Abstract
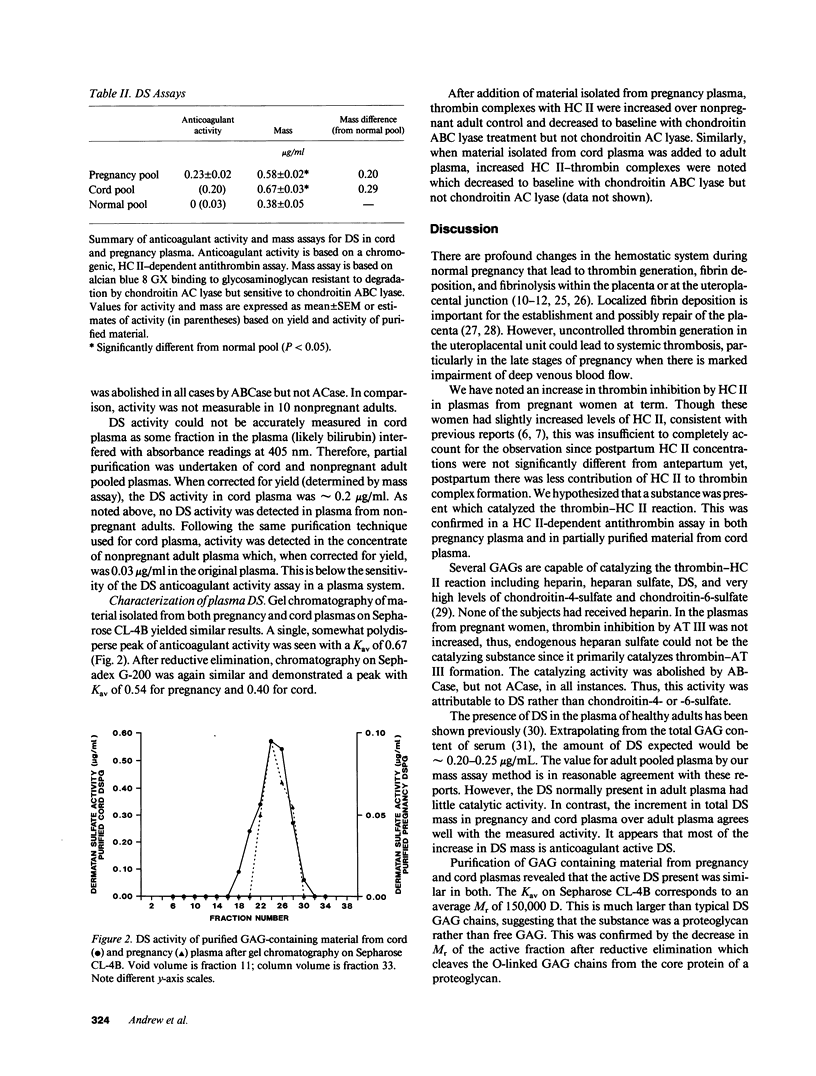
Investigation of the in vitro ability of plasma from pregnant women to inhibit exogenous thrombin (25 nM) demonstrated that heparin cofactor II inhibited more thrombin (3.0 +/- 0.7 nM, mean +/- SD) than plasma from women 3-5 d postpartum (1.9 +/- 0.5 nM) or plasma from nonpregnant adults (1.5 +/- 0.4 nM). Levels of heparin cofactor II were only slightly increased over normal in both pregnant and postpartum women and did not account for the observed increase in thrombin bound to heparin cofactor II. Assay of pregnancy plasma for dermatan sulfate anticoagulant activity demonstrated the presence of activity equivalent to 0.23 +/- 0.02 micrograms/ml of porcine mucosal dermatan sulfate. This activity could not be demonstrated in normal adult plasma or plasma from women on the contraceptive pill. The mass of dermatan sulfate in pregnancy and umbilical cord plasmas was increased over adult control plasma by 0.20 micrograms/ml (53%) and 0.29 micrograms/ml (76%), respectively. The glycosaminoglycan-containing fraction of plasma was isolated and an assay for anticoagulant dermatan sulfate confirmed its presence in both pregnancy and cord plasmas but minimal activity in adult plasma. Gel chromatography of isolated fractions from both pregnancy and cord plasmas revealed a polydisperse, active species with apparent Mr 150,000 D. Reductive elimination decreased the apparent Mr of the active species on gel chromatography to 31,000 D for cord and 21,000 D for pregnancy products. This confirmed the presence of an anticoagulant active dermatan sulfate proteoglycan circulating in the plasmas of pregnant women at term and fetuses at delivery.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaro L. A., Juergens J. L. Thrombophlebitis and pulmonary embolism as complications of pregnancy. Med Clin North Am. 1974 Jul;58(4):829–834. doi: 10.1016/s0025-7125(16)32126-5. [DOI] [PubMed] [Google Scholar]
- Andrew M., Ofosu F., Fernandez F., Jefferies A., Hirsh J., Mitchell L., Buchanan M. R. A low molecular weight heparin alters the fetal coagulation system in the pregnant sheep. Thromb Haemost. 1986 Jun 30;55(3):342–346. [PubMed] [Google Scholar]
- Andrew M., Paes B., Milner R., Johnston M., Mitchell L., Tollefsen D. M., Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987 Jul;70(1):165–172. [PubMed] [Google Scholar]
- Ballegeer V., Mombaerts P., Declerck P. J., Spitz B., Van Assche F. A., Collen D. Fibrinolytic response to venous occlusion and fibrin fragment D-dimer levels in normal and complicated pregnancy. Thromb Haemost. 1987 Dec 18;58(4):1030–1032. [PubMed] [Google Scholar]
- Bartold P. M., Page R. C. A microdetermination method for assaying glycosaminoglycans and proteoglycans. Anal Biochem. 1985 Nov 1;150(2):320–324. doi: 10.1016/0003-2697(85)90517-2. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Pierschbacher M. D., Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. J Biol Chem. 1984 Nov 25;259(22):13742–13750. [PubMed] [Google Scholar]
- Comp P. C., Thurnau G. R., Welsh J., Esmon C. T. Functional and immunologic protein S levels are decreased during pregnancy. Blood. 1986 Oct;68(4):881–885. [PubMed] [Google Scholar]
- Dalaker K., Prydz H. The coagulation factor VII in pregnancy. Br J Haematol. 1984 Feb;56(2):233–241. doi: 10.1111/j.1365-2141.1984.tb03951.x. [DOI] [PubMed] [Google Scholar]
- Dupouy D., Sié P., Dol F., Boneu B. A simple method to measure dermatan sulfate at sub-microgram concentrations in plasma. Thromb Haemost. 1988 Oct 31;60(2):236–239. [PubMed] [Google Scholar]
- Ezenagu L. C., Brandt J. T. Laboratory determination of heparin cofactor II. Arch Pathol Lab Med. 1986 Dec;110(12):1149–1151. [PubMed] [Google Scholar]
- Fernandez F. A., Buchanan M. R., Hirsh J., Fenton J. W., 2nd, Ofosu F. A. Catalysis of thrombin inhibition provides an index for estimating the antithrombotic potential of glycosaminoglycans in rabbits. Thromb Haemost. 1987 Jun 3;57(3):286–293. [PubMed] [Google Scholar]
- Fernandez F., Van Ryn J., Ofosu F. A., Hirsh J., Buchanan M. R. The haemorrhagic and antithrombotic effects of dermatan sulphate. Br J Haematol. 1986 Oct;64(2):309–317. doi: 10.1111/j.1365-2141.1986.tb04124.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi T., Heimark R. L., Hendrickson L. E., McMullen B. A., Fujikawa K. Human placental anticoagulant protein: isolation and characterization. Biochemistry. 1987 Aug 25;26(17):5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. R., Lund C. J., Creasman W. T. Antepartum pulmonary embolism. Am J Obstet Gynecol. 1972 Feb 15;112(4):476–486. doi: 10.1016/0002-9378(72)90308-0. [DOI] [PubMed] [Google Scholar]
- Hoppensteadt D., Racanelli A., Walenga J. M., Fareed J. Comparative antithrombotic and hemorrhagic effects of dermatan sulfate, heparan sulfate, and heparin. Semin Thromb Hemost. 1989 Oct;15(4):378–385. doi: 10.1055/s-2007-1002734. [DOI] [PubMed] [Google Scholar]
- Horne M. K., 3rd, Stein C. A., LaRocca R. V., Myers C. E. Circulating glycosaminoglycan anticoagulants associated with suramin treatment. Blood. 1988 Feb;71(2):273–279. [PubMed] [Google Scholar]
- Iwasaki A., Suda M., Nakao H., Nagoya T., Saino Y., Arai K., Mizoguchi T., Sato F., Yoshizaki H., Hirata M. Structure and expression of cDNA for an inhibitor of blood coagulation isolated from human placenta: a new lipocortin-like protein. J Biochem. 1987 Nov;102(5):1261–1273. doi: 10.1093/oxfordjournals.jbchem.a122165. [DOI] [PubMed] [Google Scholar]
- KERR M. G., SCOTT D. B., SAMUEL E. STUDIES OF THE INFERIOR VENA CAVA IN LATE PREGNANCY. Br Med J. 1964 Feb 29;1(5382):532–533. doi: 10.1136/bmj.1.5382.522. [DOI] [PubMed] [Google Scholar]
- Khoory M. S., Nesheim M. E., Bowie E. J., Mann K. G. Circulating heparan sulfate proteoglycan anticoagulant from a patient with a plasma cell disorder. J Clin Invest. 1980 Mar;65(3):666–674. doi: 10.1172/JCI109712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof B. R., Muller A. D., Hemker H. C. An inhibitor of clot formation in cord plasma. Thromb Res. 1979;15(3-4):389–403. doi: 10.1016/0049-3848(79)90146-4. [DOI] [PubMed] [Google Scholar]
- Kondo S., Noguchi M., Funakoshi T., Fujikawa K., Kisiel W. Inhibition of human factor VIIa-tissue factor activity by placental anticoagulant protein. Thromb Res. 1987 Nov 15;48(4):449–459. doi: 10.1016/0049-3848(87)90402-6. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Gudinchet A., Hauert J., Nicoloso G., Genton C., Welti H., Bachmann F. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987 Feb;69(2):460–466. [PubMed] [Google Scholar]
- Maggi A., Abbadini M., Pagella P. G., Borowska A., Pangrazzi J., Donati M. B. Antithrombotic properties of dermatan sulphate in a rat venous thrombosis model. Haemostasis. 1987;17(6):329–335. doi: 10.1159/000215765. [DOI] [PubMed] [Google Scholar]
- Mannucci P. M., Viganò S., Bottasso B., Candotti G., Bozzetti P., Rossi E., Pardi G. Protein C antigen during pregnancy, delivery and puerperium. Thromb Haemost. 1984 Oct 31;52(2):217–217. [PubMed] [Google Scholar]
- Marlar R. A., Montgomery R. R., Broekmans A. W. Diagnosis and treatment of homozygous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J Pediatr. 1989 Apr;114(4 Pt 1):528–534. doi: 10.1016/s0022-3476(89)80688-2. [DOI] [PubMed] [Google Scholar]
- Massouh M., Jatoi A., Gordon E. M., Ratnoff O. D. Heparin cofactor II activity in plasma during pregnancy and oral contraceptive use. J Lab Clin Med. 1989 Dec;114(6):697–699. [PubMed] [Google Scholar]
- McKillop C., Forbes C. D., Howie P. W., Prentice C. M. Soluble fibrinogen/fibrin complexes in pre-eclampsia. Lancet. 1976 Jan 10;1(7950):56–58. doi: 10.1016/s0140-6736(76)90150-1. [DOI] [PubMed] [Google Scholar]
- Merton R. E., Thomas D. P. Experimental studies on the relative efficacy of dermatan sulphate and heparin as antithrombotic agents. Thromb Haemost. 1987 Oct 28;58(3):839–842. [PubMed] [Google Scholar]
- Mitra S. K., Blau K. An improved determination of total glycosaminoglycans in body fluids by formation of complexes with quinacrine: changes in amniotic fluid total glycosaminoglycans during normal pregnancies and in pregnancies at risk for mucopolysaccharidoses. Clin Chim Acta. 1978 Oct 2;89(1):127–134. doi: 10.1016/0009-8981(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Murata K., Horiuchi Y. Molecular weight-dependent distribution of acidic glycosaminoglycans in human plasma. Clin Chim Acta. 1977 Feb 15;75(1):59–69. doi: 10.1016/0009-8981(77)90500-9. [DOI] [PubMed] [Google Scholar]
- Nelson D. M., Crouch E. C., Curran E. M., Farmer D. R. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am J Pathol. 1990 Apr;136(4):855–865. [PMC free article] [PubMed] [Google Scholar]
- Ofosu F. A., Sie P., Modi G. J., Fernandez F., Buchanan M. R., Blajchman M. A., Boneu B., Hirsh J. The inhibition of thrombin-dependent positive-feedback reactions is critical to the expression of the anticoagulant effect of heparin. Biochem J. 1987 Apr 15;243(2):579–588. doi: 10.1042/bj2430579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S., Abbate R., Rostagno C., Bruni V., Rosati D., Neri Serneri G. G. Increased thrombin generation in normal pregnancy. Acta Eur Fertil. 1988 Sep-Oct;19(5):263–267. [PubMed] [Google Scholar]
- Schmidt B., Mitchell L., Ofosu F. A., Andrew M. Alpha-2-macroglobulin is an important progressive inhibitor of thrombin in neonatal and infant plasma. Thromb Haemost. 1989 Dec 29;62(4):1074–1077. [PubMed] [Google Scholar]
- Stirling Y., Woolf L., North W. R., Seghatchian M. J., Meade T. W. Haemostasis in normal pregnancy. Thromb Haemost. 1984 Oct 31;52(2):176–182. [PubMed] [Google Scholar]
- Tefferi A., Owen B. A., Nichols W. L., Witzig T. E., Owen W. G. Isolation of a heparin-like anticoagulant from the plasma of a patient with metastatic bladder carcinoma. Blood. 1989 Jul;74(1):252–254. [PubMed] [Google Scholar]
- Tollefsen D. M., Pestka C. A., Monafo W. J. Activation of heparin cofactor II by dermatan sulfate. J Biol Chem. 1983 Jun 10;258(11):6713–6716. [PubMed] [Google Scholar]
- Uldbjerg N., Malmström A., Ekman G., Sheehan J., Ulmsten U., Wingerup L. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J. 1983 Feb 1;209(2):497–503. doi: 10.1042/bj2090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner C. P., Brandt J. Plasma antithrombin III activity in normal pregnancy. Obstet Gynecol. 1980 Nov;56(5):601–603. [PubMed] [Google Scholar]
- Weiner C. P., Kwaan H., Hauck W. W., Duboe F. J., Paul M., Wallemark C. B. Fibrin generation in normal pregnancy. Obstet Gynecol. 1984 Jul;64(1):46–48. [PubMed] [Google Scholar]
- Wright J. G., Cooper P., Astedt B., Lecander I., Wilde J. T., Preston F. E., Greaves M. Fibrinolysis during normal human pregnancy: complex inter-relationships between plasma levels of tissue plasminogen activator and inhibitors and the euglobulin clot lysis time. Br J Haematol. 1988 Jun;69(2):253–258. doi: 10.1111/j.1365-2141.1988.tb07630.x. [DOI] [PubMed] [Google Scholar]
- Yuen P. M., Yin J. A., Lao T. T. Fibrinopeptide A levels in maternal and newborn plasma. Eur J Obstet Gynecol Reprod Biol. 1989 Mar;30(3):239–244. doi: 10.1016/0028-2243(89)90007-5. [DOI] [PubMed] [Google Scholar]
- de Boer K., ten Cate J. W., Sturk A., Borm J. J., Treffers P. E. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989 Jan;160(1):95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- de Prost D., Katlama C., Pialoux G., Karsenty-Mathonnet F., Wolff M. Heparin-like anticoagulant associated with AIDS. Thromb Haemost. 1987 Apr 7;57(2):239–239. [PubMed] [Google Scholar]
- van der Heiden R., Hatton M. W., Moore S. Extraction and analysis of glycosaminoglycans from intima-media of single rabbit aortae: effect of balloon catheter de-endothelialization on the content and profile of glycosaminoglycans. Atherosclerosis. 1988 Oct;73(2-3):203–213. doi: 10.1016/0021-9150(88)90043-3. [DOI] [PubMed] [Google Scholar]