels (MRLs) | Exposure Limits* | Reference | |
|---|---|---|---|---|---|---|---|
| TOXIC METALS | |||||||
| Aluminum | Blood 1-3 µg/L Urine mean value 23.7 µg/L |
For 70 kg adult 30–50 mg/ kg body weight | 1 mg aluminum/kg/day for chronic oral exposure (≥365 days) |
NIOSH REL TWA 10 mg/m3 | [205, 207] | ||
| Arsenic | ≤ 1 ppm in nails ≤ 1 ppm in hair Blood < 1 μg/L in Urine 0.0-35 µg/L |
Toxicity of arsenic depends upon exposure | 0.005 mg As/kg/day for acute oral exposure (≤14 days) to inorganic arsenic; 0.0003 mg As/kg/day for chronic oral exposure (≥1 year) to inorganic arsenic |
NIOSH REL TWA 0.002 mg/m3 | [23, 207] | ||
| Cadmium | Blood level 0.315 µg/L Urine level 0.193 µg/g creatinine (0.185 µg/L) |
For 70 kg adult, 10-50 mg/ kg body weight | 1 X 10-5 mg Cd/m3 has been derived for chronic inhalation exposure to cadmium (≥1 year). 3 X 10-5 mg Cd/m3 has been derived for acute- inhalation exposure to cadmium (≤14 days) |
NIOSH REL TWA 0.002 mg/m3 | [207, 208] | ||
| Cobalt | Blood levels 0.05–0.19 µg/dL Urine levels 0.04–2 µg/dL Air levels at unpolluted sites <1–2 ng/m3 |
For 70 kg adult, 1.1–1.5 mg/ kg body weight, with 0.11 mg in the liver. | 0.0001 mg cobalt/m3 for chronic inhalation exposure (>365 days) to cobalt 0.01 mg Co/kg-day for intermediate oral exposure (<365 days) |
NIOSH REL TWA 0.05 mg/m3 | [48, 207] |
||
| Copper | Serum copper 151.6 µg/100 mL Urine 18 µg/ g dry weight |
For 70 kg adult, 50–70 mg/ kg body weight | 0.01 mg/kg/day for acute (1–14 days) and intermediate oral exposure (15– 365 days) oral exposure to copper | NIOSH REL TWA 0.1 mg/m3 | [85, 207] | ||
| Iron | Serum ferritin 128.58±13.85 µg% | 4.333 mg/kg body weight for women | - | NIOSH REL TWA 1 mg/m3 | [209, 210] |
||
| Lead | Blood 1.5 μg/dL for adults 20–59 years Urine 0.677 μg/L for ≥ 6 years of age |
For 70 kg adult, 22.0-441.8 mg | No MRLs derived because more sensitive effects have not been established in humans | NIOSH REL TWA (8 hour) 0.050 mg/m3 | [207, 211] | ||
| Manganese | Serum (≥16 years) 0.6-2.3 ng/mL Urine (aged 6–88 years) 1.19 µg/L |
For 70 kg adult, 10 to 20 mg | No MRLs derived interim guidance value 0.16 mg manganese/kg/day |
NIOSH REL TWA 1 mg/m3 | [207, 212] | ||
| Mercury | Blood – below 5 ng/mL Urine – below 20 ng/mL |
In exposed individuals blood 9.8 ± 2.2 µg/L | Chronic inhalation 0.2 µg/m3 for metallic mercury; oral acute 0.007 mg/kg/day and intermediate 0.002 mg/kg/day duration exposures to inorganic mercury |
NIOSH REL TWA (vapour) 0.05 mg/m3 | [207, 213, 214] |
||
| Selenium | Serum 0.125 mg/L | 15 mg in Americans | No MRL was derived for acute exposure 0.005 mg Se/kg/day was derived for chronic oral exposure (≥1 year) low adverse effect level of dietary selenium 1,540–1,600 mg daily |
NIOSH REL TWA 0.2 mg/m3 | [207, 215, 216] | ||
| Zinc | Serum 1 µg/mL Urine 0.5 mg/g creatinine |
For 70 kg adult, 0.66 -2.63 g/kg body weight | 0.3 mg Zn/kg/day for intermediate oral exposure (15–364 days). | NIOSH REL TWA 10 mg/m3 | [207, 211] | ||
| PESTICIDES and INSECTICIDES | |||||||
|
Organochlorine pesticides (OCPs) include hexachlorocyclohexane (lindane, HCH), aldrin, dieldrin, endosulfan, p,p’-dichlorodiphenyl-dichloroethylene (pp’-DDE), o,p’-DDE, p,p’-dichlorodipheny-ltrichloroethane (pp’-DDT), o,p’-DDT, p,p’-dichlorodiphenyl-dichloroethane (p,p’-DDD) and o,p’-DDD |
Serum aldrin 0.004 mg/L and dieldrin 0.002 mg/L in India; serum HCH 0.0182 mg/L; dieldrin 0.0161 mg/L pp′-DDE 1.31 mg/L in unexposed New Zealand adults; serum dieldrin (>12-year-old and older) 0.146 mg/L (whole blood) in USA adults; urine DDT+ DDE 400 µg/L |
Serum dieldrin in farmers 127±27.2 µg/g fat; serum SHCH in farmers 4.3 ± 0.1 ng/g fat; serum SDDT in farmers 7.6 ± 1.7 ng/g fat |
0.002 mg/kg/day for acute exposure to aldrin (≤14 days); 0.00003 mg/kg/day for chronic exposure to aldrin (≥1 year); 0.0001 mg/kg/day for intermediate exposure to dieldrin (15–364 days); 0.00005 mg/kg/day for chronic exposure to dieldrin (≥1 year); acute oral 0.0005 mg/kg/day for DDT; intermediate 0.0005 mg/kg/day for DDT |
NIOSH REL TWA Dieldrin 0.25 mg/m3 [skin]; NIOSH REL TWA HCH, 0.5 mg/m3 [skin]; NIOSH REL TWA Endosulfan 0.1 mg/m3 [skin]; DDT, 0.5 mg/m3 |
[207, 217, 220] | ||
| Organophosphate insecticides (OPIs) including methyl parathion, dimethyl parathion, trichlorfon (TCF), chlorpyrifos (CPF) | No reference values | After exposure, 0.156 mg/L serum methyl parathion | 0.0007 mg/kg/day for intermediate oral exposure (15–364 days) to methyl parathion; 0.0003 mg/kg/day for chronic oral exposure (365 days or more) to methyl parathion |
NIOSH REL TWA 0.05 mg/m3 [skin] for dimethyl parathion | [207] | ||
| Carbamates (e.g., carbofuran) | Urine 28 µg/kg (farmers) | Blood carbofuran 0.4 -18 µg/mL poisoning | Mild effects at 0.1 mg/kg body weight/day | NIOSH REL TWA 0.1 mg/m3 | [221], 223 | ||
| Bipyridyles (paraquat, diquat) | No reference values | Serum 0.4 - 4.0 µg/mL level after paraquat poisoning | Less than 20 mg paraquat ion per kg body weight | NIOSH REL TWA 0.1 mg/m3 (resp) [skin] for paraquat | [224] | ||
| Rotenone | No reference values | Toxicity or poisoning values vary | 40 mg/L drinking water | NIOSH REL TWA 5 mg/m3 | [225] | ||
| Fipronil | No reference values | Toxicity or poisoning values vary | No MRL | Fipronil NOAEL 0.025 mg/kg bw per day; human chronic RfD 300 ng/L/day |
[226] | ||
| Pyrethroids (permethrin deltamethrin) | No reference values | Not evaluated | 0.3 mg/kg/day acute oral exposure to permethrin (≤14 days); 0.2 mg/kg/day intermediate oral exposure to permethrin (15–364 days); no chronic-duration oral MRLs |
EPA exposure limits 0.005-0.05 mg/kg/day | [207] | ||
| Neonicotinoids (acetamiprid, imidacloprid) | No reference values | Due to poisoning plasma acetamiprid 10.58 ng/L; plasma acetamiprid < 44.6 ng/L; plasma imidacloprid 2.3-59.8 mg/L |
Acute dietary acetamiprid exposure 0.039 mg/kg; acetamiprid 600 mg/L (children 1-6 years) |
Acetamiprid NOAEL 10 mg/kg; imidacloprid acute 14 mg/kg/day; RfD 0.057 mg/kg/day | [227, 228] | ||
| OTHER INDUSTRIAL and COMMERCIAL CHEMICALS | |||||||
| Alkylphenol-polyethoxylates (APEOs) including Octylphenol (OP) |
95th percentile 2.2 (1.6–3.2) µg/L | 4-tert-Octylphenol exposed blood serum1.4 ng/g (wet weight) | No MRL | [229] | |||
| Brominated flame retardants (BFRs) including hexabromocyclo-dodecane (HBCD), tetrabromobisphenol-A (TBBPA), decabromodiphenyl ether (DBDE), polybrominated diphenyl ethers (PBDEs) |
Serum congeners BDE-47[geometric mean 20.5 ng/g lipid]; BDE-153 [5.7 ng/g lipid]; BDE-99 [5.0 ng/g lipid; BDE-100 [3.9 ng/g lipid]; BB-153 [2.3 ng/g lipid]; and BDE-28 [1.2 ng/g lipid]. | Breast milk PBDEs 4-419 ng/g lipid PBDE group (BDE-47, 99, 100, 153, 154) 330-2500 ng/kg fat |
0.006 mg/m3 for intermediate inhalation exposure (15–364 days) to lower brominated BDEs; 10 mg/kg/day has derived for intermediate oral exposure (15–364 days) to DBDE |
HBCD NOAEL 14.8 mg/kg-day; TBBPA NOAEL inhalation > 18mg/L; NOAEL oral >100 mg/kg to >2500 mg/kg; pentaPBDE NOAEL 1mg/kg/d |
[207, 236, 230] | ||
| Dioxins (e.g.. 2,3,7,8-Tetrachloro-dibenzo-p-dioxin (TCDD)), Polychlorinated biphenyls (PCBs), polychlorinated dibenzofurans (PCDFs), polychlorinated hydrocarbons (PAHs e.g.. naphthalene) | Serum TCDD 2.34 ng/L ( 0.58 – 5.5 ) Serum PCBs 6.2 mg/L |
TCDD body burden 20 ng/L; in individuals 13 years after exposure: serum PCDFs 1,030 ng/kg lipid; serum PCBs 2,220 ng/kg lipid |
0.03 µg/kg/day intermediate oral exposure (15–364 days) to PCBs; 0.02 µg/kg/day chronic oral exposure (365 days or more) to PCBs; No acute, or chronic oral MRLs were derived for PAHs; 0.6 mg/kg/day intermediate oral exposure (15-364 days) to acenaphthene |
NIOSH REL TWA for PCB 0.001 mg/m3 | [207, 237, 238] | ||
| Bisphenol A (BPA) | Serum BPA unexposed 0.276 mg/L Urine BPA unexposed 2000 ug/L |
Serum BPA median 3.198 mg/L | 0.02 µg/kg bw/day minimal health risk to infants and children | NOAEL 5 mg/kg body weight/day | [204, 219, 239, 240] | ||
| Phthalates (monoethyl phthalate (MEP), mono-2-ethylhexyl phthalate (MEHP), monobutyl phthalate (MBP), and monobenzyl phthalate (MBzP), diethylhexyl phthalate (DEHP), di-n-butyl phthalate (DBP), benzylbutyl phthalate (BzBP), and diethyl phthalate (DEP) | In blood; MEP (148000 ng/L), MBP (15100 ng/L), MBzP (7000 ng/L), MEHP (5400 ng/L), and MMP (4500 ng/L); urine DEHP <3.6 µg/kg body weight/day; total phthalates 437.9 ng/mL (8.9-47585 ng/mL) |
- | DEHP 0.1 mg/kg/day for intermediate oral exposure (15–364 days); DEHP 0.06 mg/kg/day was derived for chronic-duration oral exposure (≥365 days) |
DBP NOAEL125 mg/kg/d; BzBP NOAEL159 mg/kg/d; DEP NOAEL 750 mg/kg/d |
[207, 241, 243] | ||
| ANTIMICROBIALS | |||||||
| Parabens (ethyl, benzyl, butyl, methyl, propyl) | Urine methyl paraben: female106- 1,230 male 25.3- 727; urine ethyl paraben: female2.0-138 male <LOD-36.4; urine propyl paraben female 20.2-361 male 2.0-134; butyl paraben female 0.300-31.8 male <LOD-2.70 |
0.03 mg/kg bw/day | No MRL | 0.79, 0.34, and 0.0016 mg/kg bw/day for methyl-, propyl- and butylparaben, respectively | [165, 244, 247] | ||
| Triclosan | Plasma TCS 11 ng/g (age 16–45 years Australians); urine TCS 6.4 mg/L (mg/g creatinine) urine 2.400-3790000 ng/L in USA; blood levels males; 136760 ng/L, females 95380 ng/L in USA |
Urine triclosan concentrations are highest during the third decade of life | No MRL | [248, 250] |
|||
| Triclocarban | Serum TCC 0.45 ng/mL; urine TCC 3.85 ng/mL |
1% to 5% by body weight | No MRL | - | [159, 162] | ||
| Hexachlorophene (HCP) | Blood 0.02-0.14 mg/L | 0-80 µg/kg adipose tissue | 6 µg/L in drinking water | - | [251] | ||
| AIR POLLUTANTS | |||||||
| Particulate matter (PM) (heavy metals and toxic elements), Ozone (O3) | Sulfate particles (pg/m3) 0.7-7.4 Inhalable PM (pg/m3) 15.4- 32.7 Sulfur dioxide (ppb) 0.2-12.9 Ozone, 24-hr avg. (ppb) 16.3-34.8 Nitric acid (ppb) 0.3-2.1 (l ppb = 40.9 nmol/m3) |
- | Relative risk associated with a 10 µg/m3 change in PM10 | PM2.5, 15.0 μg/m3/ year, 35 μg/m3/24 hours; PM10, 150 μg/m3/24 hours; ozone 0.075 ppm carbon monoxide 9 ppm (10mg/m3)/8 hours; sulfur Dioxide 75 ppb/1 hour; lead 0.15 μg/m3/3 months; nitrogen dioxide 53 ppb/year; 100 ppb/ hour |
[173, 252, 253] | ||
| Volatile Organic Compounds (VOCs, e.g., naphthalene, toluene, xylene) | Blood benzene 0.28 ± 0.34 ng/mL; blood m,p-xylene 0.98 ± 0.93 ng/mL; urine 1,3-butadiene 4.24 ± 12.16 urine benzene 0.75 ± 4.23; urine toluene 0.11 ± 0.14; urine ethylbenzene 0.66 ± 1.17; urine m,p-xylene 0.83 ± 1.18; urine o-xylene 1.22 ± 2.01 |
Benzene 0.009 ppm for acute-duration inhalation exposure (≤14 days); benzene 0.006 ppm for intermediate-duration inhalation exposure (15–364 days); benzene 0.003 ppm for chronic-duration inhalation exposure (≥1 year); xylenes NOAEL 50 ppm; no MRLS for butadiene; toluene acute 1 ppm; chronic 0.08 ppm; ethylbenzene acute 1 ppm, chronic 0.1 ppm |
Benzene NIOSH REL TWA 0.1 ppm; air xylenes NIOSH REL 435 mg/m3; (total xylenes) drinking water 10 mg/L maximum contaminant level; ethylbenzene NIOSH REL TWA 100 ppm | [207, 254] | |||
TOXIC METALS
Human exposure to toxic metals is a common condition worldwide, resulting from multiple exposure pathways including inhalation of contaminated air, dermal absorption of metals contained for example in soil, and ingestion of contaminated water and foodstuff, such as agricultural crops, meat and seafood. Exposure to metals has been documented to cause acute and chronic toxicity, with outcomes including degenerative diseases and cancer [33-35]. Aggregation of amyloid-β protein on neuronal cells following exposure to aluminium, zinc, copper, iron and cadmium chloride salts indicates that metal exposures may trigger AD-like pathologies [36]. Although some metals consumed in moderation are required for maintaining good human health, excessive or insufficient amounts are known to cause adverse health effects (Tables 1 and 2).
A possible linkage of aluminium (Al) neurotoxicity with AD was discovered in cats and deceased human subjects [37]. Aluminium induced neuropathy includes neurofibrillary degeneration, oxidative stress and inflammatory response. Although Al may act as a cross-linker for in vitro amyloid-β oligomerization, whether or not Al plays a role in human AD pathogenesis is still uncertain and controversial [20, 36].
Trace amounts of copper (Cu) in the diet were found to induce amyloid-β plaques and learning deficits in an AD rabbit model, including structural changes in amyloid-β
protein with formation of plaques-like structures [30, 38]. Long term exposure of mice to high levels of Cu was shown to result in increased levels of brain amyloid-β protein and in neuroinflammation, two phenomena viewed as hallmarks of AD development [39]. Cu is physiologically complexed with essential enzymes such as superoxide dismutase, cytochrome c oxidase, and ceruloplasmin, and it has been posited that the decrease in Cu in brain regions severely affected by AD can be associated with a decreased abundance of these enzymes in the regions of the brain [40]. Human studies demonstrated altered Cu metabolism to be associated with oxidative pathology in AD [41].
Epidemiological studies have revealed high deposition of iron (Fe) from unknown sources in the hippocampus, amygdala and cerebral cortex regions of AD subjects [31, 40, 42]. In vitro studies have illustrated that iron together with aluminium is involved in the formation of aggregates of Aβ 42 to form amyloid fibrils with β-pleated sheet conformations [43]. A recent study employing magnetic resonance imaging reported increased iron levels and decreased tissue integrity in the hippocampus region of AD subjects [44].
Inorganic lead (Pb) is the current environmental hazard for humans as the organic forms of Pb have been phased out [45]. Early exposure to Pb is known to impact physiological development. This may possibly increase the susceptibility later in life to neurodegeneration and AD pathology; reported experimental effects of Pb exposure include an increased expression of amyloid precursor protein and increased production of amyloid-β protein [21]. A longitudinal cohort of men evaluated by magnetic resonance spectroscopy revealed hippocampal gliosis; it is known that the hippocampus is affected early and severely in AD [46]. Toxicological and population based research has implicated environmental Pb exposure as a cause of general neurotoxicity with decline in cognition in both children and adults [47].
Industrial exposure to cobalt (Co) was observed to cause an increase in the accumulation and concentration of this metal in humans, eliciting associated adverse effects on neuromuscular transmission [48] and neurological status [49]. Compared to age-matched controls, elevated levels of Co were observed in post-mortem AD brain tissue, especially in the nucleus basalis of Meynert, a region commonly affected in AD [31].
Elevated exposure to cadmium (Cd), particularly in occupational settings has been linked to neurological symptoms and neurobehavioral problems involving loss of attention, psychomotor speed and memory [50]. Higher, and significantly different Cd contents were seen in hippocampal tissues and in the cerebral cortex of AD subjects, when compared to age-matched control subjects [42]. In vitro experiments have illustrated that Cd causes self-aggregation of the tau peptide R3, thereby potentially impacting AD pathogenesis [51] by mechanisms including astrocyte and neural cell toxicity [49].
Brain biopsy of a single human subject with high manganese (Mn) level revealed multiple neuritic plaques and neurofibrillary tangles, which are characteristic of AD [52]. However, the high level of Mn and its association for development of AD warrants further investigations in other AD patients. In a murine study, intranasally administered Mn was shown to cause toxicity in the CNS, targeting astrocytes and leading to an increased abundance of the glial fibrillary acidic protein [53]. Previously, chronic Mn exposure in non-human primates had been observed to cause cellular stress and neurodegenerative changes, including diffuse amyloid-β protein plaques in the frontal cortex [54]. More recently, AD-like pathology and cognitive dysfunction with impairment of visuospatial associative learning were observed to be associated with Mn exposure in macaques [55].
Mercury (Hg) is a well known neuro toxin and also has been reported to be a risk factor for the development of AD. Animal and in vitro studies have demonstrated that mercury causes tau protein hyperphosphorylation, and the increased formation of amyloid-β protein [56]. Hg ions disrupt membrane structural integrity of neurites and neuron growth cones [57] and also inhibit binding of guanosine triphosphate (GTP) to β-tubulin reducing the biological activity, causing abnormal partition and ultimately microtubule degeneration as shown in AD brain homogenates [58].
Arsenic (As) has been speculated to represent an essential trace element in human nutrition, but its toxicity at higher doses in people and animals is much more firmly established [59]. Geological and epidemiological data indicate that environmental arsenic concentrations in topsoils (7–18 ppm range) are positively correlated with the prevalence and mortality of AD and dementias in countries like Italy, Spain, Belgium, France, Norway and Switzerland [60]. In another study, rat cerebellar granule neurons exposed to arsenic illustrated neurotoxicity, apoptosis and activation of p38 and JNK3 MAP kinases in the signalling pathways [61]. Epidemiological data from 434 human participants found low-level arsenic exposure linked to poorer neuropsychological functioning [62]; however, another study indicated a positive correlation between serum arsenic and cognitive ability, suggesting that seafood consumption of arsenic in addition to docosahexaenoic acid plays a role in delaying AD [23]. Since, arsenic and its compounds are used in pesticides, insecticides and herbicides, exposure to contaminated food, water and air may induce brain neuronal apoptosis; however, there is no direct evidence linking As with AD [63]. Thus, there is an absence of verified cases of human morbidity or mortality resulting from exposure to low levels of arsenic in topsoils as well as its correlation to cognitive functioning.
Selenium (Se) is both an essential nutrient and, at elevated concentrations, an environmental toxicant [64]. Epidemiological studies have observed Se deficiency in AD patients when compared with an age matched control group as evidenced by Se levels measured in plasma, erythrocytes and nails [65]. However, there is a need for confirmatory studies correlating Se status and AD etiology. In contrast, a study in Caenorhabditis elegans has shown that high Se concentrations induce oxidative stress, with reduced cholinergic signalling and degeneration of cholinergic neurons by depleting glutathione [66]. The study also points out that the environmental toxicant Se induces general neurodegeneration. As direct experimental evidence is lacking for a link between Se intake, absorption and onset of AD, further studies and clinical interventions are needed [67, 68].
Zinc (Zn) is another essential trace mineral, playing a role in the metabolic activity of some 300 human enzymes and influencing physiologies as diverse as wound healing, cell division and synthesis of DNA and proteins [69]. Deficiency of Zn in blood serum has been associated with pathogenic AD mechanisms [23], affecting microtubule polymerization and microtubule networks [70]. However, further confirmatory studies are required to establish this possible relationship. Aberrant extracellular and intracellular zinc levels suggestive of dyshomeostasis in AD have been observed in several brain regions of individuals with normal levels of Zn in their diet [71]. These studies revealed that zinc in the brain may serve twin contrasting roles. Excess zinc in senile plaques and vascular amyloid deposits may initiate amyloid deposition affecting polymerized microtubule stability; and at the same time it may also counter oxidative stress and neurotoxicity, thereby preventing neurodegeneration and cognitive impairment in a process of potential therapeutic use.
Metal mixtures also are believed to play a role in the development of neurodegenerative diseases, potentially acting synergistically rather than displaying simple, additive effects. Many studies have linked long term or short term toxic metal exposure to AD at low levels [42, 72] that point to the possibility of synergistic co-toxicity, possibly altering metabolism, oxidative stress response and neurotoxic potency. For example, one study showed significantly higher levels of Cu in the frontal cortex of macaque brains following Mn exposure [73], suggesting a synergistic effect between co-exposure to metals and metal dyshomeostasis.
INSECTICIDES AND PESTICIDES
Population growth and the increased demand in industrial food production have resulted in widespread use of synthetic pesticides, with exposure to some of which having been linked to AD [19, 74]. Increased use of pesticides in industrialized agriculture has polluted the natural and built environment, resulting in bioaccumulation of toxicants and affecting human health (Tables 1 and 2). The use of insecticides/pesticides in household and agricultural areas has exponentially increased over the course of the past four decades [75]. Resultant environmental exposure to these insecticides and pesticides has also been linked to the development of neurodegenerative disorders like Parkinson’s disease [76, 77]. Many pesticides target the nervous system of insect pests, and similarly are neurotoxic to humans by adversely affecting cell signaling, disturbing neurochemical processes, and causing neurotoxicity [78]. While the use of organophosphates, carbamates and pyrethroids has decreased over the years, the use of neonicotinoids and other compounds is still increasing [79]. Acute, chronic and long term exposures to pesticides have been associated with neurological disorders including AD [80]. Informative work includes a French cohort study called PAQUID (Personnes Agées QUID) that followed 3,777 individuals aged 65 years or older since 1988 until the present time; univariate analysis of data from follow-up exams spaced 5 and 10 years apart showed that AD and occupational pesticide exposure were significantly associated with increased risk (odds ratio of 2.9); this relationship remained significant even after adjusting for education and smoking status (relative risk = 2.4, 95% confidence interval [CI]=1.0-5.6) [19].
Certain pesticides may be harmless as a single exposure, but when mixed with other pesticides, they can be toxic and alter the body metabolism of animals as well as humans [81, 82]. Some pesticides are cholinesterase inhibitors or have similar effects on other molecular targets causing long-term, lasting toxic effects on the CNS [83].
Epidemiological studies illustrate that exposure to organochlorines (Hazard Ratio=1.49; 95% CI of 0.99–2.24) and organophosphates (Hazard Ratio=1.53; 95% CI of 1.05–2.23) are associated with an increased risk of dementia and AD later in life; this association was identified in The Cache County Study using the Cox proportional hazards model [84]. Among the organochlorine pesticides are hexachlorocyclohexane (HCH) and aldrin, two extremely persistent pesticides. When humans are exposed through food or drinking water to HCH isomers (α-HCH, β-HCH and γ-HCH) and aldrin, the slow metabolism and excretion of these pesticides together with their notable hydrophobicity promote bioaccumulation. A pilot study in a population of North Indians reported that increased blood levels of β-HCH and the organochlorine compound dieldrin were associated with significant increases in AD risk, independent of the genetic risk factor, with odds ratios of 2.78 (95% CI of 1.35–5.69) and 2.34, respectively [85]. Epidemiological studies and experimental studies showed that these pesticides induce oxidative stress and neurotoxicity [24]. Similarly, organophosphate insecticides like parathion were shown to cause morphological changes and affect non-cholinesterase targets like motor proteins, neuronal cytoskeleton and axonal transport [86]. Organochlorine and organophosphate insecticides have been documented to affect glucose and lipid metabolism and the endocrine system [87]. Although, severe acute poisoning can be rectified [88], long term exposure can cause neurobehavioral effects [89] and, at the cellular level, can induce decreased cell viability due to lipid peroxidation and genotoxicity [90]; these adverse effects ultimately may increase the risk of developing AD.
Carbamates such as carbofuran, along with organophosphates, are a group of cholinesterase inhibiting pesticides. Mammalian laboratory experiments have demonstrated that neuronal nicotinic acetylcholinesterase receptors are susceptible to toxicity induced by carbamate pesticides and may contribute to long-term disruption of the nervous system [91]. Gestational and postnatal exposure of mice to bipyridyles (paraquat) in combination with carbamate showed reduced levels of dopamine and loss of nigral dopamine neurons [92]. Further, mice exposed to paraquat showed mitochondrial dysfunction in cerebral cortex, which in turn is known to promote impairment of cognition function with elevated levels of Aβ protein [93]. Rotenone is another pesticide that causes mitochondrial dysfunction; it is an inhibitor of mitochondrial complex I, destabilizes microtubules, and is strongly associated with Parkinson disease etiology [94]. The role of rotenone in the pathogenesis of AD has not been studied in depth, but the compound’s ability to induce mitochondrial dysfunction may constitute a causative factor for AD [95].
Fipronil, a phenylpyrazole insecticide, is a neurological agent that selectively inhibits insect gamma-aminobutyric acid (GABA) receptors. Experiments in zebrafish embryos suggest that fipronil impairs spinal locomotor pathways and causes neurodegeneration [96]. In humans, exposure to fipronil causes an increased risk of mild, temporary health effects, including neurological symptoms [97]. Examination of the human AD brain showed functional remodeling of GABAergic neurotransmission similar to fipronil toxicity [98] suggesting that long term exposure to fipronil may be a predisposing factor for AD.
Pyrethroid pesticides commonly used in agriculture and urban settings are known neurotoxicants and can be transformed into neurotoxic degradates. These pesticides induce cognitive abnormalities, imbalanced tau phosphorylation and AD-like pathology in rats [99], pathological cell death and neurotrophic effects on neurons in human cell lines [100, 101]. A study in Ecuadorian children confirmed that maternal occupational exposure to pesticides like pyrethroids and organophosphates induces developmental neurotoxicity during pregnancy, which is an important risk factor for impaired neurobehavioral development in offspring [102]. Importantly, exposure to pesticides has been related to development of CNS disorders including AD [19]. However, the role of pyrethroid pesticides as agents directly causing AD is uncertain and requires further research. For example, a rat study showed neonatal exposure to permethrin or cypermethrin to induce long-lasting developmental effects, including behavioral changes, altered dopaminergic activity, and increased oxidative stress [103]. It has been established that in utero exposures to neurotoxic chemicals reduce the number of neurons in critical areas of the developing brain, causing altered dopamine levels with advancing age which are also associated with PD and AD [104]. These observations have spawned hypotheses that environmental pesticides may contribute to AD development.
Furthermore, in the 1990s, a new generation of pesticides called neonicotinoids were introduced which selectively bind to insect receptors for nicotinic acetylcholine. Neurotoxic insecticides that may bioaccumulate in the food chains were observed to cause changes in the mobility of lotic macroinvertebrates measured in continuous flow microcosms as downstream drift [105]. In vitro experiments with peripheral human blood lymphocytes showed neonicotinoid pesticides to cause cytotoxicity and genotoxicity [106]. Commonly used neonicotinoids like acetamiprid and imidacloprid act in the same manner as nicotine, readily passing through the blood-brain barrier and causing adverse effects in neonatal rat cerebellar cultures, suggesting potential risks to developmental stages in humans [107].
OTHER INDUSTRIAL AND COMMERCIAL POLLUTANTS
Urbanization and industrialization certainly have contributed to increases in environmental contamination, causing multiple health hazards to humans and other organisms [108]. Whether naturally occurring or being of anthropogenic origin, contaminants in air, water, soil, and food as well as in drugs can potentially harm or cause adverse effects to humans. Many of these contaminants tend to bioaccumulate in living organisms where they may cause toxicity (Tables 1 and 2). An overview of these contaminants and their potential role in the etiology of AD is presented in the following sections.
Brominated flame retardants (BFRs) are widely used in commerce with polybrominated diphenyl ethers (PBDEs) representing the historically most widely used compounds, found in electrical appliances, building materials, and textiles. Adult mice exposed to PBDEs showed altered spontaneous behavior, impaired learning and memory, and decreased hippocampal cholinergic receptors [26]. In vitro studies showed that PBDEs are neurotoxic and amyloidogenic specifically causing Ca2+-ATPase inhibition, amyloid-β peptide release, and apoptosis a key neuro-degenerative pathology observed in AD [109]. Multiple health effects and permanent aberrations in spontaneous behavior have been reported in neonatal and adult animals after exposure to commercial PBDE mixtures causing developmental neurotoxicity [26, 110]. Over the years, biomonitoring of the level and effects of toxins and ensuing regulatory interventions have helped to curb the use and exposure to these BFRs; however, lingering large quantities of these compounds still render many populations vulnerable to toxic exposures and effects [111, 112]. Recent research on amniotic fluid contamination highlights the potential for fetal exposure, suggesting that younger generations are at risk of neurodevelopmental toxicity similar to that seen in animal studies [113]. Some commonly used BFRs have been reported to cause neuronal cell death leading to production of β-amyloid peptide a key feature of AD [109]. Based on these results, flame retardants are believed to potentially increase the risk of AD, but more studies are needed to explore their role and importance as AD risk factors. Meanwhile, multiple studies have highlighted the importance of early life exposure to environmental agents as a risk factor for programming and developing adult-onset disease [14, 114].
Alkylphenol polyethoxylates (APEOs) are found in the paper, paint, pesticides and textile industry. Nonylphenol (NP) and octylphenol (OP) are degradates and transformation products of detergents formulated from alkylphenol polyethoxylates. NP has been shown to cause long-term harmful effects on reproductive and developmental physiology, as it binds to estrogen receptors and exerts estrogenic actions in bovine oocytes [115]. Similarly, estrogenic effects were seen in OP exposed turtles together with increased expression of amyloid-like precursor protein-2 and amyloid precursor protein, accumulation of which causes neuronal degeneration in AD brains [116]. Deposition of alkylphenols in aquatic and terrestrial environments and subsequent bioaccumulation in animals and food crops increases the likelihood of human exposure as well as ensuing risks to human health especially through ingestion of certain fish species [117, 118]. Thus far, studies on the toxic effects of alkylphenols have focused mostly on animal models where endocrine-disrupting effects have been observed [119]. Alkylphenols together with other endocrine disrupting chemicals have been linked to a number of diseases including AD [120], with an important concern being the risk of programming diseases in adult life via early exposure during windows of susceptibility.
Dioxins are naturally occurring and unintentionally produced byproducts of chemical manufacturing comprised of various toxic congeners of polychlorinated di-benzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and dioxin-like chemicals, including certain congeners of polychlorinated biphenyls (PCBs). Together with similarly behaving but structurally distinct polycyclic aromatic hydrocarbons (PAHs), dioxins exhibit toxicity and biological effects mediated through their binding to the aryl hydrocarbon receptor (AhR) and signalling pathways [121, 122]. Dioxins are very stable, lipophilic organohalogen compounds and are known to alter neurotransmitter functions in the CNS, affect Ca2+ homeostatic processes, and induce oxidative stress [49]. The most potent dioxin congener, 2, 3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), was observed to increase in neuronal cells calcium levels and tau phosphorylation via up-regulation of phospho-glycogen synthase kinase-3 β. These in vitro changes are similar to the pathologies of post-mortem brain tissues of AD patients [123]. TCDD also was observed to impact gene expression in the developing brain [121], induce neurotoxicity and neuronal apoptosis in the rat brain cortex and in PC12 cell lines through down-regulation of the Wnt/β-catenin signalling pathway [124]; furthermore, it was shown to disrupt murine adult neurogenesis which potentially may affect memory processes [125] via toxicity to neuronal processes. Other widely used, dioxin-like chemicals are specific congeners of polychlorinated biphenyls (PCBs). These mass-produced but now banned organochlorines are known to trigger health effects in humans following bioaccumulation in wildlife and food animals and other exposure routes [126]. An epidemiological study from Eastern Slovakia showed a significant association between exposure to dioxin-like PCBs and decreased cognitive development as well as decreased motor skills in children and their mothers. The study suggested that these effects, especially for children, may be the result of endocrine disruption, modification of neurotransmitter functions, or reduced thyroid hormones in the brain development in utero [127, 128] suggestive of early exposure leading to origins of diseases in later life which may be true for AD development as indicated by the LEARn model. Another study linked the presence of PCBs to decreased sperm motility in humans [129]. Case studies showed that exposure to PCBs produces certain clinical features consistent with AD type dementia [130] and cohort studies revealed that women occupationally exposed to PCBs are more susceptible to Parkinson’s and AD than PCB-exposed men [131]. Although PCBs have been strongly associated with neuropathology observed in Parkinson’s disease [132], the role of PCBs in adverse neurodevelopment and neurodegeneration relating to AD is not well understood and requires further research.
Bisphenol A (BPA) and phthalates are used in water bottles and food cans as plasticizers and plastic building blocks, which can migrate into water and food stuff [133] and may affect human health over time as they cause epigenetic modifications [134] and other effects [135]. BPA has been linked to developmental, reproductive impairments and changes in brain and behaviour in experimental animals [136]. Importantly, BPA has been shown to interfere with spine synapse formation in the prefrontal cortex and hippocampus, which may have clinical implications resembling the events in AD [137]. BPA can disrupt expression of the Kcc2 gene through epigenetic mechanisms causing neurodevelopmental toxicity [138]. BPA mimics estrogenic activity and affects dopaminergic neurotransmission [139]. BPA exposure was shown to have an adverse effect on the brain of primates, causing spine synaptic remodeling suggestive of a critical impact on cognition and mood [140]. BPA is an endocrine disrupting compound and was shown to trigger decreased immune function in a study conducted on samples from the 2003-2006 National Health and Nutrition Examination Survey or NHANES [141]. A urinary maternal and childhood BPA cohort study revealed that gestational exposure to BPA causes anxious, depressive, and hyperactive behaviours in children and especially girls [142]. Similarly, prenatal phthalate exposure was associated with social impairment and poor cognition in children [143] with girls being more vulnerable to the neurotoxic effects of phthalates than boys [144]. Fish exposed to diethylhexyl phthalate (DEHP) showed reduced neurotransmitter activity and behavioral changes [145]. DEHP was shown to significantly inhibit acetylcholinesterase activity and upregulate glial fibrillary acidic protein as well as myelin basic protein in zebrafish embryos [146]; it also was observed to cause cognitive dysfunction and increased phosphorylation of tau protein in aged rats prenatally exposed to DEHP [147]. Efforts are ongoing to reduce and replace the use of these chemicals, which also may reduce potential adverse impacts on human health of DEHP acting alone or in synergy with other common industrial compounds such as BPA. The use of BPA in baby bottles and phthalates in children's toys has been banned but several consumer goods still contain these chemicals with the magnitude of risk posed being subject to debate and discontent. Since humans are exposed to numerous chemicals, it is not surprising that their exposure might influence various metabolisms and functions in the body. For example, one study proposed synergistic toxicity of phthalates and PCBs as a potential cause of decreased sperm motility [129] but similar studies on the effect of mixtures of plasticizers and plastic components in the context of AD are lacking.
ANTIMICROBIALS
Antimicrobials including non-halogenated and polychlorinated organic compounds have been in widespread, high-volume use for more than five decades as preservatives and as active ingredients of antimicrobial consumer and personal care products. These compounds possess immunotoxic, neurotoxic and brain damaging properties and hence are of potential concern to the human health [148-150] especially with respect to AD etiology. Overuse of cleaning products can lead to hyper-hygienic conditions (known to be associated with low lymphocyte turnover), which in turn can lead to immune-dysregulation as seen in autoimmunity similar to the inflammation observed in AD. Countries with greater degree of sanitation and lower degree of pathogen prevalence have higher age-adjusted AD rates, suggesting that AD risk is inversely related to microorganism exposure [151]. Overuse of antimicrobials and disinfectants has been suggested to induce conditions which may lead to AD or AD-like pathology but definitive research studies on such associations are lacking. Listed in (Tables 1 & 2) are animal and human studies identifying relevant body burdens of antimicrobial chemicals. Major antimicrobials are discussed in the following sections.
Hexachlorophene (HCP) is a polychlorinated binuclear aromatic compound historically used at high volume as a disinfectant and more recently exclusively as a preservative. In the 1970s it was established that HCP causes developmental, neurotoxic and brain damaging effects, triggering a ban of the compound in 1972 from high-volume uses as an antimicrobial [152]. Human exposure to HCP in the U.S. population peaked in the 1970s and has been much reduced since, with usage of the chemical as a preservative constituting the only documented remaining exposure route.
In vitro studies on the effects of HCP exposure on the murine brain showed decreased activity of brain succinate dehydrogenase [153]. Studies on sheep supported the finding that HCP causes changes in brain metabolism [154]. Since the use of HCP was prevalent prior to 1973, studies using premature infant brains and follow-up work in children showed vacuolar encephalopathy after intensive exposures of prematurely and newly born infants [155, 156] from repeated whole body bathing using formulations containing 3% HCP. In part due to limited epidemiological studies and data, researchers thus far have been unable to establish whether HCP and structurally related antimicrobials may play a role in the induction and pathology of AD or AD-like symptoms. Uses of HCP have since been replaced by the two antimicrobials, triclosan and triclocarban, that show structural similarity to HCP and share some of its human health concerns [152].
Triclocarban (TCC) and triclosan (TCS) are antimicrobial agents used in personal care products, primarily in liquid and bar soaps and some uses of TCS in toothpastes. In vitro assays illustrate that TCC and its analogs enhance hormone-dependent induction of estrogen and androgen-dependent gene expression, whereas TCS causes disruption of brain Ca2+ homeostasis by altering the ryanodine (Ry) receptor type 1; this may contribute to neurotoxicity as well as altered neurodevelopment and neuroplasticity [148]. Anuran studies have revealed that TCS modulates thyroid hormone associated gene expression, causing alterations in transcript levels of the brain thyroid hormone receptor α in premetamorphic tadpoles and disrupting postembryonic development [29]. Similarly, murine studies demonstrated that TCC levels can disrupt hormone signalling pathways and that in utero exposure impairs neurogenesis and neurobehavioral development [157], affecting the survival rate in female rat neonates [158]. Increased aromatase and estrogens levels were seen in developing brains of zebrafish embryos with combined effects of BPA and TCC, although individual compound exposure did not show any effect [159]. While muscle function has been associated with mild cognitive impairment with AD [160], one study reported that TCS impairs excitation–contraction coupling in striated muscle dynamics of fish and mice [161] which may be of concern to both susceptible populations and environmental health. Human studies showed detectable urinary levels of TCC [162] and TCS [163], confirming ongoing continuous exposure of human populations to anthropogenic antimicrobials and potential chronic and acute health effects [152]. Previous research has reported levels of TCS and TCC in maternal urine and cord blood plasma in an US urban population [164]. The relevance of these exposures to the development of AD is still uncertain and deserves further study.
Parabens (methyl, benzyl, butyl, propyl, and ethyl) are antifungal and bactericidal chemicals widely used in personal care products including soaps, cosmetics and perfumes. Parabens are endocrine disruptors causing mitochondrial toxicity [165]. Recent reports suggest that paraben exposure may lead to diminished ovarian reserve in women [166] and induction of oxidative stress biomarkers such as 8-hydroxy-2-deoxyguanosine and malondialdehyde, both detectable in the urine of mothers and their newborns [167]. Whereas urinary concentrations can inform on the body burden in human populations [168, 169], such data by themselves are not suitable for revealing the relationship to neurotoxicity or neurodevelopmental disorders, and by extension, potential roles in AD. Animal studies on paraben exposure show bioaccumulation in fish brain, causing reduced neurotransmitter activity and leading to behavioral changes as a result of neurotoxicity and neurodevelopmental disturbances [28]. Rat offspring prenatally exposed to butyl paraben showed neurodevelopmental disorders [150], adversely affecting adult behavior with outcomes including anxiety and learning disabilities following exposure [170]. These results suggest that even small doses of parabens can cause changes in metabolism of animals and potentially of humans, increasing the risk of behavioral changes and neurological symptoms triggered in the wake of these exposures.
It has been suggested that exposure to environmental microorganisms is important for the regulation and proper functioning of the immune system, and that maintaining a hyper hygienic behaviour may increase the incidence of AD [151]. In addition, several researchers have indicated a possible role of endocrine disruptors in the progression of AD [32]. But again, more studies are needed to confirm or refute these working hypotheses.
AIR POLLUTANTS
Oxidative stress and free radicals are generated as a result of imbalances in metabolism caused by biological, physical or chemical exposures [171]. Free radicals may accumulate over an individual's lifetime and later induce neuroinflammation and neuropathology [172]. Recent studies have implicated particulate matter (PM, composed of particles measuring in diameter 2.5 µm or smaller and collectively termed PM2.5) in the causation of AD and other neurodegenerative disorders. Researchers have examined whether toxic metals including nickel, vanadium, lead and certain gases such as CO, NOx, and SO2 present in polluted air may cause reactive oxygen species (ROS) production, oxidative stress, chronic neuroinflammation, cerebrovascular damage, Aβ peptide accumulation, and neuron damage/loss contributing to AD pathogenesis [173]. AD associated amyloid-β40 and amyloid-β42 levels were increased in mice brains of mice exposed to a nickel nanoparticle model of air pollution [174]. In Mexico City, children exposed to severely polluted environments demonstrated neuronal accumulation of misfolded proteins similar to the anatomy observed in the early stages of both AD and Parkinson’s disease [12]. Another study by the same group revealed that 56% children had prefrontal white matter hyperintense lesions caused by PM-induced damage to the CNS by PM in early life, which may be a predisposing factor for the development of neuroinflammation and neurodegeneration later in life [175]. The authors suggest that particle exposure activates the pathogen sensors and reactive oxygen species, thereby generating brain inflammation [176]. Ozone is the main component of photochemical pollution and adult Wistar rats chronically exposed to ozone showed increases in ROS, memory deficiency, dysregulation of inflammatory processes, progressive neurodegeneration, as well as impaired brain repair in the hippocampus, an area heavily affected in AD brains [177]. Importantly, human and animal studies suggest that air pollution (PM, gases, organic compounds, and metals) may cause an increased expression of markers associated with neurodegenerative disease pathologies and also may cause developmental neurotoxicity contributing to the etiology of neurodevelopmental disorders [178]. Epidemiological, observational, clinical, and experimental studies have reported that air pollution causes diseases of the CNS including AD [179].
Laboratory animal experiments have shown that volatile organic compounds (VOCs) including volatile solvents like phenol, a simple aromatic alcohol contained in cleaning agents and fuels can cause morphologic changes in neurons and biochemical changes in synapses and neurotransmitters. A case-control study from 1995 showed that occupational exposure to VOCs can cause an imbalance in the neurotransmitter system thereby potentially influencing the onset of AD. Exposure to one or more VOCs (benzene and toluene; phenols and alcohols; ketones; other solvents) yielded an adjusted AD odds ratio of 2.3 (95% CI of 1.1–4.7); among men, the odds ratio was higher at 6.0 (95% CI of 2.1–17.2) [180]. Exposure to hydrocarbon fuels induces neurological symptoms including depression, frequent headaches, numbness, and dizziness [181]. A recent study using gas chromatography and mass spectrometry confirmed that AD patients have higher levels of VOCs than healthy controls [182].
CONCLUSION
Though environmental exposures are known to play a role in the development of AD, the specific agents and exposure thresholds remain an area of both investigation and speculation. A spectrum of organic and inorganic substances have been associated with AD risk but irrefutable evidence from human studies to date still remains elusive in many cases. Agents of established neurotoxicity deserve further study for their potential role in promoting the development of AD but, at the same time, only a small fraction of these neurotoxins ultimately may be associated with AD. Yet, the percentage of the population affected by AD cases is high and projected to increase further in the future. Multiple classes of environmental chemicals have been hypothesized to play a role in the etiology of AD. A substantial body of literature exists, identifying both inorganic and organic chemicals as possible risk factors for AD development. In contrast, only a few studies are available thus far exploring the role of exposure to environmental mixtures of chemicals on the etiology of AD. With future increases in human life expectancy projected, AD as a late-in-life onset disease is destined to rise, as shown here in a hypothetical schematic diagram (Fig. 4). Efforts are under way to limit the production of and human exposure to neurotoxic and AD-risk posing chemicals contained in cosmetics and consumer products [152, 183, 184]. For example, several countries are phasing out the use of harmful chemicals including phthalates, BPA, TCS and TCC [152, 255]. However, environmental exposures to complex mixtures of organic and inorganic AD risk factors will continue into the future due to both the large spectrum of AD-related chemicals in commercial use and the essential services some of these chemicals provide to humanity. Adding to a delay in exposure reduction is the fact that most of the environmental contaminants have been tested in vitro and in animal models only and sometimes at concentrations orders of magnitude higher than those experienced by the general population; thus, there remains considerable uncertainty and scepticism as to the relevance of single and composite exposures for adverse neurological effects observed in human populations. Moreover, the decade-long time delay between exposure and onset of disease render studies on AD etiology inherently challenging. Also, genetic susceptibility combined with the presence of environmental factors will modify the magnitude of any effects and complicate the analysis of population data. While many environmental contaminants are documented to contribute to toxic body burdens in human populations, pinpointing subtle and time-delayed effects through epidemiological studies and linking them to AD constitutes a supreme challenge. Very large, detailed epidemiological studies tracking lifetime exposure to environmental contaminants are needed to confirm the role of specific chemicals and chemical mixtures in AD etiology. These studies ideally should be flanked with laboratory studies concentrating on a determination of toxic body burdens in human tissue (e.g., fat, brain, and bone) of victims presumed to suffer from AD. Since misdiagnosis in AD patients is known to be rampant, neuropathologically diagnosed AD should be confirmed postmortem via brain autopsies. Careful analyses of autopsy confirmed AD cases, their corresponding laboratory determined toxic body burdens and their genetic risk factors will be essential for expanding the still limited knowledge of the role of environmental chemical agents in the etiology of AD. Finally, this review emphasises the need for adequate funding of future studies required to firmly establish associations between environmental causes and development of AD and other neurodegenrative disorders.
Fig. (4).
Hypothetical schematic diagram depicting the expected increasing incidence of Alzheimer’s disease due to increased human life expec-tancy
ACKNOWLEDGEMENTS
This project was supported in part by research grants R01ES015445, R01ES020889 and their supplements awarded by the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH). We thank Dr. Matthew Scotch, Dr. Benny Pycke and Jing Chen for providing helpful input for this manuscript.
Glossary
ABBREVIATIONS
- AhR =
Aryl hydrocarbon receptor
- ALS =
Amyotrophic Lateral Sclerosis
- APEOs =
Alkylphenol polyethoxylates
- BFRs =
Brominated flame retardants
- BPA =
Bisphenol A
- b.w. =
body weight
- CNS =
central nervous system
- MUFA =
DEHP = Diethylhexyl phthalate
- GABA =
gamma-aminobutyric acid
- GTP =
guanosine triphosphate
- HCH =
hexachlorocyclohexane
- HCP =
Hexachlorophene
- LEARn =
Latent Early–life Associated Regulation
- NHANES =
National Health and Nutrition Examination Survey
- NP =
Nonylphenol
- OP =
Octylphenol
- PAHs =
Polycyclic aromatic hydrocarbons
- PAQUID =
Personnes Agées QUID
- PBDEs =
Polybrominated diphenyl ethers
- PCBs =
Polychlorinated biphenyls
- PCDDs =
Dibenzo-p-dioxins
- OP =
Octylphenol
- PAHs =
Polycyclic aromatic hydrocarbons
- PCDDs =
Dibenzo-p-dioxins
- PCDDs =
Polychlorinated dibenzofurans
- PD =
Parkinson's disease
- PM =
Particulate matter
- ROS =
Reactive oxygen species
- TCC =
Triclocarban
- TCDD =
2, 3,7,8-tetrachlorodibenzo-p-dioxin
- PCDDs =
Dibenzo-p-dioxins
- TCS =
Triclosan
- VOCs =
Volatile organic compounds
REFERENCES
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer's Dement. 2007;3(3 ):186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.World-Life-Expectancy. Alzheimer's/Dementia-Death Rate Per 100,000 Age Standardized-Data source WHO 2011. 2014; Available from: http://www.worldlifeexpectancy.com/cause-ofdeath/alzheimers-dementia/by-country/ . 2014.
- 3.Murphy SL, Xu J, Kochanek KD. Deaths Final Data for 2010. Natl Vital Stat Rep. 2013;61(4 ):1–118. [PubMed] [Google Scholar]
- 4.Alzheimer's-Association. Overview of Alzheimer’s Disease: Facts and Figures2013 Contract No.: 2. 2013;Contract No [Google Scholar]
- 5.Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's Disease Progress and Problems on the Road to Therapeutics. Science. 2002;297(5580 ):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the us population Prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8 ):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer's-Association Younger/Early Onset Alzheimer's & Dementia. 2013 Available from http://www.alz.org/alzheimers_disease_early_onset.asp .
- 8.Perl DP. Neuropathology of Alzheimer's Disease. Mt Sinai J Med. 2010;77(1 ):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6(4 ):487–98. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 10.Coon KD, Myers AJ, DW C, Webster JA, Pearson JV, Lince DH , et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. J Clin Psychiat. 2007;68(4 ):613–8. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54(6 ):1316–23. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 12.Calderón-Garcidueñas L, Reed W, Maronpot RR, Henriquez-Roldán C, Delgado-Chavez R, Calderón-Garcidueñas A , et al. Brain Inflammation and Alzheimer's-Like Pathology in Individuals Exposed to Severe Air Pollution. Toxicologic Pathol. 2004;32(6 ):650–8. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH. How and when environmental agents and dietary factors affect the course of Alzheimer's disease the "LEARn" model (latent early-life associated regulation) may explain the triggering of, AD. Curr Alzheimer Res. 2007;4(2 ):219–28. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- 14.Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol. 2010;45(4 ):291–6. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377(9770 ):1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 16.Avramopoulos D. Genetics of Alzheimer's disease recent advances. Genome Med. 2009;1(3 ):34. doi: 10.1186/gm34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prusiner SB. A Unifying Role for Prions in Neurodegenerative Diseases. Science. 2012;336(6088 ):1511–3. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahiri DK. Prions A Piece of the Puzzle?. Science. 2012;337(6099 ):1172. doi: 10.1126/science.337.6099.1172-a. [DOI] [PubMed] [Google Scholar]
- 19.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues J-F, Brochard P. Neurodegenerative Diseases and Exposure to Pesticides in the Elderly. Am J Epidemiol. 2003;157(5 ):409–14. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 20.Campbell A. The potential role of aluminium in Alzheimer's disease. Nephrol Dialysis Transplan. 2002;17(2 ):17–20. doi: 10.1093/ndt/17.suppl_2.17. [DOI] [PubMed] [Google Scholar]
- 21.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge -W et al. The Fetal Basis of Amyloidogenesis Exposure to Lead and Latent Overexpression of Amyloid Precursor Protein and ß-Amyloid in the Aging Brain. J Neurosci. 2005;25(4 ):823–9. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative Lead Dose and Cognitive Function in Adults A Review of Studies That Measured Both Blood Lead and Bone Lead. Environ Health Perspectives. 2007;115(3 ):483–92. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum L, Chan I, Cheung S-K, Goggins W, Mok V, Lam L, et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals. 2010;23(1 ):173–9. doi: 10.1007/s10534-009-9277-5. [DOI] [PubMed] [Google Scholar]
- 24.Singh N, Chhillar N, Banerjee B, Bala K, Basu M, Mustafa M. Organochlorine pesticide levels and risk of Alzheimer’s disease in north Indian population. Human ExpToxicol. 2013;32(1 ):24–30. doi: 10.1177/0960327112456315. [DOI] [PubMed] [Google Scholar]
- 25.Kamel F, Hoppin JA. Association of Pesticide Exposure with Neurologic Dysfunction and Disease. Environ Health Perspect. 2004;112(9 ):950–8. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153):disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192(2 ):95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 27.Zaman T, editor. The Prevalence and Environmental Impact of Single Use Plastic Products. Public Health Management & Policy An Online Textbook 11th edition Retrieved November. 2010;23:2011. [Google Scholar]
- 28.Barse AV, Chakrabarti T, Ghosh TK, Pal AK, Kumar N, Raman RP et al. Vitellogenin Induction and Histo-metabolic Changes Following Exposure of Cyprinus carpio to Methyl Paraben. Asian-Australasian J Animal Sci. 2010;23(12 ):1557–65. [Google Scholar]
- 29.Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, et al. The bactericidal agent triclosan modulates thyroid hor-mone-associated gene expression and disrupts postembryonic anuran development. Aquatic Toxicol. 2006;80(3 ):217–27. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Sparks DL, Schreurs BG. Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc Natl Acad Sci USA. 2003;100(19 ):11065–9. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson CM, Markesbery WR, Ehmann WD, Mao YX, Vance DE. Regional brain trace-element studies in Alzheimer's disease. Neurotoxicology. 1988;9(1 ):1–7. [PubMed] [Google Scholar]
- 32.Weiss B. Can endocrine disruptors influence neuroplasticity in the aging brain?. Neurotoxicology. 2007;28(5 ):938–50. doi: 10.1016/j.neuro.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luch A, Tchounwou P, Yedjou C, Patlolla A, Sutton D. Heavy Metal Toxicity and the Environment. Molecular Clinical and Environmental Toxicology Springer Basel. 2012;p:133–64. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chhabra D, Oda K, Jagannath P, Utsunomiya H, Takekoshi S, Nimura Y. Chronic heavy metal exposure and gallbladder cancer risk in India, a comparative study with Japan. Asian Pac J Cancer Prev. 2012;13(1 ):187–90. doi: 10.7314/apjcp.2012.13.1.187. [DOI] [PubMed] [Google Scholar]
- 35.Alissa EM, Ferns GA. Heavy Metal Poisoning and Cardiovascular Disease. J Toxicol. 2011;2011 doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawahara M, Kato-Negishi M. Link between Aluminum and the Pathogenesis of Alzheimer's Disease The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int J Alzheimers Dis. 2011;276393 doi: 10.4061/2011/276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crapper DR, Krishnan SS, Dalton AJ. Brain Aluminum Distribution in Alzheimer's Disease and Experimental Neurofibrillary Degeneration. Science. 1973;180(4085 ):511–3. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- 38.Syme CD, Nadal RC, Rigby SEJ, Viles JH. Copper Binding to the Amyloid-ß (Aß) Peptide Associated with Alzheimer's Disease folding, coordination geometry, pH dependence, stoichiometry, and affinity of Aß-(1–28): insights from a range of complementary spectroscopic techniques. J Biol Chem. 2004;279(18 ):18169–77. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- 39.Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD , et al. Low levels of copper disrupt brain amyloid-ß homeostasis by altering its production and clearance. Proc Nat Acad Sci. 2013;110(36 ):14771–6. doi: 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J Neurol Sci. 1996;143(1-2 ):137–42. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 41.Squitti RPR. Copper phenotype in Alzheimer's disease dissecting the pathway. Am J Neurodegener Dis. 2013;2(2 ):46–56. [PMC free article] [PubMed] [Google Scholar]
- 42.Ward NI, Mason JA. Neutron activation analysis techniques for identifying elemental status in Alzheimer's disease. J Radioanalytical Nuclear Chem1. 1987;13(2 ):515–26. [Google Scholar]
- 43.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Aß42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis. 2004;6(3 ):291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 44.Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G. Increased Iron Levels and Decreased Tissue Integrity in Hippocampus of Alzheimer's Disease Detected in vivo with Magnetic Resonance Imaging. J Alzheimers Dis. 2013;37(1 ):127–36. doi: 10.3233/JAD-130209. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Goyer RA, Waalkes MP, editors. New York: The McGraw Hill-Companies, Inc. 2008. Casarett & Doull's Toxicology The Basic Science of Poisons. [Google Scholar]
- 46.Weisskopf MG, Proctor SP, Wright RO, Schwartz J, Spiro AI, Sparrow D, et al. Cumulative Lead Exposure and Cognitive Performance Among Elderly Men. Epidemiology. 2007;18(1 ):59–66. doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 47.Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H. Alzheimer's Disease and Environmental Exposure to Lead The Epidemiologic Evidence and Potential Role of Epigenetics. Curr Alz-heimer Res. 2012;9(5 ):563–73. doi: 10.2174/156720512800617991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Gibb HJ, Howe PD. WHO Concise International Chemical Assessment Document 69. Cobalt and Inorganic Cobalt Compounds. 2006 [Google Scholar]
- 49.Matés JM, Segura JA, Alonso FJ, Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radical BiolMed. 2010;49(9 ):1328–41. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Viaene MK, Masschelein R, Leenders J, De Groof M, Swerts LJVC, Roels HA. Neurobe-havioural effects of occupational exposure to cadmium a cross sectional epidemiological study. Occup Environ Med. 2000;57(1 ):19–27. doi: 10.1136/oem.57.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang L-F, Yao T-M, Zhu Z-L, Wang C, Ji L-N. Impacts of Cd(II) on the conformation and self-aggregation of Alzheimer's tau fragment corresponding to the third repeat of microtubule-binding domain. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2007;1774(11 ):1414–21. doi: 10.1016/j.bbapap.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Banta RG, Markesbery WR. Elevated manganese levels associated with dementia and ex-trapyramidal signs. Neurology. 1977;27(3 ):213–7. doi: 10.1212/wnl.27.3.213. [DOI] [PubMed] [Google Scholar]
- 53.Henriksson J, Tjälve H. Manganese Taken Up into the CNS via the Olfactory Pathway in Rats Affects Astrocytes. Toxicological Sci. 2000;55(2 ):392–8. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- 54.Guilarte TR. APLP1, Alzheimer's-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neuro Toxicology. 2010;31(5 ):572–4. doi: 10.1016/j.neuro.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider JS, Williams C, Ault M, Guilarte TR. Chronic manganese exposure impairs visuospatial associative learning in non-human primates. Toxicol Lett. 2013;221(2 ):146–51. doi: 10.1016/j.toxlet.2013.06.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mutter J, Curth A, Naumann J, Deth R, Walach H. Does Inorganic Mercury Play a Role in Alzheimer's Disease? A Systematic Review and an Integrated Molecular Mechanism. J Alzheimers Dis. 2010;22(2 ):357–74. doi: 10.3233/JAD-2010-100705. [DOI] [PubMed] [Google Scholar]
- 57.Leong CCW, Syed NI, Lorscheider FL. Retrograde degeneration of neurite membrane structural integrity of nerve growth cones following in vitro exposure to mercury. Neuro Report. 2001;12(4):733–7. doi: 10.1097/00001756-200103260-00024. [DOI] [PubMed] [Google Scholar]
- 58.Haley BE. The relationship of the toxic effects of mercury to exacerbation of the medical condition classified as Alzheimer’s disease. Medical Veritas. 2007;4:1510–24. [Google Scholar]
- 59.Nielsen FH. Importance of making dietary recommendations for elements designated as nu-tritionally beneficial, pharmacologically beneficial, or conditioinally essential. The J Trace Elements Exp Med. 2000;13(1 ):113–29. [Google Scholar]
- 60.Dani SU. Arsenic for the fool An exponential connection. Sci Total Environm. 2010;408(8 ):1842–6. doi: 10.1016/j.scitotenv.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 61.Namgung U, Xia Z. Arsenic Induces Apoptosis in Rat Cerebellar Neurons via Activation of JNK3 and p38 MAP Kinases. Toxicol App Pharmacol. 2001;174(2 ):130–8. doi: 10.1006/taap.2001.9200. [DOI] [PubMed] [Google Scholar]
- 62.O’Bryant SE, Edwards M, Menon C, Gong G, Barber R. Long-Term Low-Level Arsenic Exposure Is Associated with Poorer Neuropsychological Functioning A Project FRONTIER Study. Intern J Environ Res Public Health. 2011;8(3 ):861–74. doi: 10.3390/ijerph8030861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gharibzadeh S, Hoseini SS. Arsenic Exposure May Be a Risk Factor for Alzheimer’s Disease. J Neuropsychiat Clin Neurosci. 2008;20(4 ):501. doi: 10.1176/jnp.2008.20.4.501. [DOI] [PubMed] [Google Scholar]
- 64.Wells EM, Navas-Acien A, Apelberg BJ, Herbstman JB, Jarrett JM, Lin YH et al. Association of selenium and copper with lipids in umbilical cord blood. J DevelopOrigins Health Dis. 2014;5(4 ):281–7. doi: 10.1017/S2040174414000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MId , Cozzolino SMF. Nutritional status of selenium in Alzheimer's disease patients. Brit Jf Nut. 2010;103(06 ):803–6. doi: 10.1017/S0007114509992832. [DOI] [PubMed] [Google Scholar]
- 66.Estevez AO, Mueller CL, Morgan KL, Szewczyk NJ, Teece L, Miranda-Vizuete A et al. Selenium induces cholinergic motor neuron degeneration in Caenorhabditis elegans. Neuro Toxicology. 2012;33(5 ):1021–32. doi: 10.1016/j.neuro.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP et al. Diet and Alz-heimer’s disease risk factors or prevention the current evidence. Expert Rev Neurother. 2011;11(5 ):677–708. doi: 10.1586/ern.11.56. [DOI] [PubMed] [Google Scholar]
- 68.Cardoso BR, Cominetti C, Cozzolino SMF. Importance and management of micronutrient deficiencies in patients with Alzheimer’s disease. Clin Intervent Aging. 2013;8:531–42. doi: 10.2147/CIA.S27983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta UC, Gupta SC. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition A Review. Pedosphere. 2014;24(1 ):13–38. [Google Scholar]
- 70.Craddock TJA, Tuszynski JA, Chopra D, Casey N, Goldstein LE, Hameroff SR, et al. The Zinc Dyshomeostasis Hypothesis of Alzheimer's Disease. PLoS ONE. 2012;7(3 ):e33552. doi: 10.1371/journal.pone.0033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang X, Cuajungco MP, Atwood CS, Moir RD, Tanzi RE, Bush AI. Alzheimer’s Disease, ß-Amyloid Protein and Zinc. J Nutr. 2000;130(5 ):1488S–92S. doi: 10.1093/jn/130.5.1488S. [DOI] [PubMed] [Google Scholar]
- 72.Bonda DJ, Lee H-g, Blair JA, Zhu X, Perry G, Smith MA. Role of metal dyshomeostasis in Alzheimer's disease. Metallomics. 2011;3(3 ):267–70. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T. Increased APLP1 expression and neurode-generation in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105(5 ):1948–59. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson Jr, Roy A, Shalat SL, von Stein RT, Hossain MM, Buckley B et al. ELevated serum pesticide levels and risk for alzheimer disease. JAMA Neurology. 2014;71(3 ):284–90. doi: 10.1001/jamaneurol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mott L, Fore D, Curtis J, Solomon G. Chapter 5- Pesticides. Available from http://www.nrdc.org/health/kids/ocar/chap5. asp#note3 . 1997.
- 76.Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE et al. Pesti-cide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60(2 ):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 77.Qi Z, Miller GW, Voit EO. Rotenone and paraquat perturb dopamine metabolism A com-putational analysis of pesticide toxicity. Toxicology. 2014;315:92–101. doi: 10.1016/j.tox.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa LG, Giordano G, Guizzetti M, Kodavanti P. Cell Signaling and Neurotoxicity Protein Kinase C In vitro and In vivo. In vitro Neurotoxicology Humana Press; 2011:307–19. doi: 10.1007/978-1-61779-170-3_21. [DOI] [PubMed] [Google Scholar]
- 79.Casida JE, Durkin KA. Neuroactive Insecticides Targets, Selectivity, Resistance, and Secondary Effects. Ann Rev Entomol. 2013;58(1 ):99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- 80.Zaganas I, Kapetanaki S, Mastorodemos V, Kanavouras K, Colosio C, Wilks MF, et al. Linking pesticide exposure and dementia What is the evidence?. Toxicology. 2013;307:3–11. doi: 10.1016/j.tox.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Mwila K, Burton MH, Van Dyk JS, Pletschke BI. The effect of mixtures of organophosphate and carbamate pesticides on acetylcholinesterase and application of chemometrics to identify pesticides in mixtures. Environ Monit Assess. 2013;185(3 ):2315–27. doi: 10.1007/s10661-012-2711-0. [DOI] [PubMed] [Google Scholar]
- 82.Rouimi P, Zucchini-Pascal N, Dupont G, Razpotnik A, Fouché E, De Sousa G, et al. Impacts of low doses of pesticide mixtures on liver cell defence systems. Toxicology. 2012;26(5 ):718–26. doi: 10.1016/j.tiv.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Laetz CA, Baldwin DH, Collier TK, Hebert V, Stark JD, Scholz NL. The Synergistic Toxicity of Pesticide Mixtures Implications for Risk Assessment and the Conservation of Endangered Pacific Salmon. Environmenl Health Perspect. 2008;117(3 ) doi: 10.1289/ehp.0800096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayden KM, Norton MC, Darcey D, Østbye T, Zandi PP, Breitner JCS, et al. Occupational exposure to pesticides increases the risk of incident AD The Cache County Study. Neurology. 2010;74(19 ):1524–30. doi: 10.1212/WNL.0b013e3181dd4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chhillar N, Singh NK, Banerjee BD, Bala K, Sharma D, Mustafa M et al. ß-hexachlorocyclohexane as a Risk for Alzheimer’s Disease A Pilot Study in North Indian Population. Am J Alzheimers Dis. 2013;1:60–71. [Google Scholar]
- 86.Terry Jr AV. Functional consequences of repeated organophosphate exposure Potential non-cholinergic mechanisms. Pharmacol Therap. 2012;134(3 ):355–65. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Androutsopoulos VP, Hernandez AF, Liesivuori J, Tsatsakis AM. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 2013;307:89–94. doi: 10.1016/j.tox.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Aygun D. Diagnosis in an acute organophosphate poisoning report of three interesting cases and review of the literature. Eur J Emergen Med. 2004;11(1 ):55–8. doi: 10.1097/00063110-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Colosio C, Tiramani M, Maroni M. Neurobehavioral Effects of Pesticides State of the Art. NeuroToxicology. 2003;24(4-5 ):577–91. doi: 10.1016/S0161-813X(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 90.Edwards FL, Yedjou CG, Tchounwou PB. Involvement of oxidative stress in methyl parathion and parathion-induced toxicity and genotoxicity to human liver carcinoma (HepG2):cells. Environ Toxicol. 2013;28(6 ):342–8. doi: 10.1002/tox.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smulders CJGM, Bueter TJH, Van Kleef RGDM, Vijverberg HPM. Selective effects of carba-mate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase. Toxicol App Pharmacol. 2003;193(2 ):139–46. doi: 10.1016/j.taap.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 92.Bjorling-Poulsen M, Andersen H, Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environm Health. 2008;7(1 ):50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen L, Yoo S-E, Na R, Liu Y, Ran Q. Cognitive impairment and increased Aß levels induced by paraquat exposure are attenuated by enhanced removal of mitochondrial H2O2. Neurobiol Aging. 2012;33(2 ):432.e15–e26. doi: 10.1016/j.neurobiolaging.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Ullrich C, Humpel C. Rotenone Induces Cell Death of Cholinergic Neurons in an Organotypic Co-Culture Brain Slice Model. Neurochem Res. 2009;34(12 ):2147–53. doi: 10.1007/s11064-009-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K , et al. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;92(2 ):147–53. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- 96.Stehr CM, Linbo TL, Incardona JP, Scholz NL. The Developmental Neurotoxicity of Fipronil Notochord Degeneration and Locomotor Defects in Zebrafish Embryos and Larvae. Toxicol Sci. 2006;92(1 ):270–8. doi: 10.1093/toxsci/kfj185. [DOI] [PubMed] [Google Scholar]
- 97.Lee S, Mulay P, Diebolt-Brown B, Lackovic M, Mehler L, Beckman J, et al. Acute illnesses associated with exposure to fipronil—surveillance data from 11 states in the United States, 2001–2007. Clin Toxicol. 2010;48(7 ):737–44. doi: 10.3109/15563650.2010.507548. [DOI] [PubMed] [Google Scholar]
- 98.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc Nat Acad Sci. 2012;109(25 ):10071–6. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen N, Luo D, Yao X, Yu C, Wang Y, Wang Q, et al. Pesticides Induce Spatial Memory Deficits with Synaptic Impairments and an Imbalanced Tau Phosphorylation in Rats. J Alzheimers Dis. 2012;30(3 ):585–94. doi: 10.3233/JAD-2012-111946. [DOI] [PubMed] [Google Scholar]
- 100.Hossain MM, Richardson Jr. Mechanism of Pyrethroid Pesticide–Induced Apoptosis Role of Calpain and the ER Stress Pathway. Toxicol Sci. 2011;122(2 ):512–25. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ihara D, Fukuchi M, Honma D, Takasaki I, Ishikawa M, Tabuchi A, et al. Deltamethrin, a type II pyrethroid insecticide, has neurotrophic effects on neurons with continuous activation of the Bdnf promoter. Neuropharmacol. 2012;62(2 ):1091–8. doi: 10.1016/j.neuropharm.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 102.Grandjean P, Harari R, Barr DB, Debes F. Pesticide Exposure and Stunting as Independent Predictors of Neurobehavioral Deficits in Ecuadorian School Children. Pediatrics. 2006;117(3 ):e546–e56. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- 103.Nasuti C, Gabbianelli R, Falcioni ML, Di Stefano A, Sozio P, Cantalamessa F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology. 2007;229(3 ):194–205. doi: 10.1016/j.tox.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 104.Liu B, Hong J-S. Role of Microglia in Inflammation-Mediated Neurodegenerative Diseases Mechanisms and Strategies for Therapeutic Intervention. J Pharmacol Exp Therap. 2003;304(1 ):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 105.Beketov M, Liess M. Potential of 11 Pesticides to Initiate Downstream Drift of Stream Macroinvertebrates. Arch Environ Contam Toxicol. 2008;55(2 ):247–53. doi: 10.1007/s00244-007-9104-3. [DOI] [PubMed] [Google Scholar]
- 106.Calderon-Segura ME, Gomez-Arroyo S, Villalobos-Pietrini R, Martinez-Valenzuela C, Carbajal-Lopez Y, Calderon-Ezquerro MC, et al. Evaluation of Genotoxic and Cytotoxic Effects in Human Peripheral Blood Lymphocytes Exposed In vitro to Neonicotinoid Insecticides News. J Toxicol. 2012;2012 doi: 10.1155/2012/612647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE. 2012;7(2 ):e32432. doi: 10.1371/journal.pone.0032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shao M, Tang X, Zhang Y, Li W. City clusters in China air and surface water pollution Fron-tiers in Ecology and the Environment. JOURNAL NAME. 2006;4(7 ):353–61. [Google Scholar]
- 109.Al-Mousa F, Michelangeli F. Some Commonly Used Brominated Flame Retardants Cause Ca2+-ATPase Inhibition, Beta-Amyloid Peptide Release and Apoptosis in SH-SY5Y Neuronal Cells. PLoS ONE. 2012;7(4 ):e33059. doi: 10.1371/journal.pone.0033059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eriksson P, Viberg H, Jakobsson E, Örn U, Fredriksson A. A Brominated Flame Retardant, 2,2`,4,4`,5-Pentabromodiphenyl Ether Uptake, Retention, and Induction of Neurobehavioral Alterations in Mice during a Critical Phase of Neonatal Brain Development. Toxicol Sci. 2002;67(1 ):98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- 111.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Intern. 2003;29(6 ):841–53. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 112.Birnbaum LS, Hubal EAC. Polybrominated diphenyl ethers a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspec. 2006;114(11 ):1770. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417-418:294–8. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental Neuropathology of Environmental Agents. Ann Rev Pharmacol Toxicol. 2004;44(1 ):87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pocar P, Augustin R, Gandolfi F, Fischer B. Toxic Effects of In vitro Exposure to p-tert-Octylphenol on Bovine Oocyte Maturation and Developmental Competence. Biol Reproduc. 2003;69(2 ):462–8. doi: 10.1095/biolreprod.102.010355. [DOI] [PubMed] [Google Scholar]
- 116.Trudeau VL, Chiu S, Kennedy SW, Brooks RJ. Octylphenol (OP) alters the expression of members of the amyloid protein family in the hypothalamus of the snapping turtle, Chelydra serpentina serpentina. Environ Health Perspec. 2002;110(3 ):269–75. doi: 10.1289/ehp.02110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jonsson B. Risk assessment on butylphenol, octylphenol and nonylphenol, and estimated human exposure of alkylphenols from Swedish fish. Uppsala Uppsala University. 2006 [Google Scholar]
- 118.Venkatesan AK, Halden RU. National inventory of alkylphenol ethoxylate compounds in U.S. sewage sludges and chemical fate in outdoor soil meso-cosms. Environ Pollut. 2013;174:189–93. doi: 10.1016/j.envpol.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watanabe H, Suzuki A, Goto M, Lubahn D, Handa H, Iguchi T. Tissue-specific estrogenic and non-estrogenic effects of a xenoestrogen, nonylphe-nol. J Mol Endocrinol. 2004;33(1 ):243–52. doi: 10.1677/jme.0.0330243. [DOI] [PubMed] [Google Scholar]
- 120.Vaiserman A. Early-life Exposure to Endocrine Disrupting Chemicals and Later-life Health Outcomes An Epigenetic Bridge?. Ageing Dis. 2014;5(3 ):1–11. doi: 10.14336/AD.2014.0500419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gohlke JM, Stockton PS, Sieber S, Foley J, Portier CJ. AhR-mediated gene expression in the developing mouse telencephalon. Reprod Toxicol. 2009;28(3 ):321–8. doi: 10.1016/j.reprotox.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chavan H, Krishnamurthy P. Polycyclic Aromatic Hydrocarbons (PAHs) Mediate Transcrip-tional Activation of the ATP Binding Cassette Transporter ABCB6 Gene via the Aryl Hydrocarbon Receptor (AhR) J Biol Chem. 2012;287(38 ):32054–68. doi: 10.1074/jbc.M112.371476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sul D, Kim H-S, Cho E-K, Lee M, Kim HS, Jung W-W , et al. 2,3,7,8-TCDD neurotoxicity in neuroblastoma cells is caused by increased oxidative stress, intracellular calcium levels, and tau phos-phorylation. Toxicology. 2009;255(1-2 ):65–71. doi: 10.1016/j.tox.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 124.Xu G, Zhou Q, Wan C, Wang Y, Liu J, Li Y, et al. 2,3,7,8-TCDD induces neurotoxicity and neuronal apoptosis in the rat brain cortex and PC12 cell line through the down-regulation of the Wnt/ß-catenin signaling pathway. NeuroToxicology. 2013;37:63–73. doi: 10.1016/j.neuro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 125.Latchney SE, Hein AM, O'Banion MK, DiCicco-Bloom E, Opanashuk LA. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem. 2013;125(3 ):430–45. doi: 10.1111/jnc.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruder AM, Hein MJ, Hopf NB, Waters MA. Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants A ten-year update. International Journal of Hygiene and Environmental Health. 2013 doi: 10.1016/j.ijheh.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevel-opmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity a birth cohort study. Environ Health. 2010;9(1):1–13. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, et al. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environmental Health Perspec. 2009;117(10 ):1600–6. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hauser R, Williams P, Altshul LM. CA Evidence of Interaction between Polychlorinated Biphenyls and Phthalates in Relation to Human Sperm Motility. Environ Health Perspec. 2005;13(4 ):425–30. doi: 10.1289/ehp.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tröster AI, Ruff RM, Watson DP. Dementia as a neuropsychological consequence of chronic occupational exposure to polychlorinated biphenyls (PCBs). Arch Clin Neuropsychol. 1991;6(4 ):301–18. [PubMed] [Google Scholar]
- 131.Steenland K, Hein MJ, Cassinelli RTI, Prince MM, Nilsen NB, Whelan EA, et al. Polychlorinated Biphenyls and Neurodegenerative Disease Mortality in an Occupational Cohort. Epidemi-ology. 2006;17(1):8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- 132.Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD. Association between polychlorinated biphenyls and Parkinson's disease neuropathology. NeuroToxicology. 2012;33(5 ):1298–304. doi: 10.1016/j.neuro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guart A, Bono-Blay F, Borrell A, Lacorte S. Migration of plasticizersphthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Additives & Contaminants Part A. 2011;28(5 ):676–85. doi: 10.1080/19440049.2011.555845. [DOI] [PubMed] [Google Scholar]
- 134.Singh S, Li SS-L. Epigenetic Effects of Environmental Chemicals Bisphenol A and Phthalates. Intern J Mol Sci. 2012;13(8 ):10143–53. doi: 10.3390/ijms130810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halden RU. Plastics and Health Risks. Ann Rev Public Health. 2010;31(1 ):179–94. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- 136.Borrell B. Toxicology The big test for bisphenol, A. Nature. 2010;464:1122–4. doi: 10.1038/4641122a. [DOI] [PubMed] [Google Scholar]
- 137.Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31(4 ):519–30. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, et al. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Nat Acad Sci. 2013;110(11 ):4315–20. doi: 10.1073/pnas.1300959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system A possible role in drug addiction. Biochem Pharmacol. 2008;76(5 ):569–81. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Nat Acad Sci. 2008;105(37 ):14187–91. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the US population NHANES 2003-2006. Environ Health Perspect. 2011;119(3 ):390–6. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011;128(5 ):873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. NeuroToxicology. 2011;32(2 ):261–7. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461-462:386–90. doi: 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barse AV, Chakrabarti T, Ghosh TK, Pal AK, Jadhao SB. Endocrine disruption and metabolic changes following exposure of Cyprinus carpio to diethyl phthalate. Pesticide Biochem Physiol. 2007;88(1 ):36–42. [Google Scholar]
- 146.Xu H, Shao X, Zhang Z, Zou Y, Chen Y, Han S, et al. Effects of Di-n-butyl Phthalate and Diethyl Phthalate on Acetylcholinesterase Activity and Neurotoxicity Related Gene Expression in Embryonic Zebrafish. Bull Environ Contam Toxicol. 2013;91(6 ):635–9. doi: 10.1007/s00128-013-1101-9. [DOI] [PubMed] [Google Scholar]
- 147.Sun W, Ban J-B, Zhang N, Zu Y-K, Sun W-X. Perinatal exposure to di-(2-ethylhexyl)-phthalate leads to cognitive dysfunction and phospho-tau level increase in aged rats. Environ Toxicol. 2014;29(5 ):596–603. doi: 10.1002/tox.21785. [DOI] [PubMed] [Google Scholar]
- 148.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, et al. In vitro biological activities of the antimicrobials triclocarban, its analogues, and triclosan in bioassay screens receptor-based bioassay screens. Environ Health Perspect. 2008;116(9 ):1203–0. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yueh M-F, Li T, Evans RM, Hammock B, Tukey RH. Triclocarban Mediates Induction of Xenobiotic Metabolism through Activation of the Constitutive Androstane Receptor and the Estrogen Receptor Alpha. PLoS ONE. 2012;7(6 ):e37705. doi: 10.1371/journal.pone.0037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ali EHA, Elgoly AHM. Combined prenatal and postnatal butyl paraben exposure produces autism-like symptoms in offspring Comparison with valproic acid autistic model. Pharmacol Biochem Behavior. 2013;111:102–10. doi: 10.1016/j.pbb.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 151.Fox M, Knapp LA, Andrews PW, Fincher CL. Hygiene and the world distribution of Alzheimer's Disease. Evolution Medicine and Public Health. 2013 August 11. 2013 doi: 10.1093/emph/eot015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Halden RU. On the Need and Speed of Regulating Triclosan and Triclocarban in the United States. Environ Sci Tech. 2014;48(7 ):3603–11. doi: 10.1021/es500495p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lokanatha V, Sailaja P, Rajendra W. In vitro kinetics of the rat brain succinate dehydrogenase inhibition by hexachlorophene. J Biochem Mol Toxicol. 1999;13(6 ):303–6. doi: 10.1002/(sici)1099-0461(1999)13:6<303::aid-jbt3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 154.Prasad GV, Indira K, Rajendra W. Inhibition of sheep brain acetylcholinesterase by hexachlorophene. Bull Environ Contam Toxicol. 1987;38(1 ):139–42. doi: 10.1007/BF01606571. [DOI] [PubMed] [Google Scholar]
- 155.Shuman RM, Leech RW, Alvord EC. Neurotoxicity of Hexachlorophene in the Human I. A Clinicopathologic Study of 248 Children. Pediatrics. 1974;54(6 ):689–95. [PubMed] [Google Scholar]
- 156.Shuman RM, Leech RW, Alvord EC. Neurotoxicity of hexachlorophene in humans: Ii. a clinicopathological study of 46 premature infants. Arch Neurol. 1975;32(5 ):320–5. doi: 10.1001/archneur.1975.00490470064009. [DOI] [PubMed] [Google Scholar]
- 157.Chen J, Ahn KC, Gee NA, Mohamed MI, Duleba AJ, Zhao L, et al. Triclocarban enhances testosterone action A new type of endocrine disruptor? Endocrinology. 2008;149(3 ):1173–9. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kennedy RC, Healy L, Fecteau K, Zhao L, Hu P, Gee NA, et al. Early Exposure to Triclocarban During Lactation Alters Survival Rate in the Female Rat Neonate. Endocrine Reviews (03 Meeting Abstracts): OR38-3 [serial on the Internet]. 2013;34 [Google Scholar]
- 159.Chung E, Genco MC, Megrelis L, Ruderman JV. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc Nat Acad Sci. 2011;108(43 ):17732–7. doi: 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11 ):1339–44. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cherednichenko G, Zhang R, Bannister RA, Timofeyev V, Li N, Fritsch EB et al. Triclosan impairs excitation–contraction coupling and Ca2+ dynamics in striated muscle. Proc Natl Acad Sci USA. 2012;109(35 ):14158–63. doi: 10.1073/pnas.1211314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL et al. Biomarkers of exposure to triclocarban in urine and serum. Toxicology. 2011;86(1-3 ):69–74. doi: 10.1016/j.tox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Calafat AM, Ye X, Wong LY, Reidy JAL. NL Urinary Concentrations of Triclosan in the US Population 2003–2004. Environ Health Perspect. 2008;116(3 ):303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pycke BFG, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU. Human Fetal Exposure to Triclosan and Triclocarban in an Urban Population from Brooklyn, New York. Environ Sci Tech. 2014;48(15 ):8831–8. doi: 10.1021/es501100w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30(2 ):301–12. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 166.Smith KW, Souter I, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. Urinary Paraben Concentrations and Ovarian Aging among Women from a Fertility Center. Environ Health Perspectives. 2013 doi: 10.1289/ehp.1205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kang S, Kim S, Park J, Kim H-J, Lee J, Choi G et al. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci Total Environ. 2013;461-462:214–21. doi: 10.1016/j.scitotenv.2013.04.097. [DOI] [PubMed] [Google Scholar]
- 168.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the US population NHANES 2005-2006. Environ Health Perspeec. 2010;118(5 ):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma W-L, Wang L, Guo Y, Liu L-Y, Qi H, Zhu N-Z, et al. Urinary Concentrations of Parabens in Chinese Young Adults Implications for Human Exposure. Arch Environ Contam Toxicol. 2013;65(3 ):611–8. doi: 10.1007/s00244-013-9924-2. [DOI] [PubMed] [Google Scholar]
- 170.Kawaguchi M, Irie K, Morohoshi K, Watanabe G, Taya K, Morita M, et al. Maternal isobutyl-paraben exposure alters anxiety and passive avoidance test performance in adult male rats. Neurosci Res. 2009;65(2 ):136–40. doi: 10.1016/j.neures.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 171.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64(17 ):2202–10. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Block ML, Calderón-Garcidueñas L. Air pollution mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9 ):506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moulton PV, Yang W. Air Pollution, Oxidative Stress, and Alzheimer's Disease. J Environm Pub Health. 2012;2012:9. doi: 10.1155/2012/472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim S, Knight E, Saunders E, Cuevas A, Popovech M, Chen L-C, et al. Rapid doubling of Alzheimer’s amyloid-ß40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000 Research. 2012;1(70 ) doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Calderon-Garciduenas L, Franco-Lira M, Mora-Tiscareno A, Medina-Cortina H, Torres-Jardon R, Kavanaugh M. Early Alzheimer's and Parkinson's Disease Pathology in Urban Children Friend versus Foe Responses-It Is Time to Face the Evidence. BioMed Res Interna. 2013;2013:16. doi: 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Calderón-Garcidueñas L, Kavanaugh M, Block M, D'Angiulli A, Delgado-Chávez R, Torres-Jardón R et al. Neuroinflammation, Hyperphosphorylated Tau, Diffuse Amyloid Plaques, and Down-Regulation of the Cellular Prion Protein in Air Pollution Exposed Children and Young Adults. J Alzheimers Dis. 2012;28(1 ):93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 177.Rivas-Arancibia S, Guevara-Guzmán R, López-Vidal Y, Rodríguez-Martínez E, Zanardo-Gomes M, Angoa-Pérez M, et al. Oxidative Stress Caused by Ozone Exposure Induces Loss of Brain Repair in the Hippocampus of Adult Rats. Toxicol Sci. 2010;113(1 ):187–97. doi: 10.1093/toxsci/kfp252. [DOI] [PubMed] [Google Scholar]
- 178.Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, Roque P. Neurotoxicants Are in the Air Convergence of Human, Animal, and In vitro Studies on the Effects of Air Pollution on the Brain. Bio Med Res Internat. 2014;2014:8. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Genc S, Zadeoglulari Z, Fuss SH, Genc K. The Adverse Effects of Air Pollution on the Nervous System. J Toxicol. 2012;2012(1-23 ):23–0. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kukull WA, Larson EB, Bowen JD, McCormick WC, Teri L, Pfanschmidt ML et al. Solvent Exposure as a Risk Factor for Alzheimer's Disease A Case-Control Study. American J Epidemiol. 1995;141(11 ):1059–71. doi: 10.1093/oxfordjournals.aje.a117370. [DOI] [PubMed] [Google Scholar]
- 181.Ritchie GD, Still KR, Alexander WK, Nordholm AF, Wilson CL, Rossi JIII, et al. A review of the neurotoxicity risk of selected hydrocarbon fuels. J Toxicol Environ Health Part B. 2001;4(3 ):223–312. doi: 10.1080/109374001301419728. [DOI] [PubMed] [Google Scholar]
- 182.Tisch U, Schlesinger I, Ionescu R, Nassar M, Axelrod N, Robertman D et al. Detection of Alzheimer’s and Parkinson’s disease from exhaled breath using nanomaterial-based sensors. Na-nomedicine. 2012;8(1 ):43–56. doi: 10.2217/nnm.12.105. [DOI] [PubMed] [Google Scholar]
- 183.Network SCA, Kelly M, Coughlin S. Procter & Gamble Eliminating Phthalates, Triclosan from Products Worldwide. The Campaign for Safe Cosmetics. 2013 [Google Scholar]
- 184.Koch W. Wal-Mart announces phase-out of hazardous chemicals. Available from http://www.usatoday.com/story/news/nation/2013/09/12/walmart-disclose-phase-out-toxic-chemicals-products-cosmetics/2805567/ 2013.
- 185.Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues J-F. Relation between Aluminum Concentrations in Drinking Water and Alzheimer's Disease An 8-year Follow-up Study. Am J Epidemiol. 2000;152(1 ):59–66. doi: 10.1093/aje/152.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues J-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer's Disease or Cognitive Decline Findings From 15-Year Follow-up of the PAQUID Cohort. Am J Epidemiol. 2009;169(4 ):489–96. doi: 10.1093/aje/kwn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gong G, O'Bryant SE. The Arsenic Exposure Hypothesis for Alzheimer Disease. Alzheimer Dis Assoc Disord. 2010;24(4 ):311–6 10. doi: 10.1097/WAD.0b013e3181d71bc7. [DOI] [PubMed] [Google Scholar]
- 188.Wang G, Hazra T, Mitra S, Lee H, Englander E. Mitochondrial DNA damage and a hypoxic response are induced by CoCl2 in rat neuronal PC12 cells. Nucleic Acids Res. 2000;28(10 ):2135–40. doi: 10.1093/nar/28.10.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, et al. Alzheimer's Disease (AD)-Like Pathology in Aged Monkeys after Infantile Exposure to Environmental Metal Lead (Pb): Evidence for a Developmental Origin and Environmental Link for AD. J Neurosci. 2008;28(1 ):3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Grashow R, Spiro A, Taylor KM, Newton K, Shrairman R, Landau A, et al. Cumulative lead exposure in community-dwelling adults and fine motor function Comparing standard and novel tasks in the VA Normative Aging Study. NeuroToxicology. 2013;35:154–61. doi: 10.1016/j.neuro.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. In Utero Exposure to Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) and Neurodevelopment Among Young Mexican American Children. Pediatrics. 2006;118(1 ):233–41. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- 192.Torres-Sánchez L, Schnaas L, Rothenberg SJ, Cebrián ME, Osorio-Valencia E, Hernández MC, et al. Prenatal p,p´-DDE Exposure and Neurodevelopment among Children 3.5–5 Years of Age. Environ Health Perspec. 2013;121(2 ) doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Landrigan PJ. Pesticides and Inner-city Children: Exposures, Risks, and Prevention: National Institute of Environmental Health Sciences. 1999 doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C et al. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environ Health Perspec. 2007;115(5 ) doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hernández YT, Rubio AEC, Barragán IR. Neurotoxic potential of trichlorfon to multiple sublethal doses in wistar rats. Acta Biol gica Colombiana. 2013;18(3 ):479–88. [Google Scholar]
- 196.Rao PS, Roberts GH, Pope CN, Ferguson PW. Comparative Inhibition of Rodent and Human Erythrocyte Acetylcholinesterase by Carbofuran and Carbaryl. Pest Biochem Physiol. 1994;8(2 ):79–84. [Google Scholar]
- 197.Kamboj S, Kumar V, Kamboj A, Sandhir R. Mitochondrial Oxidative Stress and Dysfunction in Rat Brain Induced by Carbofuran Exposure. Cell Mol Neurobiol. 2008;28(7 ):961–9. doi: 10.1007/s10571-008-9270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mishra D, Tiwari SK, Agarwal S, Sharma VP, Chaturvedi RK. Prenatal Carbofuran Exposure Inhibits Hippocampal Neurogenesis and Causes Learning and Memory Deficits in Offspring. Toxicol Sci. 2012;127(1 ):84–100. doi: 10.1093/toxsci/kfs004. [DOI] [PubMed] [Google Scholar]
- 199.Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Khan WA, Dechkovskaia AM et al. Imidacloprid Induces Neurobehavioral Deficits and Increases Expression of Glial Fibrillary Acidic Protein in the Motor Cortex and Hippocampus in Offspring Rats Following in Utero Exposure. J Toxicol Environ Health Part A. 2008;71(2 ):119–30. doi: 10.1080/15287390701613140. [DOI] [PubMed] [Google Scholar]
- 200.Negishi T, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Inhibition of staurosporine-induced neuronal cell death by bisphenol A and nonylphenol in primary cultured rat hippocampal and cortical neurons. Neurosc Lett. 2003;353(2 ):99–102. doi: 10.1016/j.neulet.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 201.Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C et al. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ Health Perspec. 2013;121(2 ) doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kakeyama M, Tohyama C. Developmental Neurotoxicity of Dioxin and Its Related Compounds. Industrial Health. 2003;41(3 ):215–30. doi: 10.2486/indhealth.41.215. [DOI] [PubMed] [Google Scholar]
- 203.Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol Teratology. 1989;11(1 ):13–9. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 204.WHO Bisphenol A (BPA) - Current state of knowledge and future actions by WHO and FAO. Geneva. 2009 [Google Scholar]
- 205.Exley C, Korchazhkina O, Job D, Strekopytov S, Polwart A, Crome P. Non-invasive therapy to reduce the body burden of aluminium in Alzheimer's disease. J Alzheimers Dis. 2006;10(1 ):17–24. doi: 10.3233/jad-2006-10103. [DOI] [PubMed] [Google Scholar]
- 206.Röllin HB, Theodorou P, Cantrell AC. Biological Indicators of Exposure to Total and Respirable Aluminium Dust fractions in a Primary Aluminium Smelter. Occup Environ Med. 1996;53(6 ):417–21. doi: 10.1136/oem.53.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.CDC ATSDR ToxGuides™ Atlanta Agency for Toxic Substances and Disease Registry. Available from http://www.atsdr.cdc.gov/toxguides/index.asp#bookmark01 . 2011.
- 208.Mahajan RK, Singh Walia TP, Kaur S. Stripping Voltammetric Determination Of Zinc, Cadmium, Lead And Copper In Blood Samples Of Children Aged Between 3 Months And 6 years. Online J Health All Sci. 2005;1(2)(2 ) [Google Scholar]
- 209.Bhagat SS, Sarkar PD, Suryakar AN, Padalkar RK, Ghone RA, Patil SM et al. Attenuation of Serum Ferritin and Iron Burden by Intake of Antioxidants in Beta Thalassemia Major. Indian J Physiol Pharmacol. 2013;57(2 ):189–94. [PubMed] [Google Scholar]
- 210.Gallagher CM, Chen JJ, Kovach JS. The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20–49 years. Environ Res. 2011;111(5 ):702–7. doi: 10.1016/j.envres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 211.Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS. Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environm Res. 1990;52(2 ):126–45. doi: 10.1016/s0013-9351(05)80248-8. [DOI] [PubMed] [Google Scholar]
- 212.Bader M, Zimmer H. Manganese [Biomonitoring Methods, 2006].. The MAK-Collection for Occupational Health and Safety:; Wiley-VCH Verlag GmbH & Co. KGaA; Germany. 2002. [Google Scholar]
- 213.Ngim CH, Foo SC, Boey KW, Jeyaratnam J. Chronic neurobehavioural effects of elemental mercury in dentists. Brit J Industrial Med. 1992;49(11 ):782–90. doi: 10.1136/oem.49.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Department-of-Health. Understanding Mercury Exposure Levels New York NYSDOH Bureau of Toxic Substance Assessment. 2013 Available from http://www.health.ny.gov/environmental/chemicals/hsees/mercury/mercury_exposure_levels.htm . 2013.
- 215.Schroeder HA, Frost DV, Balassa JJ. Essential trace metals in man Selenium. J Chronic Dis. 1970;23(4 ):227–43. doi: 10.1016/0021-9681(70)90003-2. [DOI] [PubMed] [Google Scholar]
- 216.Whanger PD. Metabolism of selenium in humans. J Trace Elements in Experim Med. 1998;11(2-3 ):227–40. [Google Scholar]
- 217.Bates MN, Buckland SJ, Garrett N, Ellis H, Needham LL, Patterson Jr DG et al. Persistent organochlorines in the serum of the non-occupationally exposed New Zealand population. Chemosphere. 2004;54(10 ):1431–43. doi: 10.1016/j.chemosphere.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 218.Nair A, Dureja P, Pillai MKK. Levels of aldrin and dieldrin in environmental samples from Delhi, India. Sci Total Environ. 1991;108(3 ):255–9. doi: 10.1016/0048-9697(91)90362-i. [DOI] [PubMed] [Google Scholar]
- 219.Aylward LL, Kirman CR, Schoeny R, Portier CJ, Hays SM. Evaluation of Biomonitoring Data from the CDC National Exposure Report in a Risk Assessment Context Perspectives across Chemicals. Environ Health Perspec. 2013;121:287–94. doi: 10.1289/ehp.1205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Loganathan BG, Lam PKS , tow WJ, Botwe BO. Contamination status of organochlorine pesticides in Ghana Global contaminant trends of persistant organic chemicals. Boca Raton Taylor & Francis Group LLC. 2012;p:393–440. [Google Scholar]
- 221.Tennakoon DASS, Karunarathna WDV, Udugampala USS. Carbofuran concentrations in blood, bile and tissues in fatal cases of homicide and suicide. Forensic Sci Intern. 2013;227(1-3 ):106–10. doi: 10.1016/j.forsciint.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 222.Baars AJ, Theelen RMC, Janssen TJCM, Hesse JM, van Apeldoorn ME, Meijerink MCM, et al. Re-evaluation of human-toxicological maximum permissible risk levels RIVM report no.711701025. National Institute of Public Health and the Environment; Bilthoven, The Netherlands. 2001. pp. 217–22. [Google Scholar]
- 223.Hussain M, Yoshida K, Atiemo M, Johnston D. Occupational exposure of grain farmers to carbofuran. Arch Environ Contam Toxicol. 1990;19(2 ):197–204. doi: 10.1007/BF01056087. [DOI] [PubMed] [Google Scholar]
- 224.USEPA. Paraquat and Diquat United States Environmental Protection Agency. 2006.
- 225.Arizona-Game-and-Fish-Department Rotenone Review Advisory Committee Final Report. Phoenix2012 Available from http://www.azgfd.gov/h_f/documents/ROTENONEFAQcommitteefinalreportsection201-6-12.pdf . 2012.
- 226.Dobozy VA. Fipronil World Health Organisation. 2001.
- 227.EFSA-PPR-Panel. EFSA Panel on Plant Protection Products and their Residues 2013 Scientific Opinion on the developmental neurotoxicity potential of acetamiprid and imidacloprid. EFSA J. 2013;11(12):3471–522. [Google Scholar]
- 228.USEPA. Pesticide Fact Sheet Acetamiprid. 2002.
- 229.Calafat AM, Ye X, Wong L, Reidy JA, Needham LL. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspec. 2008;116(1 ):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Geyer HJ, Schramm K, Darnerud PO, Aune MA, Feicht EA, Fried KW, et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Brominated organohalogen compounds: biotic levels, trends, effects. 2004;66 [Google Scholar]
- 231.Toms LL, Guerra P, Eljarrat E, Barceló D, Harden FA, Hobson P et al. Brominated flame retardants in the Australian population 1993–2009. Chemosphere. 2012;89(4 ):398–403. doi: 10.1016/j.chemosphere.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 232.Costa L, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants environmental contamination, human body burden and potential adverse health effects. Acta Bio-Medica Atenei Parmensis. 2008;79(3 ):172–83. [PubMed] [Google Scholar]
- 233.Sjödin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum Concentrations of Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyl (PBB) in the United States Population 2003–2004. Environ Sci Techn. 2008;42(4 ):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 234.Birnbaum LS, EA CH. Polybrominated diphenyl ethers a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114(11 ):1770–5. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.USEPA. Flame Retardant Alternatives For Hexabromocyclododecane (HBCD) 2013.
- 236.Morf L, Zurich RT, Daxbeck H, Vienna RS. Environmentally hazardous substances Selected polybrominated flame retardants PBDEs and TBBPA. Berne. 2003;Contract No:338. [Google Scholar]
- 237.Aylward LL, Hays SM. Temporal trends in human TCDD body burden Decreases over three decades and implications for exposure levels. J Exposure Analysis & Environ Epidemiol. 2002;12(5 ):319. doi: 10.1038/sj.jea.7500233. [DOI] [PubMed] [Google Scholar]
- 238.Guo YL, Ryan JJ, Lau BPY, Yu ML, Hsu CC. Blood Serum Levels of PCBs and PCDFs in Yucheng Women 14 Years After Exposure to a Toxic Rice Oil. Arch Environ Contam Toxicol. 1997;33(1 ):104–8. doi: 10.1007/s002449900230. [DOI] [PubMed] [Google Scholar]
- 239.WHO-Health-Canada. Health Risk Assessment of Bisphenol A from Food Packaging Applications; Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch; Canada. 2008. pp. 1–14. [Google Scholar]
- 240.Zhou Q, Miao M, Ran M, Ding L, Bai L, Wu T et al. Serum bisphenol-A concentration and sex hormone levels in men. Fertility and Sterility. 2013;100(2 ):478–82. doi: 10.1016/j.fertnstert.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 241.Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Per-spec. 2010;118(7 ):998–1003. doi: 10.1289/ehp.0901712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Aylward LL, Hays SM, Gagné M, Krishnan K. Derivation of Biomonitoring Equivalents for di-n-butyl phthalate (DBP), benzylbutyl phthalate (BzBP), and diethyl phthalate (DEP). Regulatory Toxicol Pharmacol. 2009;55(3 ):259–67. doi: 10.1016/j.yrtph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 243.Anderson WAC, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Additives & Contaminants. 2001;18(12 ):1068–74. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- 244.Genuis SJ, Birkholz D, Curtis L, Sandau C. Paraben Levels in an Urban Community of Western Canada. ISRN Toxicology. 2013;2013:8. doi: 10.1155/2013/507897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspec. 2006;114(12 ):1843–6. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.CDC-USDHHS. Fourth National Report on Human Exposure to Environmental Chemicals-National Health and Nutrition Examination Survey (NHANES) Atlant; (NHANES) Atlanta. 2013. [Google Scholar]
- 247.Cowan-Ellsberry CE, Robison SH. Refining Aggregate Exposure Example using Parabens. Reg Toxicol Pharmacol. 2009;55(3):321–9. doi: 10.1016/j.yrtph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 248.Krishnan K, Gagné M, Nong A, Aylward LL, Hays SM. Biomonitoring Equivalents for triclosan. Reg Toxicol Pharmacol. 2010;58(1 ):10–7. doi: 10.1016/j.yrtph.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 249.Allmyr M, Harden F, Toms L-ML, Mueller JF, McLachlan MS, Adolfsson-Erici M, et al. The influence of age and gender on triclosan concentrations in Australian human blood serum. Science of The Total Environment. 2008;393(1 ):162–7. doi: 10.1016/j.scitotenv.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 250.Lankester J, Patel C, Cullen MR, Ley C, Parsonnet J. Urinary Triclosan is Associated with Elevated Body Mass Index in NHANES. PLoS ONE. 2013;8(11 ):e80057. doi: 10.1371/journal.pone.0080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.NCBI Hexachlorophene-Pubchem. Available from http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3598# . 2014.
- 252.WHO Particulate matter. Copenhagen Denmark: WHO Regional Office for Europe. 2000.
- 253.Dockery DW, Cunningham J, Damokosh AI, Neas LM, Spengler JD, Koutrakis P , et al. Health effects of acid aerosols on North American children respiratory symptoms. Environ Health Per-spect. 1996;104:500–5. doi: 10.1289/ehp.96104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tietze D, Donnelly KC, McDonald T. Houston Air Toxics Biomarkers Of Exposure Study (HATBES). Houston. 2009 [Google Scholar]
- 255.Halden RU. Epistemology of Contaminants of Emerging Concern and Literature Meta-analysis. J. Hazardous Materials. 2015;282:2–9. doi: 10.1016/j.jhazmat.2014.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]