Abstract
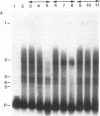
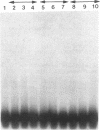
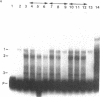
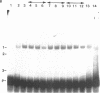
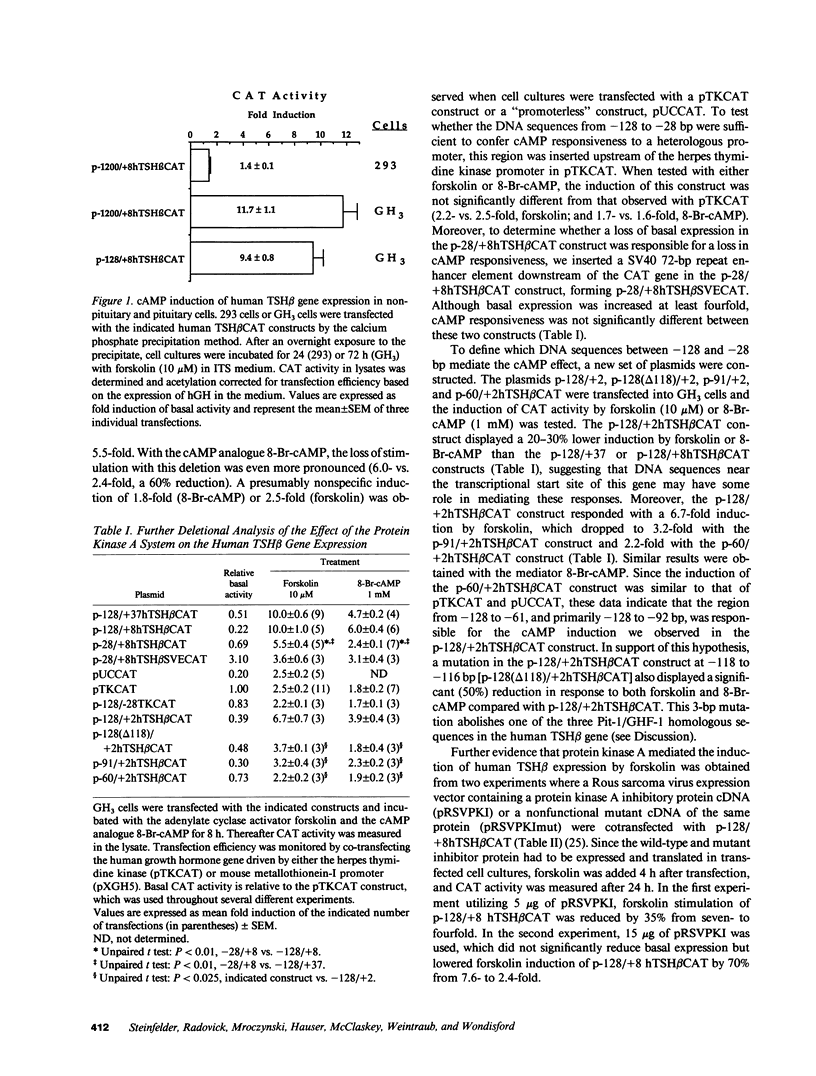
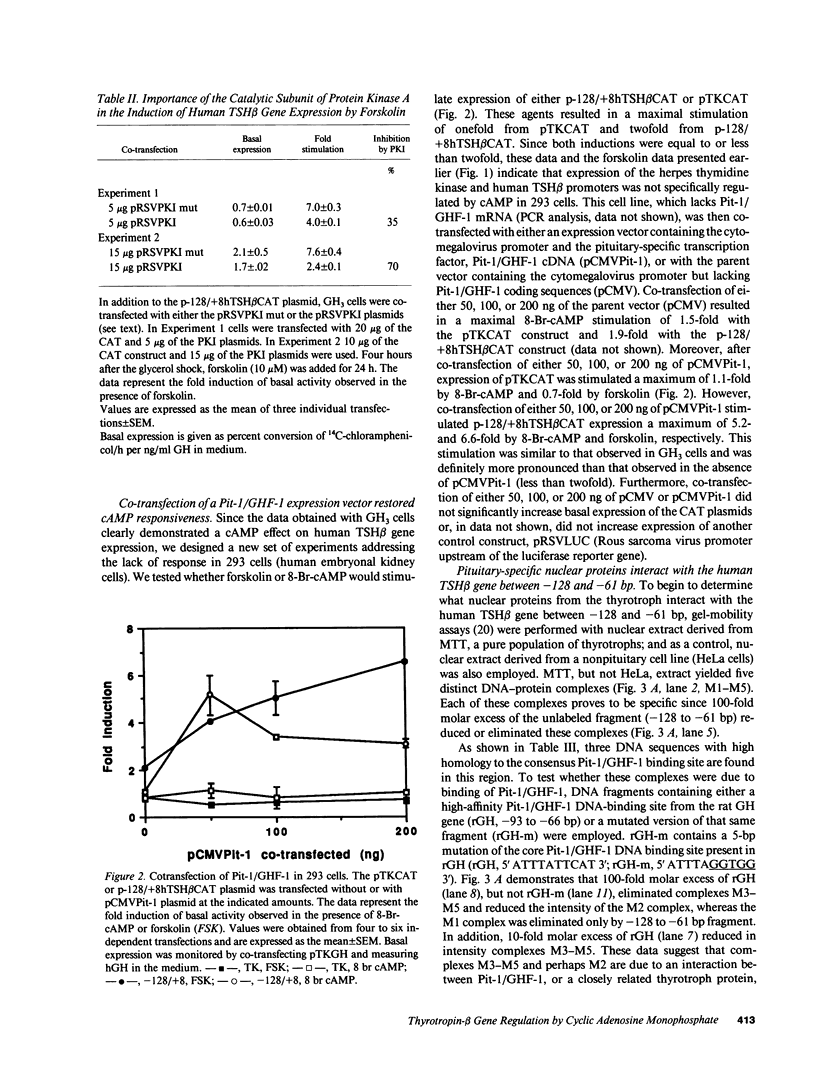
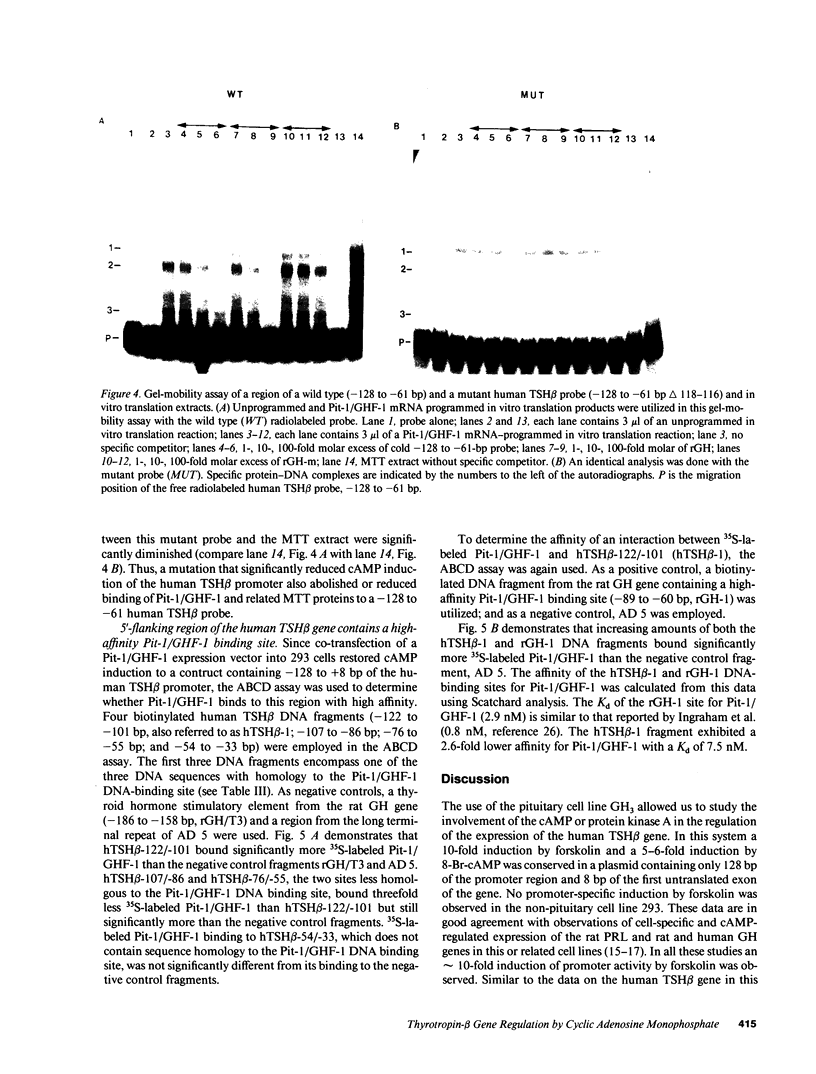
cAMP regulation of the human thyrotropin-beta (TSH beta) gene cAMP was studied in two heterologous cell lines, a human embryonal kidney cell line (293) and a rat pituitary cell line (GH3). In 293 cells, human TSH beta gene expression was not stimulated by the adenylate cyclase activator forskolin or the cAMP analogue 8-bromo-cAMP (8-Br-cAMP). On the other hand, these agents induced human TSH beta gene expression 4-12-fold in GH3 cells. Deletion analysis demonstrated that the regions from +3 to +8 bp and from -128 to -61 bp were both necessary for cAMP stimulation. The latter region contains three DNA sequences homologous to a pituitary-specific transcription factor, Pit-1/GHF-1, DNA-binding site. Gel-mobility assays demonstrated that a radiolabeled human TSH beta probe (-128 to -61 bp) formed five specific DNA-protein complexes with mouse thyrotropic tumor (MTT) nuclear extract and two specific complexes with in vitro translated Pit-1/GHF-1. Four of the five MTT complexes and both in vitro Pit-1/GHF-1 complexes were reduced or eliminated by excess of an unlabeled Pit-1/GHF-1 DNA-binding site from the rat growth hormone gene, but not a mutated version of the same DNA fragment, suggesting that Pit-1/GHF-1 or a closely related thyrotroph protein binds to these DNA sequences. In 293 cells, co-transfection of an expression vector containing the Pit-1/GHF-1 cDNA restored cAMP-responsiveness to the human TSH beta promoter (5.2- and 6.6-fold maximal stimulation by 8-Br-cAMP and forskolin, respectively) but not the herpes virus thymidine kinase promoter (1.2-fold maximal stimulation by either agent). Thus we conclude that the human TSH beta gene is positively regulated by cAMP in GH3 but not 293 cells. Since the human TSH beta gene contains at least one high-affinity binding site for Pit-1/GHF-1 in a region necessary for cAMP stimulation and cAMP stimulation could be restored to the human TSH beta promoter in a previously nonresponsive cell line by the addition of Pit-1/GHF-1, this suggests that Pit-1/GHF-1, or a closely related protein in the thyrotroph, may be a trans-acting factor for cAMP stimulation of the TSH beta gene.
Full text
PDF



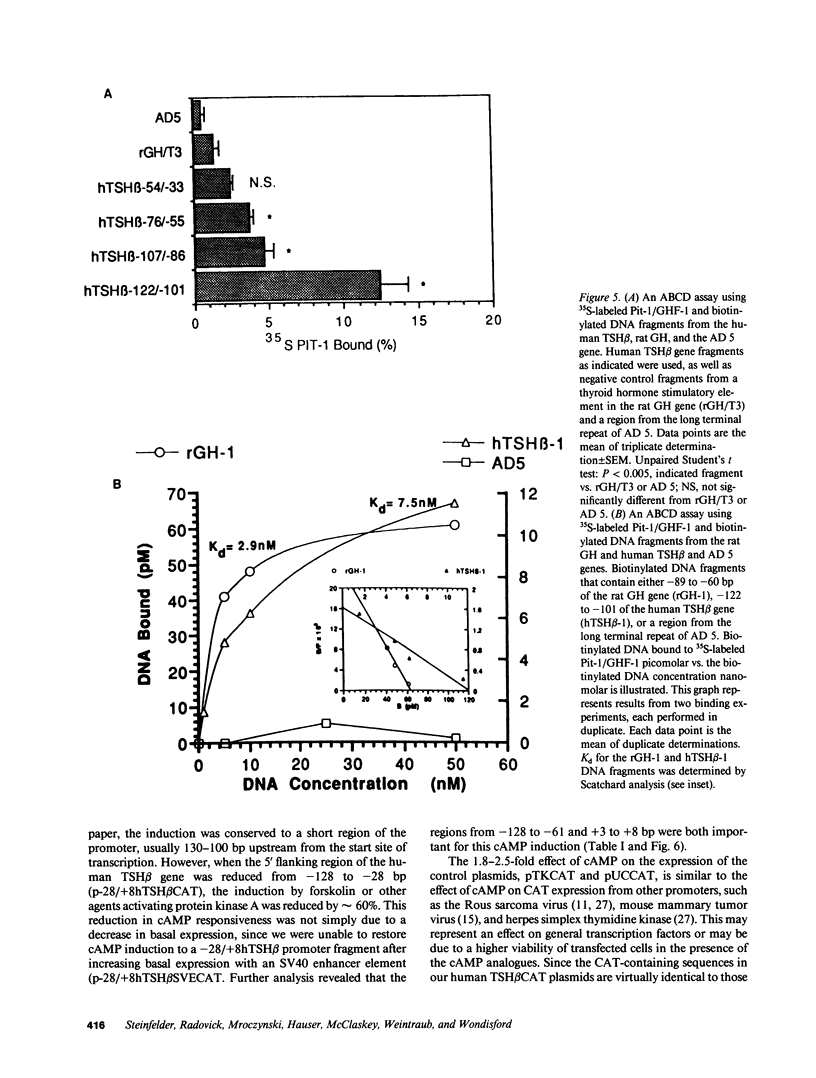



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander L. M., Williamson D. J., Wood W. M., Gordon D. F., Ridgway E. C., Gutierrez-Hartmann A. Activation of the murine thyrotropin beta-subunit promoter by GH4 rat pituitary cell-free extracts. Mol Endocrinol. 1990 Dec;4(12):1887–1896. doi: 10.1210/mend-4-12-1887. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Harney J. W., Moore D. D., Larsen P. R. Multihormonal regulation of the human, rat, and bovine growth hormone promoters: differential effects of 3',5'-cyclic adenosine monophosphate, thyroid hormone, and glucocorticoids. Mol Endocrinol. 1988 Sep;2(9):792–798. doi: 10.1210/mend-2-9-792. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Shupnik M. A., Burnside J., Chin W. W. Thyrotropin-releasing hormone stimulates the activity of the rat thyrotropin beta-subunit gene promoter transfected into pituitary cells. Mol Endocrinol. 1989 Apr;3(4):717–724. doi: 10.1210/mend-3-4-717. [DOI] [PubMed] [Google Scholar]
- Copp R. P., Samuels H. H. Identification of an adenosine 3',5'-monophosphate (cAMP)-responsive region in the rat growth hormone gene: evidence for independent and synergistic effects of cAMP and thyroid hormone on gene expression. Mol Endocrinol. 1989 May;3(5):790–796. doi: 10.1210/mend-3-5-790. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Kalla K., Simmons D. M., Swanson L. W., Rosenfeld M. G. Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev. 1989 Jul;3(7):959–972. doi: 10.1101/gad.3.7.959. [DOI] [PubMed] [Google Scholar]
- Dana S., Karin M. Induction of human growth hormone promoter activity by the adenosine 3',5'-monophosphate pathway involves a novel responsive element. Mol Endocrinol. 1989 May;3(5):815–821. doi: 10.1210/mend-3-5-815. [DOI] [PubMed] [Google Scholar]
- Day R. N., Walder J. A., Maurer R. A. A protein kinase inhibitor gene reduces both basal and multihormone-stimulated prolactin gene transcription. J Biol Chem. 1989 Jan 5;264(1):431–436. [PubMed] [Google Scholar]
- Deutsch P. J., Jameson J. L., Habener J. F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987 Sep 5;262(25):12169–12174. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenstermaker R. A., Milsted A., Virgin J. B., Miller W. L., Nilson J. H. The transcriptional response of the human chorionic gonadotropin beta-subunit gene to cAMP is cycloheximide sensitive and is mediated by cis-acting sequences different from that found in the alpha-subunit gene. Mol Endocrinol. 1989 Jul;3(7):1070–1076. doi: 10.1210/mend-3-7-1070. [DOI] [PubMed] [Google Scholar]
- Fuh V. L., Burrin J. M., Jameson J. L. Cyclic AMP (cAMP) effects on chorionic gonadotropin gene transcription and mRNA stability: labile proteins mediate basal expression whereas stable proteins mediate cAMP stimulation. Mol Endocrinol. 1989 Jul;3(7):1148–1156. doi: 10.1210/mend-3-7-1148. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Rebecchi M. J., Geras E., Arevalo C. O. Thyrotropin-releasing hormone (TRH) action in mouse thyrotropic tumor cells in culture: evidence against a role for adenosine 3',5'-monophosphate as a mediator of TRH-stimulated thyrotropin release. Endocrinology. 1980 Sep;107(3):665–670. doi: 10.1210/endo-107-3-665. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Flynn S. E., Voss J. W., Albert V. R., Kapiloff M. S., Wilson L., Rosenfeld M. G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990 Jun 15;61(6):1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M. Isolation and characterization of the human chorionic gonadotropin beta subunit (CG beta) gene cluster: regulation of transcriptionally active CG beta gene by cyclic AMP. Mol Cell Biol. 1988 Dec;8(12):5100–5107. doi: 10.1128/mcb.8.12.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff M. S., Farkash Y., Wegner M., Rosenfeld M. G. Variable effects of phosphorylation of Pit-1 dictated by the DNA response elements. Science. 1991 Aug 16;253(5021):786–789. doi: 10.1126/science.1652153. [DOI] [PubMed] [Google Scholar]
- Keech C. A., Gutierrez-Hartmann A. Analysis of rat prolactin promoter sequences that mediate pituitary-specific and 3',5'-cyclic adenosine monophosphate-regulated gene expression in vivo. Mol Endocrinol. 1989 May;3(5):832–839. doi: 10.1210/mend-3-5-832. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lumpkin M. D., Samson W. K., McCann S. M. Arginine vasopressin as a thyrotropin-releasing hormone. Science. 1987 Feb 27;235(4792):1070–1073. doi: 10.1126/science.2881350. [DOI] [PubMed] [Google Scholar]
- McCormick A., Brady H., Theill L. E., Karin M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature. 1990 Jun 28;345(6278):829–832. doi: 10.1038/345829a0. [DOI] [PubMed] [Google Scholar]
- Milsted A., Cox R. P., Nilson J. H. Cyclic AMP regulates transcription of the genes encoding human chorionic gonadotropin with different kinetics. DNA. 1987 Jun;6(3):213–219. doi: 10.1089/dna.1987.6.213. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H., Fawcett C. P., McCann S. M. Enzymatic dissociation and short-term culture of isolated rat anterior pituitary cells for studies on the control of hormone secretion. Endocrinology. 1976 Feb;98(2):278–288. doi: 10.1210/endo-98-2-278. [DOI] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Rosenzweig B. A., Showers M. O. Interactions of thyrotropin-releasing hormone, phorbol ester, and forskolin-sensitive regions of the rat thyrotropin-beta gene. Mol Endocrinol. 1990 Jun;4(6):829–836. doi: 10.1210/mend-4-6-829. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfelder H. J., Hauser P., Nakayama Y., Radovick S., McClaskey J. H., Taylor T., Weintraub B. D., Wondisford F. E. Thyrotropin-releasing hormone regulation of human TSHB expression: role of a pituitary-specific transcription factor (Pit-1/GHF-1) and potential interaction with a thyroid hormone-inhibitory element. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3130–3134. doi: 10.1073/pnas.88.8.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Fink J. S., Mandel G., Goodman R. H. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987 Jun 25;262(18):8743–8747. [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Wondisford F. E., Farr E. A., Steinfelder H. J., Radovick S., Gesundheit N., Gyves P. W., Taylor T., DeCherney G. S. Pre-translational and post-translational regulation of TSH synthesis in normal and neoplastic thyrotrophs. Horm Res. 1989;32(1-3):22–24. doi: 10.1159/000181235. [DOI] [PubMed] [Google Scholar]
- Wondisford F. E., Farr E. A., Radovick S., Steinfelder H. J., Moates J. M., McClaskey J. H., Weintraub B. D. Thyroid hormone inhibition of human thyrotropin beta-subunit gene expression is mediated by a cis-acting element located in the first exon. J Biol Chem. 1989 Sep 5;264(25):14601–14604. [PubMed] [Google Scholar]
- Wondisford F. E., Usala S. J., DeCherney G. S., Castren M., Radovick S., Gyves P. W., Trempe J. P., Kerfoot B. P., Nikodem V. M., Carter B. J. Cloning of the human thyrotropin beta-subunit gene and transient expression of biologically active human thyrotropin after gene transfection. Mol Endocrinol. 1988 Jan;2(1):32–39. doi: 10.1210/mend-2-1-32. [DOI] [PubMed] [Google Scholar]