Abstract
Innate immune responses are regulated in the intestine to prevent excessive inflammation. Here we show that a subset of mouse colonic macrophages constitutively produce the anti-inflammatory cytokine IL-10. In mice infected with Citrobacter rodentium, a model for enteropathogenic Escherichia coli infection in humans, these macrophages are required to prevent intestinal pathology. IL-23 is significantly increased in infected mice with a myeloid cell-specific deletion of IL-10, and the addition of IL-10 reduces IL-23 production by intestinal macrophages. Furthermore, blockade of IL-23 leads to reduced mortality in the context of macrophage IL-10 deficiency. Transcriptome and other analyses indicate that IL-10-expressing macrophages receive an autocrine IL-10 signal. Interestingly, only transfer of the IL-10 positive macrophages could rescue IL-10 deficient infected mice. Therefore, these data indicate a pivotal role for intestinal macrophages that constitutively produce IL-10, in controlling excessive innate immune activation and preventing tissue damage after an acute bacterial infection.
Introduction
Interleukin 10 (IL-10) is an immunoregulatory cytokine that limits mucosal immune responses and minimizes immunopathology. Indeed, mutations in the IL-10 receptor gene (Il10r) were found in some patients with early-onset colitis1, 2. Similarly, mice deficient for IL-10 (Il10−/−) or IL-10R (Il10rb−/−) developed spontaneous inflammation of the large intestine, a process that was T cell dependent and dominated by a pathogenic T helper type 1 and type 17 (Th1 and Th17) immune responses 3, 4, 5.
A number of cell types can produce IL-10, including lymphocytes, monocytes, macrophages, mast cells, keratinocytes, and intestinal epithelial cells. In several colitis models, the role of T cell-derived IL-10 has proven to be central 6, 7, 8, and in fact, mice with a conditional deletion of IL-10 in the CD4+ T cell subset develop spontaneous inflammation of the intestine, as do those with a deletion in regulatory T cells (Treg) mediated by a Foxp3 driven Cre 9, 10. However, we found IL-10 from macrophages plays an indispensable role in the maintenance of Foxp3 expression by regulatory T cells (Treg) and their function in mice with colitis 11. Despite this, mice with a conditional deletion of IL-10 or IL- 10R in myeloid cell subsets12, 13, or of IL-10 specifically in macrophages 14, did not spontaneously develop aggressive colitis, although they were susceptible to excessive inflammation following systemic LPS exposure12, 13. These data suggest that myeloid cell derived IL-10 might have a pivotal role in controlling mucosal immune responses after bacterial infection.
In the context of acute mucosal infections, the lack of IL-10 could be protective because of an enhanced inflammatory response with increased IL-12, TNF and other cytokines15, 16, 17, 18, 19, 20. However, the absence of IL-10 also could lead to excessive inflammation17, 21, 22, 23. Therefore, in the context of acute bacterial infection of the intestine, it remains to be determined which cell type(s) produce IL-10, if IL-10 is protective or harmful, and which pathways are activated or inhibited by IL-10 secretion. To explore these issues, we have analyzed mice with cell type-specific deletion of Il10 that were infected with Citrobacter rodentium (C. rodentium). This is a gram-negative bacterium that is considered a model for enteropathogenic and enterohemorrhagic E. coli infections in humans. C. rodentium causes attaching and effacing mechanism of epithelial infection, leading to intestinal inflammation and diarrhea. The bacteria normally are cleared in wild type mice due to the actions of innate and adaptive immunity and the intestinal inflammation ultimately resolves24, 25, 26. Here we show that a unique subset of macrophages in the colonic lamina propria that constitutively produces IL-10 plays a critical role in preventing excessive inflammation following acute bacterial infection by limiting innate immunity, and that a major pathway by which IL-10 acts is through controlling IL-23 production.
Results
Myeloid cell IL-10 is important for survival from Citrobacter rodentium
To assess the function of IL-10 in regulating mucosal immune responses after intestinal bacterial challenge, we used Il10−/−, Il10rb−/− and wild type (wt) recipient mice that were infected with a sublethal dose of C. rodentium by oral gavage. The onset and severity of colitis in Il10−/− mice is strongly influenced by the genetic background3, and we used mice on the C57BL6/J background that are more protected from inflammation under steady state conditions. Indeed, none of the groups developed spontaneous colitis in our mouse colony (Supplementary Fig. 1). However, we found that all Il10−/− and Il10rb−/− mice died 7 to 12 days after infection, whereas all wt mice survived (Fig. 1a). At day 6 after infection, Il10−/− and Il10rb−/− mice showed severe colonic inflammation, characterized by epithelial cell destruction, infiltration of mononuclear cells (Supplementary Fig. 1). These indicate that the role of IL-10 in modulating the initial mucosal immune response against C. rodentium is indispensable for survival and mucosal damage.
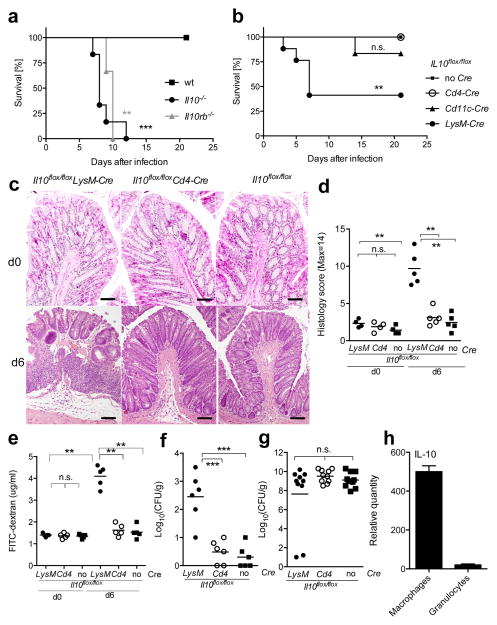
Figure 1. Myeloid cell-derived IL-10 is crucial for survival after Citrobacter rodentium infection.
(a) Il10−/− (black circle), Il10rb−/− (gray triangle) and C57BL/6J (black square) mice or (b) Il10flox/floxLysM-Cre (black circle), Il10flox/floxCd4-Cre (open circle), Il10flox/floxCd11c-Cre (black triangle) and Il10flox/flox (black square) mice were infected orally with C. rodentium and survival was monitored. (a) Two independent experiments with 6 mice per group and (b) 3 independent experiments with 12–17 mice per group are presented. (a,b) Log-Rank test, **p<.01, ***p<.001 (c,d) Inflammation of middle colon was assessed by histology before and 6 days after infection. Scale bars represent 100 μm. (e) Epithelial barrier permeability was determined before and 6 days after infection. (f,g) Titers of C. rodentium in (f) spleen and (g) feces were quantified 6 days of infection. (eg) Each symbol represents a measurement from a single mouse. Student’s t test, **p<.01, ***p<.001 (h) Analysis of Il10 mRNA in macrophages or granulocytes at day 3 after infection. Results were representative of two independent experiments.
To identify which cell type(s) produce the IL-10 that is essential for host protection, we analyzed mice with cell type-specific deletions of the Il10 gene. These included Il10flox/floxLysM-Cre, with deletion targeted primarily to CD11b+ myeloid cells, including macrophages, monocytes and neutrophils, Il10flox/floxCd4- Cre targeting mostly CD4+ T cells, and Il10flox/floxCd11c-Cre mice, which acts predominantly in dendritic cells (DC). We used Il10flox/floxCre− (Il10flox/flox) littermates for controls. We found that Il10flox/floxLysM-Cre mice started to die early after C. rodentium infection, with less than half surviving beyond day seven. In contrast, most of the Il10flox/floxCd11c-Cre and all of the Il10flox/floxCd4-Cre recipients survived to 21 days after infection (Fig. 1b). The average histology scores of each group were similar before C. rodentium infection. With the dose of bacteria we used, at day 6 we observed mild inflammation in Il10flox/floxCd4-Cre and Il10flox/flox recipients, manifested by epithelial cell hyperplasia and some cellular infiltration. However, Il10flox/floxLysM-Cre recipients, most of which survived to day 6, developed significantly more severe colonic inflammation, characterized by epithelial cell destruction, infiltration of mononuclear cells and submucosal edema in the large intestine (Fig. 1c and d). In parallel with this, we found increased colonic epithelial permeability and bacterial dissemination to the spleen after infection in Il10flox/floxLysM-Cre recipients (Fig. 1e and f). Despite this, bacterial numbers in the feces of Il10flox/floxLysM-Cre recipients at day 6 after bacterial challenge were similar compared to Il10flox/floxCd4-Cre and Il10flox/flox mice (Fig. 1g). These data suggest intestinal epithelial cell destruction by excessive mucosal immune responses after infection in Il10flox/floxLysM-Cre recipients probably contributed to bacterial dissemination and lethality.
Macrophages are the principal IL-10 producing cell
We assessed the effectiveness and specificity of the deletion of Il10 in the various cell populations by measuring the decrease in Il10 mRNA at steady state. Indeed, we detected a decrease of approximately 90% in Il10 mRNA transcripts in the CD11cintCD11b+ macrophage population from the colon of Il10flox/floxLysM- Cre mice compared to Il10flox/flox controls, with little decrease in CD11c+CD11b− cells or CD4+ T cells (Supplementary Fig. 2). Similarly, we detected a specific and nearly complete loss of Il10 transcripts in CD4+ T cells in Il10flox/floxCd4-Cre mice and a similar decrease in the CD11c+ CD11b− population in Il10flox/floxCd11c- Cre mice. There also was a 15–20% decrease in Il10 mRNA in the CD11cintCD11b+ population in Il10flox/floxCd11c-Cre mice (Supplementary Fig. 2). The intermediate level of CD11c expression by macrophages in the CD11cintCD11b+ population likely is responsible for the incomplete disruption of IL-10 expression and the preservation of protective function in the Il10flox/floxCd11c-Cre strain. Therefore, we conclude that the absence of a strong effect on survival and inflammation in the Il10flox/floxCd4-Cre and Il10flox/floxCd11c- Cre strains is not due to inefficient removal of the Il10 gene.
Because the LysM promoter drives Cre recombinase expression in macrophages, monocytes and neutrophils, we determined which of these cell types produced IL-10 after infection. Consistent with a recent report20, IL-10 mRNA was most highly expressed in CD11b+CD11cint macrophages, which also express F4/80 and MHC class II, but was undetectable in granulocytes (Fig. 1h) in the large intestine. Therefore, the reduced survival in Il10flox/floxLysM-Cre mice is very likely due to the loss of IL-10 production by macrophages.
A significant proportion of macrophages were IL-10 competent, GFP+ cells in the large intestine before infection. By flow cytometry, these cells were more frequent in the colon compared to the small intestine (Supplementary Fig. 3a). When analyzed by immunohistology, both the GFP+ (IL-10+) and GFP− (IL-10−) macrophages were scattered from the top of villi to the crypt in both small and large intestine (Supplementary Fig. 3b). This population is maintained through day 6 of infection (Supplementary Fig. 4), although the frequency tended to decrease.
To confirm that IL-10 producing macrophages regulate mucosal innate immune responses independently of adaptive immunity, we analyzed intestinal inflammation triggered by C. rodentium using Rag−/− and Il10−/−Rag−/− mice. Il10−/− Rag−/− mice had more severe mortality and severe colonic inflammation at day 6 after infection compared to Rag−/− mice (Supplementary Fig. 5). Together, these data suggest that macrophage derived IL-10 plays a nonredundant role in limiting the innate mucosal immune responses against intestinal infection with C. rodentium.
Macrophage IL-10 regulates myeloid cell recruitment to the colon
To determine if macrophage derived IL-10 regulates inflammatory cell recruitment to the large intestine after C. rodentium infection, we examined the kinetics of cell accumulation in the colon lamina propria in Il10flox/floxLysM-Cre, Il10flox/floxCd4-Cre and Il10flox/flox recipients during C. rodentium infection, and we characterized the infiltrating cell types by flow cytometric analysis. The total cell number in the colon lamina propria was comparable in all groups before C. rodentium injection, and CD11b+Ly6G−Ly6Chigh monocytes were undetectable (Fig. 2a). Six days after infection, we detected a significant increase in total cell numbers in Il10flox/floxLysM-Cre mice compared to Il10flox/floxCd4-Cre and Il10flox/flox recipients, and the population in the large intestine lamina propria consisted mainly of CD11b+Ly6G−Ly6Chigh monocytes and CD11b+Ly6GhighLy6Clow granulocytes (Fig. 2a). There was no increase in CD4+ T cells in any of these groups at this early time point (Fig. 2a).
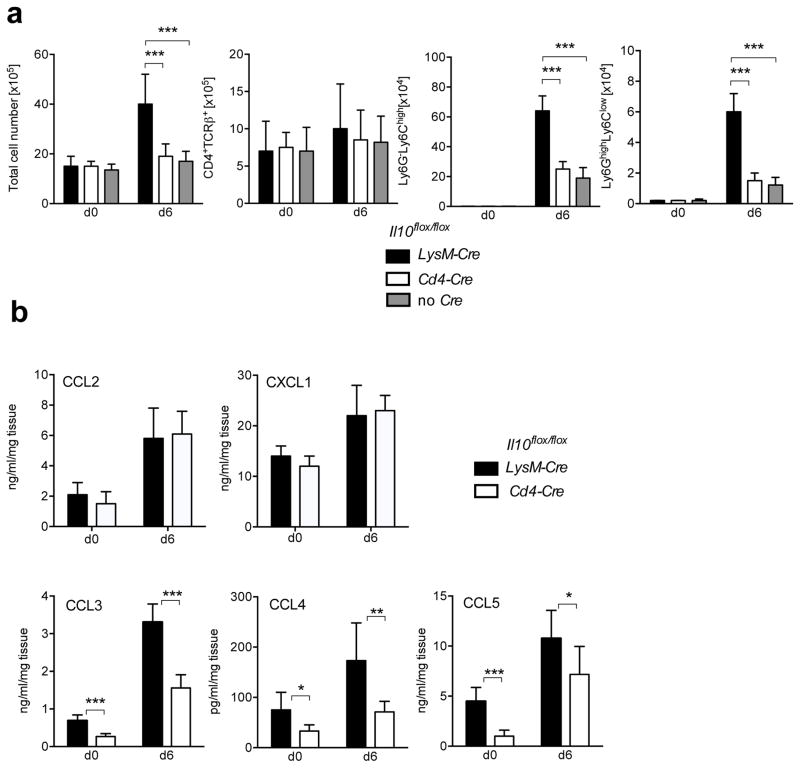
Figure 2. Macrophage IL-10 regulates myeloid cell recruitment to the colon.
Il10flox/floxLysM-Cre (black), Il10flox/floxCd4-Cre (white) and Il10flox/flox (grey) mice were analyzed before a day 6 after C. rodentium infection. (a) Lamina propria cells were isolated at the indicated times and total cell numbers were counted. Frequencies of CD4+ T cells (CD4+TCRβ+), monocytes (Ly6G−Ly6Chigh) and granulocytes (Ly6GhighLy6Clow) were measured by flow cytometry and cell numbers were calculated according to the respective total cell number. Cell numbers are presented as averages and SD from 9 mice per group from 3 independent experiments. (b) At the indicated time points, 3mm fragments of the middle colon of Il10flox/floxLysM-Cre (black), Il10flox/floxCd4-Cre (white) mice were cultured for 24h. Chemokine production was measured in the supernatants by Multi-Plex cytokine assay and normalized to the weight of the corresponding tissue fragments. Data are presented as averages with SD from two independent experiments with 6 mice in each group. Student’s t test, * p<.05, ** p<.01, *** p<.001
Because myeloid cell infiltrates were increased in infected Il10flox/floxLysM- Cre mice, we tested if chemokines that would attract these cells were also increased. Because Il10flox/floxCd4-Cre and Il10flox/flox recipients were similar in survival and degree of inflammation, we compared Il10flox/floxLysM-Cre mice to Il10flox/floxCd4-Cre mice. Although CCL2 and CXCL1, known to recruit monocytes and granulocytes, respectively, were similar between Il10flox/floxLysM-Cre mice and Il10flox/floxCd4-Cre mice (Fig. 2b), other chemokines that recruit monocytes and neutrophils, including CCL3, CCL4 and CCL5, were significantly increased in colon fragment culture supernatants from Il10flox/floxLysM-Cre mice. This was true even before C. rodentium infection (Fig. 2b), although this increased level before infection apparently was not sufficiently high to recruit monocytes and granulocytes and cause detectable inflammation. These data suggest that macrophage-derived IL-10 regulates the chemokine dependent recruitment of monocytes and granulocytes to the large intestine during intestinal bacterial infection.
Increased pro-inflammatory cytokines in Il10flox/floxLysM-Cre mice
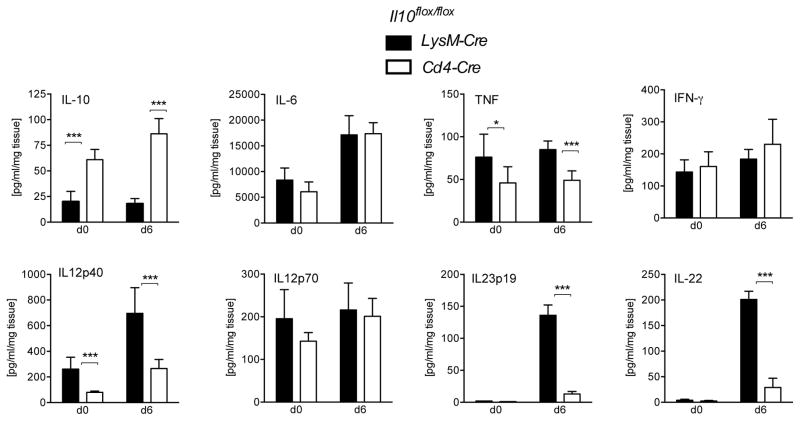
We examined if the increased colitis in C. rodentium infected Il10flox/floxLysM-Cre mice was associated with excessive production of pro- inflammatory cytokines using a Bio-Plex multi-cytokine assay. As illustrated (Fig. 3), IL-10 production was reduced before infection and early after infection in Il10flox/floxLysM-Cre compared to Il10flox/floxCd4-Cre mice, suggesting that the majority of IL-10 in the colon under these conditions is produced by macrophages. Conversely, the production of TNF and IL-12p40 was increased in Il10flox/floxLysM-Cre recipients in colon fragment cultures before, and more strongly during infection, while IL-6 and IFN-γ levels were comparable. We used a different method, ELISA, to explore expression of IL-12 family cytokines further. We detected a striking increase in IL-23p19 by day 6 after infection in Il10flox/floxLysM-Cre mice. However neither IL-23 nor IL-12p70 were significantly increased in Il10flox/floxLysM-Cre before infection, suggesting that IL-12p40 monomers or dimers, known to be produced in other contexts 27, 28, likely were produced in the intestine under lack of macrophage derived IL-10, although the function of IL-12p40 homodimers is uncertain.
Figure 3. Increased pro-inflammatory cytokines in the large intestine in Il10flox/floxLysM-Cre mice.
Il10flox/floxLysM-Cre (black) and Il10flox/floxCd4-Cre (white) mice were analyzed at day 0 or day 6 after C. rodentium infection. At the indicated time points, 3mm fragments of the middle colon were cultured for 24h. Cytokine production was measured in the supernatants by Multi-Plex or ELISA and normalized to the weight of the corresponding tissue fragments. Averages and SD from two independent experiments with 6 mice in each group are presented. Student’s t test, * p<.05, *** p<.001
It has been reported that IL-23 induces IL-22 production from innate lymphoid cells29, 30. Indeed, consistent with the significant increase in IL-23 by day six after infection, in colonic fragment cultures from infected Il10flox/floxLysM- Cre mice, we detected a profound increase in IL-22 production as well (Fig. 3).
Macrophage IL-10 influences mortality through regulation of IL-23p19
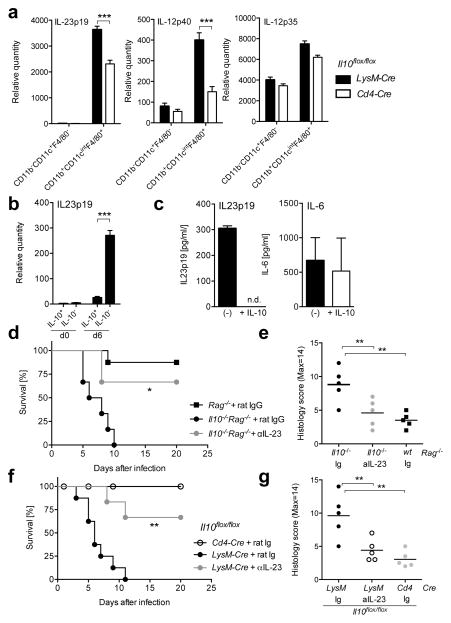
We assessed if IL-10 acts on the IL-23p19 producing cells activated by C. rodentium. It has been reported IL-23 is produced by macrophages and DCs29, 31, 32. To determine the dominant source of IL-23 producing cells, we sorted CD11b+CD11cintF4/80+ colonic macrophages and CD11b−CD11c+F4/80− DCs from two strains of IL-10 deficient mice at day 6 after infection, and compared the Il23p19, Il12p40 and Il12p35 mRNA transcripts. Macrophages tended to have higher amounts of each of these transcripts, but importantly, mice with a LysM Cre-mediated deletion of IL-10 had increased amounts of Il23p19 and Il12p40 mRNA, while Il12p35 mRNA transcripts were similar to mice with a CD4 Cre- mediated deletion (Fig. 4a). To determine if IL-10 by macrophages regulates IL-23 production, we sorted GFP+ (IL-10+) and GFP− (IL-10−) macrophages from Il10gfp mice before and after C. rodentium infection. We detected significantly increased amounts of Il23p19 mRNA in GFP− macrophages compared to those in GFP+ cells after infection, suggesting an autocrine role for IL-10 in the reporter positive cells. There were very few Il23p19 mRNA transcripts in either type of macrophages before infection, but even in the GFP+ macrophages there was a small increase in Il23p19 mRNA following exposure to C. rodentium (Fig. 4b). We also sorted macrophages from the large intestine of mice with a germline Il10 deficiency at day three after infection, cultured them with or without the addition of recombinant IL-10, and analyzed the supernatants by ELISA. Exogenous IL-10 completely abrogated IL-23p19 production by colonic macrophages from infected mice; but had little effect on IL-6 production (Fig. 4c). IL-12p70 was undetectable at this time, with or without the addition of IL-10 (data not shown). These data suggest that IL-10 might inhibit IL-23 production in vivo following infection, in part by acting directly on the IL-23 producing macrophages.
Figure 4. Macrophage-derived IL-10 influences mortality through regulation of IL-23p19.
(a) Analysis of gene expression by RT-PCR in sorted CD11b−CD11c+F4/80− DC or CD11b+CD11c+F4/80+ macrophages from large intestines in Il10flox/floxLysM- Cre or Il10flox/floxCd4-Cre mice at day 6 after C. rodentium infection. Averages and SD from two independent experiments with 6 mice in each group are shown. (b) Analysis of Il23p19 mRNA transcripts by RT-PCR in large intestine lamina propria macrophages, including GFP+(IL-10+) and GFP−(IL-10−), sorted from five to eight Il10gfp mice before and after 6 days after C. rodentium infection. These data are from 2 independent experiments. (c) Large intestine lamina propria macrophages were sorted from five to eight infected Il10−/−Rag−/− mice and cultured for 24h in the absence (black bars) or presence (white bars) of 100ng/ml recombinant mouse IL-10. Supernatants were analyzed for IL23p19 and IL-6 by ELISA. These data are from 2–3 independent experiments. (d,e) Il10−/−Rag−/− and Rag−/− mice or (f,g) Il10flox/floxLysM-Cre and Il10flox/floxCd4-Cre mice were infected with C. rodentium. (d–g) At the time of infection, 100μg anti-IL-23 or rat IgG were injected per mouse intravenously. (d, f) Survival curve and (e,g) histology score from middle colon at day 6 after infection are shown. These are from 2 independent experiments with 3–4 mice per group. Student’s t test, * p<.05, ** p<.01, *** p<.001.
To elucidate if increased IL-23 production in the absence of IL-10 is involved in intestinal damage and mortality, independent of adaptive immunity, we analyzed C. rodentium infected Rag−/− and Il10−/−Rag−/− recipients that were treated with IL-23p19 blocking or control antibodies. Il10−/−Rag−/− recipients treated with control antibodies died between 5 and 10 days after infection, however, a neutralizing IL-23p19 antibody treatment rescued the survival of the infected mice (Fig. 4d). Furthermore, IL-23p19 antibody treatment prevented tissue damage at day 6 after infection compared to control antibodies (Fig. 4e). These data show that in the absence of IL-10 the innate immune response is unchecked, and excessive IL-23 contributes to early morbidity and death.
To verify that blocking IL-23 also can reduce mortality in fully immune competent mice lacking only macrophage-derived IL-10, we analyzed C. rodentium infected Il10flox/floxLysM-Cre mice, and as a control, Il10flox/floxCd4-Cre mice. Il10flox/floxLysM-Cre mice treated with the isotype control antibody began to die at day 3 after infection, and none survived beyond day 11. However IL-23p19 antibody treatment had a significant effect on promoting the survival and preventing the epithelial cell damage of the infected Il10flox/floxLysM-Cre mice (Fig. 4f and g). Altogether, these data indicate macrophage derived IL-10 plays a dominant role in controlling the amount of IL-23 production by macrophages in the large intestine during the early phases of C. rodentium infection, and IL-23 is responsible, at least in part, for morbidity and mortality and is a marker for the excessive response by innate immune cells.
Gene signature of IL-10 competent large intestine macrophages
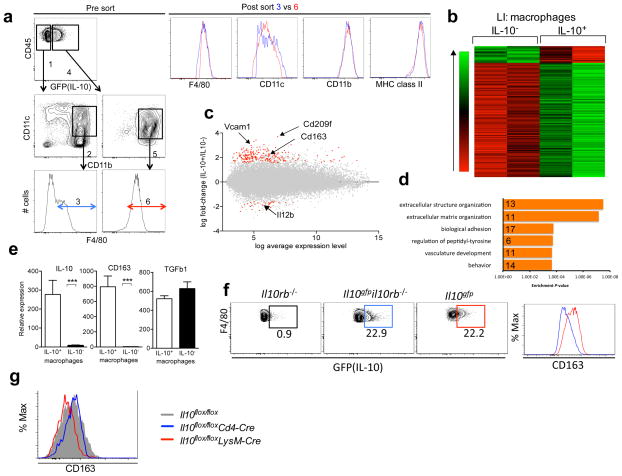
Macrophages are heterogeneous, and therefore we determined which subset was responsible for preventing excessive intestinal damage after C. rodentium infection. We reported previously that IL-10 competent GFP+ cells in the large intestine from Il10gfp reporter mice were mainly CD11b+, CD11cint and F4/80+ 11. Therefore we first gated GFP+ or GFP− cells, then sorted IL-10 competent, GFP+ CD11b+CD11cintF4/80+ macrophages (IL-10+) or their GFP− counterparts (IL-10−) from the large intestine of Il10gfp reporter mice, and performed microarray analyses to obtain insight into their degree of relatedness (Fig. 5a). F4/80, CD11b and MHC class II expression levels in post sorted GFP+ (IL-10+) and GFP− (IL-10−) populations were similar. While CD11c expression in the GFP− population had a broad distribution, we sorted those cells that had an amount of CD11c comparable to the GFP+ population. Heatmap and MA plots showed that these two populations were closely related, with 242 mRNA transcripts that were differentially expressed (Fig. 5b and c). 211 genes were upregulated in the GFP+ cells, including the cell surface markers, Vcam1, Cd209f and Cd163. In addition, 31 genes, including Il12b, were down regulated (Fig. 5c).
Figure 5. Comparison of IL-10-producing and non-producing large intestinal macrophages.
(a) Gating strategy for the microarray analysis of GFP+(IL10+) and GFP−(IL-10−) colonic lamina propria macrophages. Cells that were CD45+, MHC class II+, CD11b+, CD11cint and F4/80+ were analyzed. As depicted, the GFP− cells (gate #1) used to provide mRNA were the subset falling in gates #2 and #3, CD11b+, CD11cint and which were F4/80+ (gate #3). The corresponding GFP+ cells (gate #4) were less heterogeneous as they were almost entirely, CD11b+CD11cint (gate #5) and F4/80+ (gate #6). After the sort, F4/80, CD11c, CD11b and MHC class II expressions of cell in gate 3 (blue) and gate 6 (red) were examined by flow cytometry. (b) Heatmap of differentially expressed probe sets (n=242) between IL-10+ and IL-10− macrophages from the large intestine lamina propria in Il10gfp reporter mice, ordered by average difference in intensity. (c) MA plot of all probe sets on the array. Red points significantly above zero represent higher expression in IL-10+ macrophages compared to IL-10− macrophages (n=211), while points significantly below zero represent the inverse relationship (n=31). Gray points indicate probe sets that did not exhibit a significant difference in expression levels, according to criteria described in Methods. (d) Enriched GO biological processes among upregulated genes, as determined by DAVID (e) IL-10+ and IL-10− macrophages (CD11b+CD11cintF4/80+) were sorted from large intestine lamina propria of Il10gfp reporter mice and analyzed for expression of Il10, Cd163 and Tgfβ1 by real time PCR. Data are presented relative to L32 expression and are presented as average and SD of two independent experiments. (f) (Left) GFP (IL-10) expression by colonic macrophages in Il10rb−/−, Il10gfpil10rb−/− and Il10gfp mice. Gated on CD45+ MHC classII+ CD11b+ CD11cint F4/80+ cells. (Right) CD163 expression by IL-10 positive macrophages in Il10gfp mice (red) and in Il10gfpil10rb−/− mice (blue). (g) CD163 expression in large intestinal macrophages (CD11b+CD11cintF4/80+ cells) from Il10flox/floxLysM-Cre (red), Il10flox/floxCd4-Cre (blue) and Il10flox/flox (gray) mice. Data are representative of one of two independent experiments.
An annotation search in DAVID yielded unexpectedly enriched functional categories among upregulated genes in GFP+ macrophages (P <0.05), including: extracellular structure organization, extracellular matrix organization, biological adhesion, regulation of peptidyl-tyrosine, vasculature development and behavior. However, no pathways were identified for the downregulated genes, likely reflecting the small number of genes with decreased expression (Fig. 5d and Supplementary Fig. 6).
We validated increased expression of Il10 and Cd163 in GFP+ colonic macrophages by real-time RT-PCR. In contrast, both macrophage populations produced Tgfb1 mRNA (Fig. 5e). We also confirmed increased expression of CD163 in GFP+ macrophages by flow cytometry (Fig. 5f).
It has been reported CD163 is an IL-10 target gene in vitro33. Therefore we compared IL-10 receptor expression in IL-10+ and IL-10− colonic macrophages. However there was no difference in receptor expression. To determine if IL-10 provides an autocrine signal in macrophages in vivo, we crossed Il10gfp mice with Il10rb−/− mice. Interestingly, we found a similar frequency of GFP+ colonic macrophages in mice that had not received any IL-10R signals in vivo (Fig. 5f). However, intestinal macrophages from Il10rb−/− mice showed reduced CD163 cell surface expression (Fig. 5f). To determine if an autocrine IL-10 signal could be responsible for the increased expression of CD163 by intestinal macrophages, we analyzed cells from Il10flox/floxLysM-Cre and control mice. Intestinal macrophages expressed increased CD163 in Il10flox/floxCd4-Cre and Il10flox/flox mice compared to Il10flox/floxLysM-Cre (Fig. 5g). These data suggest that autocrine IL-10 may play a role in the induction of the phenotype of the IL-10 producing colonic macrophage subset, but IL-10 signals, either autocrine or paracrine, are not required for the induction of IL-10 competence in these cells.
IL-10 competent macrophages are protective
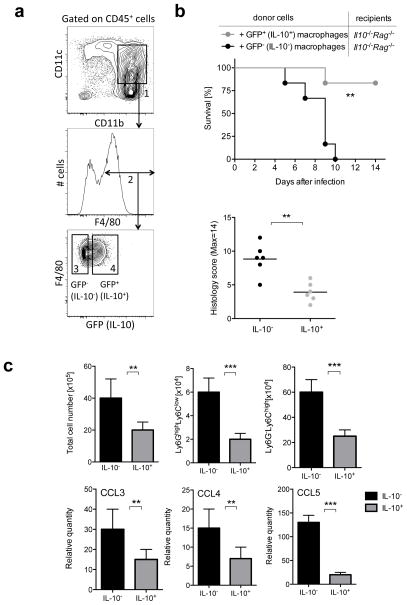
To determine if the reporter positive subset of large intestinal macrophages is required for preventing excessive inflammation after C. rodentium infection, we transferred sorted, reporter expressing GFP+ (IL-10+) or GFP− (IL-10−) colonic macrophages from Il10gfp mice into C. rodentium infected Il10−/−Rag−/− recipients (Fig. 6a). When GFP+ (IL-10+) macrophages were transferred, we detected a dramatic increase in survival and protection of tissue damage in the intestine compared to recipients of GFP− cells (Fig. 6b). Indeed we detected significant reduction of inflammatory cell recruitment, including monocytes and granulocytes, in the large intestine of recipients of GFP+ colonic macrophages after C. rodentium infection (Fig. 6c). Furthermore, chemokines potentially involved in the recruitment of monocytes and neutrophils to the large intestine, including CCL3, CCL4 and CCL5, were significantly decreased in the recipients of GFP+ cells (Fig. 6c). These data show that IL-10 from macrophages in the large intestine is indispensable for limiting excessive inflammation by regulating the inflammatory cell recruitment during the innate response.
Figure 6. IL-10 competent macrophages can rescue survival after infection.
Il10−/− Rag−/− mice were infected with C. rodentium. On day 1 after infection, macrophages (CD11b+CD11cintF4/80+ cells) were sorted from large intestine of Il10gfp reporter mice and 5×105 cells per mouse of GFP+ (IL-10+) or GFP− (IL10−) populations were injected intravenously. (a) Gating strategy for obtaining GFP+ (IL-10+) and GFP− (IL-10−) colonic macrophages for adoptive cell transfer. CD45+ colonic lamina propria cells were selected on the basis of CD11b+, CD11cint expression (gate #1) and F4/80 expression (gate #2) and further separated into GFP+(IL-10+) (gate #4) or GFP− (IL-10−)(gate #3) populations. (b) Survival and Histology score at day 6 after infection were monitored and is presented as a summary of two independent experiments with 3–5 mice per group. (c) (Top) Total cell numbers were counted from large intestine at day 6 after infection. Frequencies of monocytes (Ly6G−Ly6Chigh) and granulocytes (Ly6GhighLy6Clow) were measured by flow cytometry and cell numbers were calculated according to the respective total cell number. Cell numbers are presented as averages and SD from 3 mice per group from 2 independent experiments. (Bottom) Analysis of Ccl3, Ccl4 and Ccl5 mRNA transcripts in the large intestine at day 6 after infection. Data are presented as averages with SD from 3 mice per group from 2 independent experiments. Student’s t test, ** p<.01, *** p<.001
DISCUSSION
A balanced innate immune response is required for protection from infection, and preventing excessive inflammation and tissue damage. Here we address what mediators control excessive inflammation and mortality during innate immune responses against mucosal bacteria, and the cell type and the mechanism responsible for the resolution of inflammatory response early during infection.
IL-10 is a potent inhibitor of the production of tumor necrosis factor (TNF), IL-1, IL-6 and IL-12 in vitro34, 35, and is therefore a good candidate for limiting excessive inflammation in the intestinal mucosae. In the context of mucosal infectious disease, studies using Salmonella choleraesuis15, Klebsiella pneumoniae16, Listeria monocytogenes18, and Candida albicans19 have reported an increase in pathogen clearance, because the lack of IL-10 enhanced pro- inflammatory cytokines. In these situations the lack of IL-10 was beneficial to the host. In contrast, during infection with Toxoplasma gondii17, 21, 22 and Trypanosoma cruzi23, infection in the absence of IL-10 caused excessive and often lethal inflammatory responses. These data highlight the importance of IL-10 in influencing the proper balance of innate immunity following infection with a variety of pathogens.
We observed that C. rodentium was cleared slightly faster in mice deficient for IL-10, but these animals suffered from high morbidity and mortality, indicative of increased inflammation. A recent study, however, reported only a beneficial effect of the lack of IL-10 following C. rodentium infection 20. The discordant findings could reflect the four-fold increased dose of bacteria we used, but more likely reflect aspects of the endogenous microbiota in different mouse colonies, as the virulence of C. rodentium is dependent on other components of the microbial flora 36. By analyzing mouse strains with T cell or myeloid cell specific deletion of IL-10, we could implicate macrophage IL-10 in limiting the destructive immune response. Consistent with this, intestinal tissue from Il10flox/floxCd4-Cre mice produced more IL-10 protein than tissue from Il10flox/floxLysM-Cre mice, both at steady state and at early time points after infection, suggesting that macrophages are major IL-10 producing cells in the colon, both before and after bacterial infection. Indeed, colonic macrophages were GFP+ before and after infection in Il10gfp reporter mice.
Macrophage IL-10 is indispensable for maintaining a balance in adaptive immune responses in the mucosae11, 37, 38, 39, 40, and here we demonstrate its importance during the innate immune response. It is possible, however, that IL-10 producing intestinal macrophages acquire inflammatory features during bacterial infection, or alternatively, that many intestinal macrophages can rapidly acquire the ability to produce IL-10. We showed that adoptive transfer of IL-10 producing intestinal macrophages from reporter mice, but not macrophage populations that did not express IL-10, rescued the survival of infected, IL-10 deficient mice. Therefore the GFP− colonic macrophages could not induce sufficient IL-10 production to attain a protective function during this acute infection. Although we have shown that IL-10 from colonic macrophages is protective, we cannot formally exclude the possibility that the GFP− cells were not protective because they could not home to the intestine or survive well after transfer. We consider this unlikely, because we did not see an increase in GFP+ cells after infection. Therefore, while we suggest that the IL-10 producing intestinal macrophages could be a relatively stable subset, further experiments will be required to prove that this is the case. Interestingly, while the transcriptomes of the two colonic macrophage subsets are similar, the GFP+ cells have increased expression of genes involved in extracellular matrix organization, vascular development, and others involved in tissue regeneration, such as the epidermal growth factor receptor and the platelet derived growth factor receptor. These data suggest that the GFP+ macrophages may have a more prominent role in tissue homeostasis and repair.
Here we have demonstrated that after a bacterial challenge the absence of IL-10 production by macrophages led to increased IL-23 synthesis, predominantly by macrophages, an enhanced innate immune response and fatal colitis. It is curious that IL-12 synthesis was not greatly affected, but indeed, it has been reported that IL-23 is associated with innate immune pathology in the intestine41, 42. Consistent with this, IL-23 transgenic mice developed systemic inflammation that included the terminal ileum and colon, with infiltration of neutrophils and macrophages43. A few other studies have demonstrated that IL- 10 negatively regulates IL-23 production, and therefore the absence of IL-10 caused an increase in IL-17 producing T cells and spontaneous intestinal inflammation 5, 44. We note, however, that the Il10flox/floxLysM-Cre did not have increased IL-23 in the intestine at steady state nor did they spontaneously develop colitis.
Our data demonstrate that exogenous IL-10 reduced in vitro IL-23 production by intestinal macrophages from C. rodentium infected mice. In vivo, there is evidence that IL-10 acts directly on the IL-10 producing macrophage subset, although IL-10 competence did not depend on any IL-10 signals. IL-10 producing macrophages expressed increased CD163, however, which was dependent on IL-10R expression by these cells. Furthermore, GFP− macrophages from infected mice produced more IL-23p19 mRNA more than GFP+ macrophages. However, in our analyses less than 40% of colonic macrophages were GFP+, and therefore the reduced inflammation and improved survival in wild type mice suggests a paracrine role for IL-10 is also possible.
Recent studies showed that IL-10 signals drive macrophages to express tolerogenic functions that prevent colitis14, 45. However, those investigations focused on either steady state or adaptive mucosal immune responses, not on innate mucosal immune responses, and they did not provide a mechanism or tie the protective role of IL-10 to macrophage IL-23 production.
In humans, there is a study showing myeloid DCs from Crohn’s disease patients produced a higher amount of IL-23 and a lower amount of IL-10 after a bacterial stimulation when compared to ulcerative colitis patients46, leading us to propose that intestinal IL-10 producing myeloid cells might regulate IL-23 production not only in Crohn’s disease, but also in bacterial infection in humans.
In summary, our data show that IL-10 synthesis by intestinal macrophages has a dramatic effect on regulating the innate immune response in mice through regulating macrophage IL-23 production after mucosal bacterial infection. Furthermore, intestinal macrophages are heterogenous, and although IL-10 competent and IL-10− macrophages are similar, we suggest that they may not rapidly interconvert in vivo.
Material and Methods
Animals
Mice were bred and housed under SPF conditions in the vivarium of the La Jolla Institute for Allergy & Immunology (LJI, La Jolla, CA). Female 8 week old Il10−/−, Il10rb−/− and Rag−/− were purchased from Jackson laboratory. Female 8 week old Il10gfp (VertX) were obtained from Christopher Karp (Cincinnati Children’s Hospital, OH) and also purchased from Jackson Laboratory. Il10−/−Rag−/− mice were described previously47. Il10flox/flox mice were obtained from Alexander Rudensky (Memorial Sloan-Kettering Cancer Center, NY) with the permission of Axel Roers (Dresden University of Technology, Germany). Il10flox/flox mice were bred to Cd4-Cre, LysM-Cre and Cd11c-Cre mice, which were all purchased from Jackson laboratory. All mouse strains were generated on the C57BL6/J background or backcrossed at least 10 generations. All procedures were approved by the LJI Animal Care and Use Committee.
Citrobacter rodentium infection
A chloramphenicol-resistant variant of the wild-type Citrobacter rodentium strain DBS100 was cultured as previously described30. Briefly, bacteria were cultured for 15–16 hours in Luria-Bertani broth at 37°C. Mice were infected with 2–3×109 c.f.u. C. rodentium by oral gavage. In some experiments functional grade IL23p19 (G23-8) antibody or control rat IgG were injected intravenously at 100μg per mouse at the time of infection. Antibodies were purchased from eBiosciences (San Diego, CA). GFP+ (IL-10+) and GFP− (IL-10−) macrophages were sorted from colon tissue of uninfected Il10gfp(VertX) mice as CD45+, MHC class II+, CD11b+, F4/80+, CD11cint cells. One day after infection, 5×105 macrophages were injected intravenously per mouse. For c.f.u. determination, fecal pellets and spleen were weighed, homogenized and plated on chloramphenicol-containing MacConkey agar plates in serial dilutions.
FITC-dextran permeability assay
In vivo permeability assays to assess intestinal barrier function were performed using FITC-labelled dextran (Sigma) as described previously30. Briefly, mice were infected with C. rodentium. At day 0 or 6 after infection, mice were gavaged with FITC-dextran (60mg per 100 g body weight) and serum was collected retro- orbitally 4 hours later. Blood cells in serum samples were removed and the fluorescence intensity of FITC-dextran was measured. FITC-dextran concentrations were determined from standard curves generated by serial dilution.
Reagents and antibodies
For flow cytometry and cell sorting the following antibodies were used: CD11b (M1/70), CD11c (N418), F4/80 (BM8), CD45 (30-F11), IA/IE (M5/114.15.2), Ly6C (HK1.4), Ly6G (1A8), CD4 (RM4-5), TCRβ (H57-597), CD163 (polyclonal). Antibodies were purchased from BD Biosciences (San Diego, CA), eBiosciences (San Diego, CA), BioLegend (San Diego, CA) or Antibodies-online.com. Cell sorting was performed on an Aria II instrument (BD Biosciences). Cytokines and chemokines were quantified using either the Bio-plex multicytokine assay (Biorad, Hercules, CA), as described previously48, IL-23p19, IL-22 or IL-12p70 ELISA kits (eBiosciences, San Diego, CA), or a IL-6 ELISA kit (BD, San Diego, CA).
Colon fragment cultures
Large intestines were harvested and 3mm colon fragments were cultured for 24h in DMEM with 10% FBS and antibiotics. Supernatants were analyzed for cytokines and chemokines in ratio to the weight of the corresponding tissue fragment.
Isolation of lamina propria cells
Large intestines were harvested, opened longitudinally and washed to remove fecal content. Intestines were cut into small pieces and incubated in a horizontal shaker at 37°C in the presence of 2.5mM EDTA three times for 20 min to remove epithelial cells. Colon pieces were minced and digested for 20min with 1mg/ml Collagenase type VIII (Sigma, St. Louis, MO) at 37°C. Lamina propria cells were filtered and stained for flow cytometric analysis or cell sorting.
Histology
Tissue sections were taken from the middle colon and fixed in 10% formalin. After embedding in paraffin, 5μm sections were cut and stained with hematoxylin- eosin (H&E). Histological scores were assigned in a blinded manner according to the following criteria: (i) mucosal architecture (0, normal; 1, focally abnormal; 2, diffusely abnormal; 3, severely abnormal); (ii) inflammatory cell infiltration of mucosa (0, normal; 1, mild infiltration; 2, moderate infiltration; 3, severe infiltration), submucosa (0, normal; 1, mild infiltration; 2 moderate infiltration; 3, severe infiltration), muscle (0, normal; 1 moderate to severe), and serosa (0, absent; 1, present); (iii) Epithelial erosions and ulcerations (0, absent; 1, present); (iv) crypt abscesses (0, absent; 1, present); and (v) goblet cell loss (0, absent; 1, present). Maximal total score for colitis severity is 14.
Relative mRNA quantification
Total RNA was extracted from sorted cells or 2–3mm long colon tissue fragments using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genomic DNA was digested with DNase I (Qiagen), cDNA was synthesized using iScript (Biorad, Hercules, CA) and real-time PCR was performed using SYBRgreen (Roche, Indianapolis, IN) on a LightCycler instrument (Roche). The primers were as follows: L32, 5′-GAA ACT GGC GGA AAC CCA-3′ (sense), 5′-GGA TCT GGC CCT TGA ACC TT-3′ (antisense), CD163, 5′-GGT GGA CAC AGA ATG GTT CTT C-3′ (sense), 5′-CCA GGA GCG TTA GTG ACA GC-3′ (antisense), TGFβ1, 5′-ACC ATG CCA ACT TCT GTC -3′ (sense), 5′-CGG GTT GTG TTG GTT GTA GA -3′ (antisense), Il10, 5′-GCC ACA TGC TCC TAG AGC TG-3′ (sense), 5′-CAG CTG GTC CTT TGT TTG AAA-3′ (antisense), Il23p19, 5′-AAT AAT GTG CCC CGT ATC CA-3′ (sense), 5′-GGA TCC TTT GCA AGC AGA AC-3′ (antisense), Il12p35, 5′-GTG AAG ACG GCC AGA GAA A-3′ (sense), 5′-GGT CCC GTG TGA TGT CTT C-3′ (antisense), Il12p40, 5′-AGC AGT AGC AGT TCC CCT GA-3′ (sense), 5′-AGT CCC TTT GGT CCA GTG TG-3′ (antisense).
Microarray data processing and analysis
All microarray datasets were processed in R, using customized scripts and Bioconductor modules49. Raw intensity data were normalized with the ‘rma’ function, with default parameters. Differential expression analysis was performed using the ‘limma’ package. Probe sets were deemed significantly differentially expressed only if their corrected P values were less than 0.05 and the difference was at least 3-fold. Heatmaps containing the differentially expressed genes in each set were built using the ‘regHeatmap’ function of the ‘Heatplus’ module and sorted by average fold-change. The 242 probe sets that were significantly differentially expressed were mined for enriched GO biological process terms using the database for Annotation, Visualization and Integrated Discovery (DAVID) Functional Classification Tool (http://david.abcc.ncifcrf.gov).
Immunofluorescence
Colons and ilea were harvested, flushed with phosphate buffered saline (PBS, Life Technologies, Carlsbad, CA) and fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS overnight at 4°C and then equilibrated in 30% w/v sucrose solution in PBS for another 24 hours. Tissues were briefly washed in PBS, frozen in OCT and stored at -80 °C. Cryostat sections were cut at 16 μm thickness, and air-dried for 30 minutes. Sections were rehydrated for 10 minutes in PBS and non-specific binding sites were saturated with 10% normal goat serum, 0.5% bovine serum albumin (both from Gemini Bio-Products, West Sacramento, CA) in the presence of 0.1% Triton X-100 in PBS for 1 hour. Tissues were incubated with anti-F4/80 rat monoclonal antibodies (50 μg/ml, BM8, eBiosciences) and anti-GFP rabbit antiserum (5 μg/ml, ab290, Abcam, Boston, MA) overnight at 4 °C. Following washing, sections were reacted with donkey anti-rat DyLight 549-labeled (1.4 μg/ml, Jackson Immunoresearch) and goat anti-rabbit F(ab′)2 fragment Alexa Fluor 647-labeled secondary antibodies (4 μg/ml, Invitrogen) for 1h at room temperature. Slides were washed, mounted in Prolong Gold antifade reagent (Invitrogen) with a cover slip and examined with FluoView FV10i confocal microscope (Olympus). Imaging was performed at room temperature, using 60x/1.35 NA oil objective. To improve feature visibility images were processed by contrast stretching using identical procedures.
Statistical analysis
The Student’s t test was used for statistical analysis except for survival and histological scores, for which the Log-Rank test and the Mann Whitney test were used respectively. Differences were considered significant at P<.05.
Supplementary Material
Acknowledgments
We thank Drs. K. Ley and R. Hanna for critical reading of this manuscript, members of our laboratory and Dr. C. Hedrick for discussions, C. Kim and K. Van Gunst for assistance with cell sorting. This work was supported by National Institutes of Health Grants R21 AI091911 to M.M., PO1 AI089624 and Crohn’s and Colitis Foundation of America, Senior research award to M.K.
Footnotes
AUTHOR CONTRIBUTIONS
M.M. and M.K. designed experiments. P.K., V.M., Y.P., U.B., Z.M., T.M. and M.M. performed experiments. M.M., P.K., J.G. and B.P. performed the bioinformatics analysis, J.S., G.K., H.C., Y.C.L. and B.P. helped with critical advice and discussion throughout. P.K., M.K. and M.M. wrote the manuscript.
Accession codes:
Microarray data has been deposited in the Gene Expression Omnibus (GEO) database under accession code GSE58677.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interest.
References
- 1.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19(1):115–123. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10- deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 4.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187(4):571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of clinical investigation. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 7.Van Montfrans C, Rodriguez Pena MS, Pronk I, Ten Kate FJ, Te Velde AA, Van Deventer SJ. Prevention of colitis by interleukin 10-transduced T lymphocytes in the SCID mice transfer model. Gastroenterology. 2002;123(6):1865–1876. doi: 10.1053/gast.2002.37067. [DOI] [PubMed] [Google Scholar]
- 8.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171(2):971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 9.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200(10):1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10(11):1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Muller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. European journal of immunology. 2006;36(12):3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 13.Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, et al. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. European journal of immunology. 2010;40(2):443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40(5):720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Arai T, Hiromatsu K, Nishimura H, Kimura Y, Kobayashi N, Ishida H, et al. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology. 1995;85(3):381–388. [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155(2):722–729. [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN- gamma and TNF-alpha. J Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 18.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158(5):2259–2267. [PubMed] [Google Scholar]
- 19.Vazquez-Torres A, Jones-Carson J, Wagner RD, Warner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67(2):670–674. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dann SM, Le C, Choudhury BK, Liu H, Saldarriaga O, Hanson EM, et al. Attenuation of intestinal inflammation in IL-10 deficient mice infected with Citrobacter rodentium. Infect Immun. 2014 doi: 10.1128/IAI.00066-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis e Sousa C, Yap G, Schulz O, Rogers N, Schito M, Aliberti J, et al. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11(5):637–647. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164(10):5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 23.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158(7):3311–3316. [PubMed] [Google Scholar]
- 24.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cellular microbiology. 2005;7(12):1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 25.Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann N Y Acad Sci. 2006;1072:28–38. doi: 10.1196/annals.1326.008. [DOI] [PubMed] [Google Scholar]
- 26.Bhinder G, Sham HP, Chan JM, Morampudi V, Jacobson K, Vallance BA. The Citrobacter rodentium mouse model: studying pathogen and host contributions to infectious colitis. Journal of visualized experiments: JoVE. 2013;(72):e50222. doi: 10.3791/50222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 28.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28(1):33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, et al. Notch2- dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nature immunology. 2013;14(9):937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, et al. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012 doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 32.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, et al. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal immunology. 2012 doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12(9):1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 34.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 36.Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science (New York, NY) 2012;336(6086):1325–1329. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 38.Ueda Y, Kayama H, Jeon SG, Kusu T, Isaka Y, Rakugi H, et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22(12):953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 39.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. The Journal of clinical investigation. 2011;121(12):4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of experimental medicine. 2012;209(1):139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166(12):7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 44.Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137(5):1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 47.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nature immunology. 2003;4(2):154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 48.Park Y, Jin HS, Lopez J, Elly C, Kim G, Murai M, et al. TSC1 regulates the balance between effector and regulatory T cells. The Journal of clinical investigation. 2013;123(12):5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome biology. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.