Abstract
Immunological responses to bacterial heat shock proteins have been implicated in the pathogenesis of arthritis in animals and humans. The predicted amino acid sequence of dnaJ, a heat shock protein from Escherichia coli, contains an 11-amino acid segment that is homologous to the third hypervariable region of the human histocompatibility antigen (HLA) DRB10401 (formerly known as HLA Dw4), the part of the molecule that carries susceptibility to rheumatoid arthritis. To test the biological significance of this finding, we expressed and purified recombinant dnaJ (rdnaJ), and determined its immunologic cross-reactivity with HLA DRB10401. A rabbit antipeptide antiserum raised against the sequence of the third hypervariable region of HLA DRB10401 specifically bound to 'dnaJ, thus confirming that a similar sequence is expressed on the bacterial protein. Of greater consequence, an antiserum to the 'dnaJ protein recognized not only a peptide from the third hypervariable region of HLA DRB10401, but also the intact HLA DRB10401 polypeptide. Furthermore, the antibody to 'dnaJ reacted with HLA DRB10401 homozygous B lymphoblasts, but not with HLA DRB11501, DRB10101, DRB10301, and DRB10701 (formerly known as HLA Dw2, DR 1, DR 3, and DR 7, in the same order) homozygous cells. These results demonstrate that exposure to a bacterial heat shock protein can elicit antibodies against the rheumatoid arthritis susceptibility sequence in the third hypervariable region of HLA DRB10401.
Full text
PDF

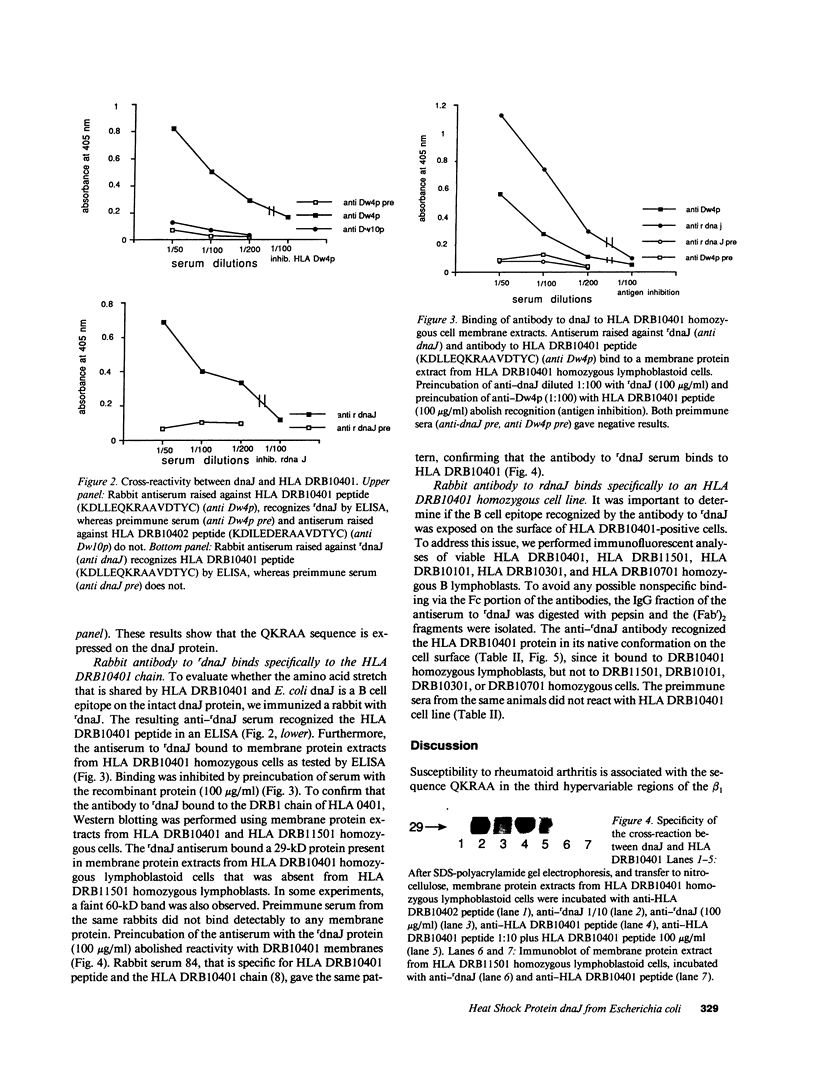
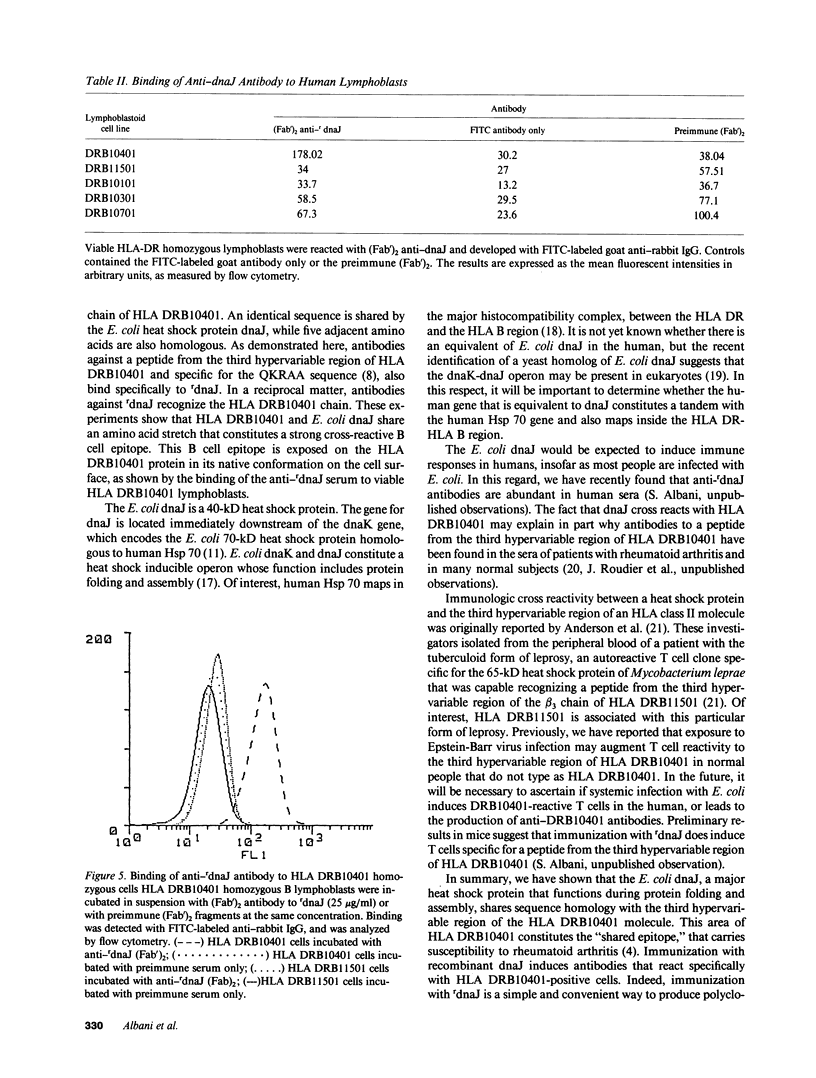

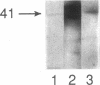
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., van Schooten W. C., Barry M. E., Janson A. A., Buchanan T. M., de Vries R. R. A Mycobacterium leprae-specific human T cell epitope cross-reactive with an HLA-DR2 peptide. Science. 1988 Oct 14;242(4876):259–261. doi: 10.1126/science.2459778. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Tilly K., Craig E., King J., Zylicz M., Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 dnaJ+ gene. A gene that encodes a heat shock protein. J Biol Chem. 1986 Feb 5;261(4):1782–1785. [PubMed] [Google Scholar]
- Blumberg H., Silver P. A. A homologue of the bacterial heat-shock gene DnaJ that alters protein sorting in yeast. Nature. 1991 Feb 14;349(6310):627–630. doi: 10.1038/349627a0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Chang W. C., Li C. H. Human beta-endorphin: synthesis and characterization of analogs iodinated and tritiated at tyrosine residues 1 and 27. Int J Pept Protein Res. 1980 Oct;16(4):311–320. doi: 10.1111/j.1399-3011.1980.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Hansen J. A., Nepom B. S. The molecular basis for HLA class II associations with rheumatoid arthritis. J Clin Immunol. 1987 Jan;7(1):1–7. doi: 10.1007/BF00915418. [DOI] [PubMed] [Google Scholar]
- Roudier J., Petersen J., Rhodes G. H., Luka J., Carson D. A. Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR beta-1 chain and the Epstein-Barr virus glycoprotein gp110. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5104–5108. doi: 10.1073/pnas.86.13.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier J., Rhodes G., Petersen J., Vaughan J. H., Carson D. A. The Epstein-Barr virus glycoprotein gp110, a molecular link between HLA DR4, HLA DR1, and rheumatoid arthritis. Scand J Immunol. 1988 Apr;27(4):367–371. doi: 10.1111/j.1365-3083.1988.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Dunham I., Trowsdale J., Campbell R. D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmbeck P. L., Yu D. T., Oldstone M. B. Autoantibodies to HLA B27 in the sera of HLA B27 patients with ankylosing spondylitis and Reiter's syndrome. Molecular mimicry with Klebsiella pneumoniae as potential mechanism of autoimmune disease. J Exp Med. 1987 Jul 1;166(1):173–181. doi: 10.1084/jem.166.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Takeuchi F., Kosuge E., Matsuta K., Nakano K., Tokunaga K., Juji T., Miyamoto T. Antibody to a specific HLA-DR beta 1 sequence in Japanese patients with rheumatoid arthritis. Arthritis Rheum. 1990 Dec;33(12):1867–1868. doi: 10.1002/art.1780331220. [DOI] [PubMed] [Google Scholar]
- Winfield J. B. Stress proteins, arthritis, and autoimmunity. Arthritis Rheum. 1989 Dec;32(12):1497–1504. doi: 10.1002/anr.1780321202. [DOI] [PubMed] [Google Scholar]
- Woodrow J. C., Nichol F. E., Zaphiropoulos G. DR antigens and rheumatoid arthritis: a study of two populations. Br Med J (Clin Res Ed) 1981 Nov 14;283(6302):1287–1288. doi: 10.1136/bmj.283.6302.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Ang D., Liberek K., Georgopoulos C. Initiation of lambda DNA replication with purified host- and bacteriophage-encoded proteins: the role of the dnaK, dnaJ and grpE heat shock proteins. EMBO J. 1989 May;8(5):1601–1608. doi: 10.1002/j.1460-2075.1989.tb03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]