Abstract
Hepatitis C virus (HCV) genotype 1 infections are significantly more difficult to eradicate with PEG-IFN/ribavirin therapy, compared to HCV genotype 2. The aim of this work is to investigate the difference of immunological impairments underlying this phenomenon. Pre-treatment NKG2D expression on peripheral CD56+CD3+ lymphocytes and CD56+CD3− NK cells from cases of chronic hepatitis C were analyzed and assessed by treatment effect. Two strains of HCV were used to co-incubate with immune cells in vitro. NKG2D expression on peripheral CD56+CD3+ lymphocytes, but not NK cells, was significantly impaired in genotype 1 infection, compared to genotype 2. When peripheral blood mononuclear cells from healthy donors were co-incubated with TNS2J1, a genotype 1b/2a chimera strain, or with JFH1, a genotype 2a strain, genotype-specific decrease of NKG2D on CD56+CD3+ lymphocytes, but not NK cells, was observed. Pre-treatment NKG2D expression on peripheral CD56+CD3+ lymphocytes significantly correlated with reduction in serum HCV RNA levels from week 0 to week 4, and predicted treatment response. Ex vivo stimulation of peripheral CD56+CD3+ lymphocytes showed NKG2D expression-correlated IFN-γ production. In conclusion, Decreased NKG2D expression on CD56+CD3+ lymphocytes in chronic HCV genotype 1 infection predicts inferior treatment response to PEG-IFN/ribavirin therapy compared to genotype 2.
Introduction
More than 70% of acute hepatitis C virus (HCV) infections become persistent and lead to chronic hepatitis [1, 2]. Longer persistency of HCV infection increases the rate of progression to liver cirrhosis [3]. There are several immunological mechanisms that favor HCV persistency, including disruption of pattern-recognition receptor function, impaired innate interferon function, increase of regulatory T cells, and exhaustion of T cells [2, 4–6]. For immune cells with cytotoxicity, including natural killer (NK) cells, natural killer T (NKT) cells, or CD8+ cytotoxic T lymphocytes (CTLs), one of the important mechanisms for the eradication of HCV-infected hepatocytes is through interferon (IFN)-γ, alongside many of the immunoregulatory functions of this cytokine [2, 4]. IFN-γ secretion can be induced by cross-linking natural killer group 2, member D (NKG2D), an activating receptor for MHC class I chain-related protein A/B (MICA/B) or other ligands [7–9]. NKG2D is previously known as an NK cell marker, however, other cytotoxic cells, including CTLs, NKT cells, or γδT cells, also express NKG2D [7, 9–13].
Human CD56+CD3+ lymphocytes are T cells expressing both T cell and NK cell markers [10]. Classical invariant NKT (iNKT) cells, which are CD1d-restricted with a very limited repertoire of T cell receptors, are more sophisticatedly studied in mice than in human [10]. Although not all CD56+CD3+ lymphocytes are classical iNKTs, by the original definition, they belong to the broader group of NKT cells. Some researchers refer human CD56+CD3+ lymphocytes as natural T (NT) cells [10, 14–16]. Because human iNKT cells are relatively sparse in frequency (about 1–2% in peripheral blood and less than 1% in livers)[17, 18], and multiple isoforms of human CD1 exist, iNKT cells are less likely to be the only effective NKT cells in human [19]. Human livers, as primary targets for HCV infection, are enriched of CD56+CD3+ lymphocytes making up about 30% of hepatic CD3+ cells [10, 15, 16]. Unlike classical T cells, CD56+CD3+ lymphocytes may produce large quantities of both Th1 and Th2 cytokines, and alongside human iNKT cells, are considered important innate immune cells as first-line defense against pathogens, including viruses [14]. Although reduced NKG2D expression on “NK cells” in acute [20] and chronic [21] HCV infections have been reported, the NKG2D expression on “CD56+CD3+ lymphocytes” and its immunological role in chronic HCV infection has not yet been fully studied.
Over the past two decades, interferon-based antiviral therapy has been the standard treatment for chronic hepatitis C. Multiple viral and host factors can affect the goal of reaching sustained virologic response (SVR). The most prominent viral factor is the genotype of HCV[2]. Genotype 1 infection is significantly more difficult to eradicate by pegylated-interferon (PEG-IFN)/ ribavirin therapy than genotype 2 [2, 22–24]. The underlying immunological differences aroused by different HCV genotypes must exist, however, they are not yet fully elucidated.
In this study, followed by the evaluation of the expression of NKG2D on peripheral blood CD56+CD3+ lymphocytes, CD56+CD3- NK cells and CD3+CD56−CD8+ CTLs between chronic HCV infections of genotype 1 and genotype 2, we revealed that NKG2D expression on CD56+CD3+ lymphocytes predicts treatment response, and may be key to the immune mechanism mediating genotype difference in chronic HCV infections.
Materials and Methods
Study Subjects
According to the ethical guidelines of the 1975 Declaration of Helsinki, the institutional review board of Keio University School of Medicine approved the study protocols specifically before we started this study. Recruited study subjects provided prior written informed consents, which included blood sampling and study participation. Study protocols were as follows:
For the evaluation of NKG2D expression, sixty-six patients with chronic HCV infection, including fifty-three with genotype 1, and thirteen with genotype 2, were included. No patient with hepatocellular carcinoma was included. NKG2D expression on peripheral blood mononuclear cells (PBMCs) was analyzed before treatment. Detailed definition of flow cytometry analyses and gating strategies are explained below. Clinical characteristics are summarized in Table 1, and the detailed statistics were included in supporting S1 Table. PBMC analyses from nine healthy volunteer donors were used as controls.
Thirty patients with chronic HCV infection, including seventeen with genotype 1 and thirteen patients with genotype 2, were analyzed for treatment response. PEG-IFNα-2b (Schering-Plough KK, Tokyo, Japan) and ribavirin (Schering-Plough KK) were administered in combination at doses according to the manufacturer’s instructions. Treatment duration was determined by the treatment guideline from the Japan Ministry of Health, Labour and Welfare [25]. Patient characteristics are shown in supporting S2, S3, and S4 Tables. Undetectable plasma HCV RNA (< 15IU/ml) at week 4 is defined as rapid viral response (RVR). Negative plasma HCV RNA at 24 weeks after the end of treatment is considered to have a sustained virological response (SVR). Patient whose HCV RNA declines more than 2 log10 at week 12 but is positive at week 24 is defined as partial responder (PR). Patient whose HCV RNA declines less than 2 log10 at week 12 is defined as null responder (NR).
Fourteen patients with chronic HCV genotype 1 infection were included for the evaluation of NGK2D expression on IFN-γ production. Clinical characteristics of these patients are summarized in supporting S5 Table.
Table 1. Clinical characteristics of cases of genotype 1 and genotype 2.
| Characteristics | Units | All cases | Genotype 1 | Genotype 2 | p |
|---|---|---|---|---|---|
| HCV genotype, 1: 2 | - | 53:13 | - | - | - |
| Patient number, n | 66 | 53 | 12 | - | |
| Age | Years | Median 62 | Median 62 | Median 60 | 0.25 a |
| (51–67) | (52–68) | (46.5–65) | |||
| Gender, M:F | - | 35:31 | 30:23 | 5:8 | 0.33 b |
| Liver histology, | - | 11/9/5/5 (30) | 7/5/4/4 (20) | 3/4/1/1 (10) | 0.71 b |
| F1/F2/F3/F4 | |||||
| (data available) | |||||
| HCV-RNA | Log | Median 6.6 | Median 6.7 | Median 6.5 | 0.34 a |
| IU/ml | (6.1–7.0) | (6.0–7.0) | (6.25–6.65) | ||
| PLT count | x1000/μl | 170±54 | 166±53 | 185±56 | 0.23 c |
| Serum albumin | g/dl | 4.0±0.4 | 4.0±0.5 | 4.0±0.3 | 0.93 c |
| Total | mg/dl | 176±36 | 177±38 | 169±28 | 0.47 c |
| Cholesterol | |||||
| ALT | IU/L | 65±61 | 60±57 | 80±74 | 0.28 c |
| ALP | IU/L | 260±116 | 249±111 | 301±133 | 0.15 c |
| WBC | /μl | 4720±1450 | 4750±1470 | 4580±1430 | 0.71 c |
| NKG2D | % | Median 75.8% | Median 72.3% | Median 90.1% | <0.0001 a |
| expression on | (64.3%-83.4%) | (62.7%-82.2%) | (51.9%-81.4%) | ||
| CD56+CD3+ | |||||
| NKG2D | % | Median 68.9% | Median 66.5% | Median 77.0% | 0.39 a |
| expression on | (50.2%-81.1%) | (49.6%-82.0%) | (51.9%-81.4%) | ||
| CD56+CD3- | |||||
| NKG2D | % | Median 87.4% | Median 84.3% | Median 92.9% | 0.0048 a |
| expression on | (77.1%-93.8%) | (74.1%-93.2%) | (90.4%–95.7%) | ||
| CD8+ T cells |
a Statistics are analyzed by Mann-Whitney U-test. IQRs are shown in the parentheses.
b Statistics are analyzed by Student’s t-test. Data are shown as mean± standard deviation.
c Statistics are analyzed by Fisher’s exact test.
Abbreviations: ALT, alanine aminotransferase, ALP, alkaline phosphatase.
Flow Cytometry Analysis
Eight to sixteen milliliters of whole blood was drawn and PBMCs were isolated with BD Vacutainer CPT (Becton Dickinson, Franklin Lake, NJ, USA), containing 1.0 ml of 0.1 M sodium citrate, 3 g of polyester gel, and 2.0 ml of polysaccharide sodium diatrizoate. After centrifugation at 1500 × g for 25 minutes, PBMCs on the gel phase were harvested, and washed twice with RPMI-1640 medium (Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% bovine albumin. Isolated PBMCs were incubated with specific fluorescence-labeled monoclonal antibodies (mAbs) at 4°C for 30 min. Anti-human mAbs including CD3 (FITC, BD), CD56 (PE-Cy7, BD), CD4 (APC, BD), CD8 (APC-Cy7, BD), 7-amino-actinomycin D (7-AAD) and NKG2D (PE, clone 1D11, BioLegend, San Diego, CA, USA) were used. Dead cells were excluded from assessment by 7-AAD. Irrelevant anti-rat isotype antibodies (BD) were used to assess background fluorescence. Stained cells were analyzed using FACS Canto II (BD), and the data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA). Gating strategies are illustrated in supporting S1 Fig NKG2D expression is defined as the percentage of NKG2D-positive cells compared to isotype controls, as illustrated in Fig 1B.
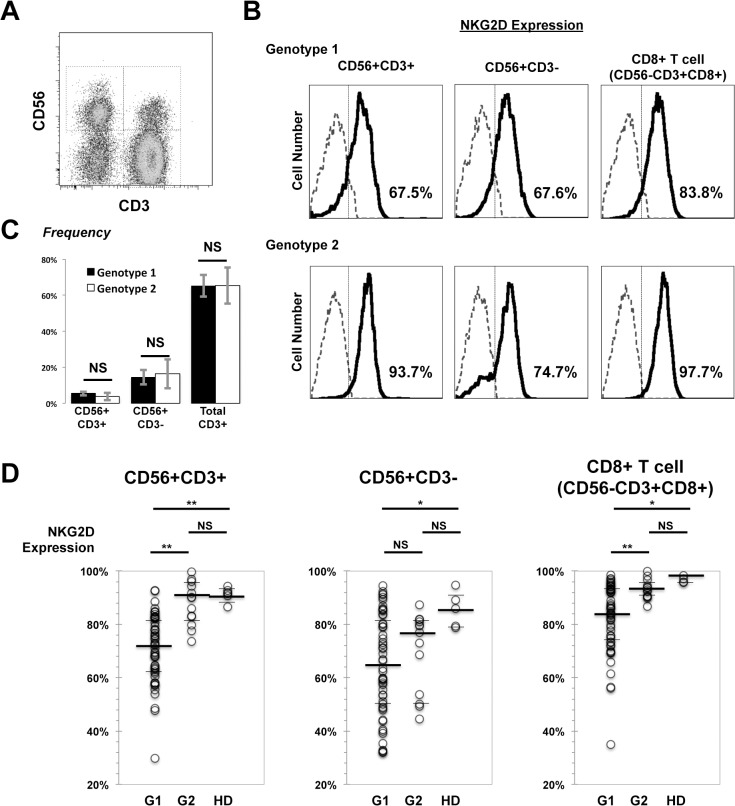
Fig 1. NKG2D expression on CD56+CD3+ lymphocytes in HCV genotype 1 infection is significantly impaired.
PBMCs were collected and analyzed by flow cytometry. (A) Representative flow cytometry plot shows CD56+CD3+ lymphocytes, NK (CD3-CD56+) cells and T lymphocytes (CD3+CD56-) gated on SSClow compartment. (B) Histograms from representative genotype 1 HCV infected (upper) and genotype 2 (lower) show NKG2D expression on CD56+CD3+ lymphocytes, NK cells and CD8+ T cells. Isotype controls are shown as dashed lines. Percentages are for NKG2D positive levels in corresponding compartments. (C) Peripheral frequency (compared to total SSClow cells) stratified by HCV genotypes. (D) NKG2D expression on CD56+CD3+ lymphocytes, NK cells and CD8+ CTLs was compared by HCV genotypes (G1, genotype 1, n = 53; G2, genotype 2, n = 13), with control data from healthy donors (HD, n = 9). Statistics were analyzed by Mann-Whitney U-test. Medians, IQRs were presented as bars. Please refer to Table 1 for exact values *P < 0.05, **P < 0.01, NS; not significant.
Intracellular IFN-γ Production
Isolated PBMCs were adjusted to 2 × 106/ml, and treated with brefeldin A (Sigma Aldrich, final concentration of 10 μg/ml), and at the same time, phorbol 12-myristate 13-acetate (PMA, Sigma Aldrich, final concentration of 10 ng/ml), and ionomycin (Sigma Aldrich, final concentration of 1 μg/ml) were added in the stimulation group. After 4 hours of incubation at 37°C with 5% CO2, cells were harvested, washed, and then stained for surface antigens including CD3 (APC), CD56 (FITC), and 7-AAD. After permeabilization and fixation with cytofix/cytoperm solution (BD) for 15 minutes, cells were stained with mAbs against IFN-γ (PE, BD) for 30 min at 4°C, washed twice, and then fixed in 1% paraformaldehyde. The samples were analyzed by flow cytometry.
HCV Genotyping
HCV genotypes were determined by enzyme immunoassay according to previously reported methods [26]. According to Simmonds classification [27], serotype 1 corresponded to genotypes 1a and 1b, whereas serotype 2 corresponded to genotypes 2a and 2b. In Japan, 1b (about 70%) and 2a (about 20%) are the majority genotypes [28].
Viral Kinetics
Plasma HCV RNA load was measured using COBAS TaqMan HCV test, with a lower limit of detection of 15 IU/ml (Roche Diagnostics Co, Ltd, Tokyo, Japan).
IL28B SNP Genotyping
A single nucleotide polymorphism (SNP) of IL28B was evaluated in patients with genotype 1 infection [29]. We selected rs8099917, which was previously reported as the predictive factor of SVR by IFN-based therapies in Japan [29, 30], to determine IL28B SNP. This SNP is located within the same haplotype block as rs12979860, reported by Ge et al. [31], and are associated with each other [30]. TT at rs8099917 is advantageous and defined as major, whereas TG or GG is minor.
Amino Acid Substitutions
The nucleotide sequences of ISDR in the nonstructural 5A region (NS5A, amino acids (aa) 2209–2248) and core protein (aa70 and aa91) were determined by direct sequencing of PCR amplified materials. Mutant-type ISDR was defined as containing two or more than two amino acid substitutions [32]. Substitutions at aa 70 of arginine (Arg70, wild type) to glutamine/histidine (Gln70/His70), and at aa 91 of leucine (Leu91, wild type) to methionine (Met91) were determined [33].
Co-incubation of HCV and PBMCs
PBMCs from healthy volunteer donors were isolated. JFH1, an HCV genotype 2a strain [34], and TNS2J1, a chimeric HCV strain of the structural region from genotype 1b and the non-structural region from genotype 2a [35], were used to infect hepatoma cell line Huh7.5. After several passages, supernatants enriched of infectious viral particles were concentrated by Amicon Ultra-15 (100,000 NMWL membrane; Merck Millipore, Billerica, MA). Supernatants from naïve Huh7.5 cultures were used as controls. Viral titers were determined as fluorescent focus forming units (FFU) in Huh7.5 cells. PBMCs were in contact with concentrated HCVs with a multiplicity of infection (MOI) of 0.5 FFU/cell. NKG2D expression on immune cells was determined by flow cytometry before treatment and after 48-hours of co-incubation.
Statistics
Data were analyzed using JMP9 (SAS Institute, Inc. Cary, NC) and are expressed as mean ± standard deviation (SD) for Student’s t-test, or median with IQRs shown for Mann-Whitney U-test as appropriate. Receiver operating characteristic (ROC) analysis was performed for confirming the usefulness of NKG2D expression on CD56+CD3+ lymphocytes to predict SVR after PEG-IFN/ ribavirin therapy. Categorical data were analyzed using Fisher’s exact test. Differences were considered statistically significant when P < 0.05.
Results
NKG2D Expression on CD56+CD3+ Lymphocytes in HCV Genotype 1 Infection is Significantly Impaired
PBMCs from patients of chronic HCV infection were isolated and analyzed for the surface expression of NKG2D by flow cytometry. The two groups of patients with HCV genotype 1 and 2 infection showed no statistical significant differences in age, gender, fibrotic stages or other characteristics (Table 1). NKG2D-expressing cells were gated as CD56+CD3− (NK cells), CD56+CD3+, and CD56−CD3+CD8+ (CTL) cells from the side-scatter low lymphocyte compartment, respectively (Fig 1). No significant differences in peripheral blood frequency of total T cells, NK cells, CD56+CD3+ lymphocytes were observed between genotype 1 and genotype 2 infections. NKG2D expression levels on CD56+CD3+ lymphocytes were significantly impaired in patients with HCV genotype 1 infection, compared to genotype 2 (Fig 1D, left). A similar tendency was observed with CD8+ T cells. However, between genotypes, no significant difference was observed in NK2GD expression on NK cells (Fig 1D). Furthermore, NKG2D expression on CD56+CD3+ lymphocytes did not correlate with age, HCV-RNA levels, or status of clinical liver fibrosis as assessed by blood platelet count and serum type 4 collagen 7s levels (Fig 2). Significantly different degrees of NKG2D expression induced by different HCV genotypes were noticed on CD56+CD3+ lymphocytes or on CD8+ T cells, but not on NK cells.
Fig 2. NKG2D expression on CD56+CD3+ lymphocytes has no significant correlation to age, HCV-RNA levels or liver fibrotic degree.
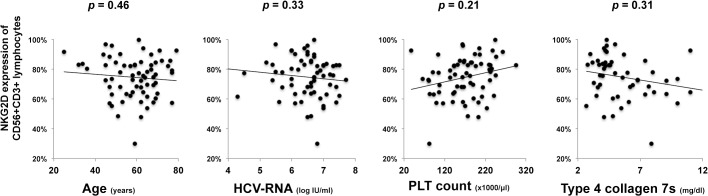
PBMCs were collected and analyzed by flow cytometry for expression of NKG2D on CD56+CD3+ lymphocytes. Statistics of data including age, HCV-RNA, peripheral platelet count, and serum type IV collagen 7s levels were analyzed for correlation. Please refer to supporting S1 Table for detailed values.
Association of Differential NKG2D Expression on CD56+CD3+ Lymphocytes from Chronic Genotype 1 Infection and Other Known Predictive Factors
Since NKG2D expression on CD56+CD3+ lymphocytes differed between genotypes 1 and 2 (Fig 1C), and showed a differential range of expression in genotype 1, we hypothesized that NKG2D expression on CD56+CD3+ lymphocytes may correlate with treatment response. Thus, we attempted to identify whether any correlation with other known predictive factors exist, including IL28B polymorphism [30, 31], ISDR amino acid mutations [24, 32, 36], and core protein substitution at aa70 or aa91 [33]. Of fifty-three patients with genotype 1 infection included in this study, IL28B SNPs at rs8099917, mutation numbers of ISDR on NS5A (aa2209-aa2248) and core protein mutation at aa70 or aa91 were determined. In this cohort, NKG2D expression on CD56+CD3+ lymphocytes showed no difference when stratified by IL28B SNPs (major vs. minor) or ISDR mutation numbers (≤1 vs. ≥2). However, a significant difference when stratified by core protein determination at aa70 and aa91 (double wild-type or not, Fig 3) was noticed. This implies that differential NKG2D expression on CD56+CD3+ lymphocytes in chronic genotype 1 infection might be more prominently influenced viral structural proteins, especially the differences in core proteins, rather than host factors.
Fig 3. Differential NKG2D expression on CD56+CD3+ lymphocytes from chronic genotype 1 infection is associated with core protein aa substitutions.
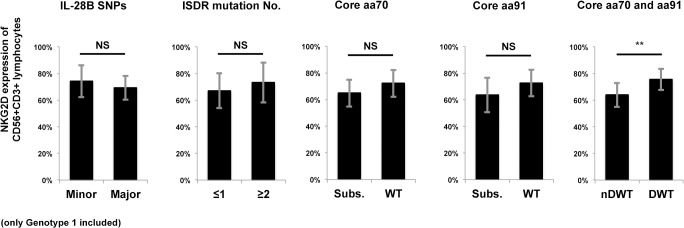
Cases of HCV genotype 1 infection were determined for the IL28B SNP polymorphism at rs8099917, mutation numbers of ISDR on NS5A (aa2209-aa2248) and core protein substitution at aa70 or aa91 before treatment. NKG2D expression on CD56+CD3+ lymphocytes was compared between IL28B genotype major (TT at rs8099917, n = 35) or minor (TG or GG, n = 18), ISDR mutation numbers ≤1 (n = 34) or ≥2 (= 19), aa70 substituted (subs, n = 17) or wild type (n = 36), aa91 substituted (n = 19) or wild type (n = 34), double wild type (DWT, n = 25) at aa70 and aa91 or non-double wild type (nDWT, n = 27). Data show mean ± SD. **P < 0.01. NS: not significant.
Ex vivo Exposure with Different Genotypes of HCV Strains Induced Genotype-specific Decrease of NKG2D Expression on CD56+CD3+ Lymphocytes
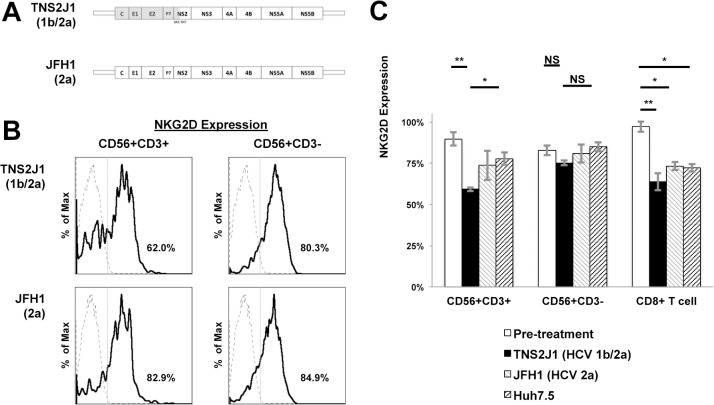
As previously described, different genotypes of HCV may influence NKG2D expression on CD56+CD3+ lymphocytes, and may related to structural proteins. Here we used two different strains of HCV genotypes, JHF1 (genotype 2a) and TNS2J1, a chimeric strain of genotype 1b in structural areas and 2a in non-structural areas (Fig 4A), and co-incubate them in vitro with PBMCs from healthy donors. We used whole isolated PBMCs because there is sufficient evidence that immune cells showed interactions between antigen-presenting cells such as dendritic cells are important in sensing viral particles [21]. Using Huh7.5 hepatoma cell line as the control, NKG2D expression on CD56+CD3+ lymphocytes was significantly decreased in the TNS2J1 group, but not in the JFH1 group. This phenomenon was not observed on NK cells (Fig 4B and 4C). In CD8 T lymphocytes, NKG2D expression decreased when co-incubated with both HCV strains; however, this was not statistically significant compared with the group co-incubated with Huh7.5 supernatants (Fig 4C).
Fig 4. Ex vivo exposure with different genotypes of HCV strains induced genotype-specific decrease of NKG2D expression on CD56+CD3+ lymphocytes.
PBMCs from healthy donors were isolated and co-incubated with HCV strains of different genotypes, or with supernatants from Huh7.5 cells for 48 hours, and were analyzed by flow cytometry for NKG2D expression. (A) Genetic schemes of two strains of HCV, TNS2J1, a genotype 1b/2a chimera virus strain, and JFH1, a genotype 2a strain. (B) Representative histograms from THS2J1 (upper) and JFH1 groups (lower) show NKG2D expression on CD56+CD3+ lymphocytes and NK cells. Isotype controls are shown as dashed lines. Percentages show positive NKG2D levels in corresponding compartments. (C) NKG2D expression on CD56+CD3+ lymphocytes, NK or CD8+ CTLs, and representative results of PBMCs from three healthy donors are shown. Data show mean ± SD. *P < 0.05, **P < 0.01, NS: not significant.
NKG2D Expression on CD56+CD3+ Lymphocytes Predict Treatment Responses to PEG-IFN/Ribavirin Therapy
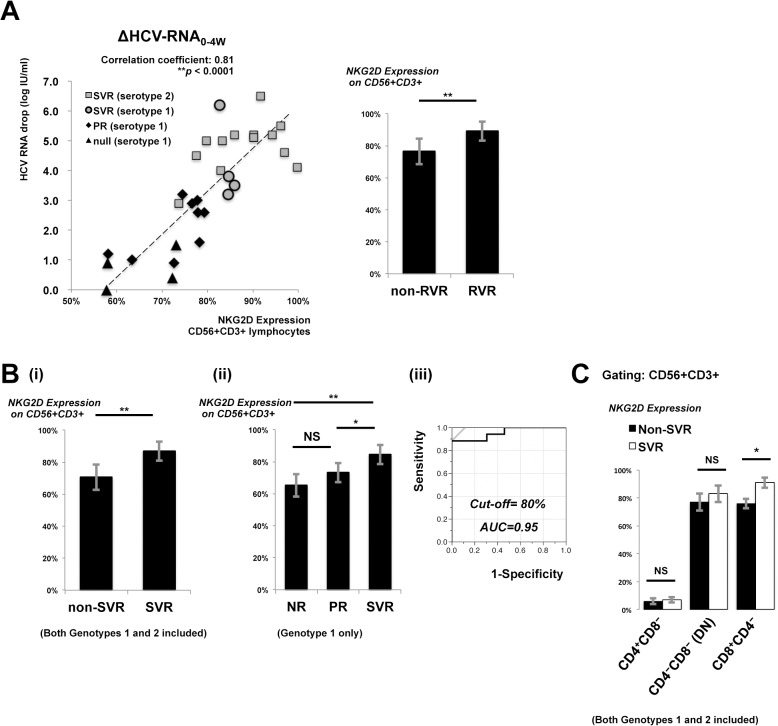
Since HCV genotype 1 infection is more difficult to eradicate with interferon-based therapies than genotype 2, we further investigated whether CD56+CD3+ lymphocytes bearing different NKG2D expressing levels influenced the treatment effect of PEG-IFN/ ribavirin therapy. Thirty patients, including 17 with genotype 1 HCV infection and 13 with genotype 2, were analyzed. PBMCs were isolated and analyzed for NKG2D expression prior to the start of treatment. Regardless of genotype differences, NKG2D expression on CD56+CD3+ lymphocytes was highly and significantly correlated to the degree of HCV-RNA reduction from week 0 to week 4 (ΔHCV-RNA0-4w; correlation coefficient, 0.81; P < 0.0001, Fig 5A, left). When stratified by RVR or not, cases that developed RVR showed significantly higher NKG2D expression on CD56+CD3+ lymphocytes than non-RVR cases (Fig 5A, right).
Fig 5. NKG2D expression on CD56+CD3+ lymphocytes predicts treatment responses to PEG-IFN/ ribavirin therapy.
PBMCs were collected and analyzed by flow cytometry for NKG2D expression on CD56+CD3+ lymphocytes before treatment. Plasma HCV-RNA levels were measured regularly to monitor viral kinetics during treatment. (A, left) Correlation of decreased plasma HCV RNA (IU/ml) from week 0 to week 4 and NKG2D expression on CD56+CD3+ lymphocytes is shown. (A, right) NKG2D expression was analyzed to compare RVR (n = 8) and non-RVR (n = 22) cases. (B) NKG2D expression on CD56+CD3+ lymphocytes was analyzed to (i) compare SVR (n = 17) and non-SVR (n = 13) cases; (ii) SVR (n = 4), PR (n = 9), and NR (n = 4) in genotype 1 infection; (iii) ROC analysis to predict SVR by NKG2D expression on CD56+CD3+ lymphocytes. (C) Pre-treatment peripheral CD56+CD3+ lymphocytes were stratified into CD4+CD8-, CD8+CD4- and CD4-CD8- double negative (DN) subpopulations. NKG2D expression on corresponding subpopulations were determined. Statistics of NKG2D expression were compared between SVR (n = 17) and non-SVR (n = 13) cases, including both genotypes. Data show mean ± SD. *P < 0.05, **P < 0.01, NS: not significant.
Significant differences of NKG2D expression on CD56+CD3+ lymphocytes between SVR and non-SVR cases were also observed (Fig 5B-i). When limited to cases of genotype 1, NKG2D expression on CD56+CD3+ lymphocytes from SVR cases was significantly higher than PR or NR (Fig 5B-ii). ROC analysis revealed that NKG2D expression on CD56+CD3+ lymphocytes above 0.80 could predict SVR by PEG-IFN/ ribavirin therapy with a high probability (area under the curve 0.95; Fig 5B-iii). Because NKG2D expression on CD8+ T cells also showed significant difference between HCV genotypes as showed in Fig 1D, we did ROC analysis of NKG2D expression on CD8+ T cells for the prediction of SVR in the same cohort of cases. The area under curve was 0.76 (ROC curve not shown), and was inferior to that of NKG2D expression on CD56+CD3+ lymphocytes.
The pre-treatment NKG2D expression of peripheral CD56+CD3+ lymphocyte subpopulations, ie, CD8+CD4-, CD4+CD8-, or CD4-CD8- double negative (DN) was also analyzed. Higher pre-treatment NKG2D expression of CD8+CD4- subpopulation significantly associated to SVR (Fig 5C).
Impaired NKG2D Expression on CD56+CD3+ Lymphocytes Correlates to Decreased IFN-γ Production
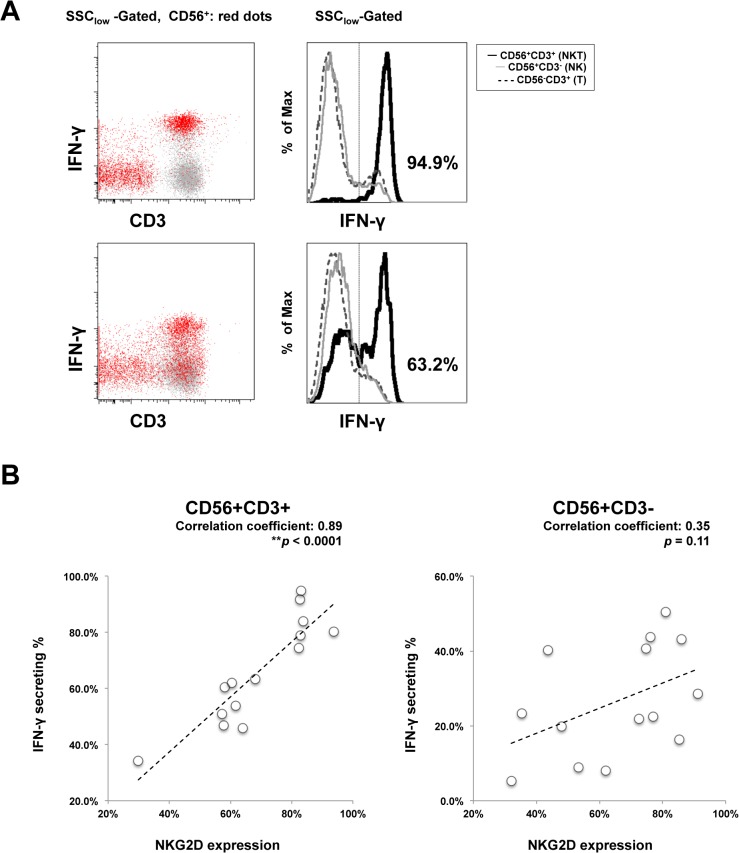
IFN-γ-mediated killing of HCV-infected hepatocytes is a mechanism of immune-mediated eradication of HCV infection [2, 37], and NKG2D is the receptor for the MICA/MICB pathway that induces IFN-γ production [9]. Therefore, differences of NKG2D expression on CD56+CD3+ lymphocytes that exist between genotypes and within genotype 1 might explain the inferior immunological response. In previous studies, CD56+CD3+ lymphocytes produce more IFN-γ upon activation, compared to NK cells [14] and CD56 negative T cells [37, 38]. To investigate whether differential NKG2D expression within genotype 1 infections on CD56+CD3+ lymphocytes influences IFN-γ production, we analyzed IFN-γ production from CD56+CD3+ lymphocytes after stimulation by PMA/ionomycin (gating strategy in Fig 6A), a known strong stimulator of human peripheral blood CD56+CD3+ lymphocytes [39]. Compared to NK cells, the frequency of IFN-γ- producing CD56+CD3+ lymphocytes was higher within population. Of 14 cases of genotype 1 infection enrolled in this experiment, higher NKG2D expression on CD56+CD3+ lymphocytes significantly correlated with higher IFN-γ production (Fig 6B; correlation coefficient, 0.89; P < 0.0001). However, NKG2D expression on CD56+CD3- NK cells showed no significant correlation with IFN-γ production.
Fig 6. Impaired NKG2D expression on CD56+CD3+ lymphocytes correlates to decreased IFN-γ production.
PBMC were collected and analyzed for surface NKG2D expression by flow cytometry in fourteen chronically HCV genotype 1 infected cases. With the same blood sample and at the same time, 4 hours of PMA/ionomycin stimulations were introduced, and then intracellular IFN-γ production levels were determined in CD56+CD3+ (NKT), CD56+CD3- (NK) and CD56-CD3+ (T lymphocyte) populations. (A) Two representative FACS plots and histograms are from the case of SVR (upper) and non-SVR (lower). CD56+ cells are shown as red dots, and other cells as gray dots. n = 14. Statistical correlation of corresponding NKG2D expression and IFN-i production was analyzed in (B).
Discussion
The current study demonstrates that NKG2D expression on CD56+CD3+ lymphocytes, rather than on CD56+CD3− NK cells, is significantly reduced in chronic HCV genotype 1 infection. NKG2D expression on CD56+CD3+ lymphocytes correlates significantly with HCV viral kinetics in the early phase of PEG-IFN/ ribavirin therapy, and predicts treatment responses.
Intrahepatic NKT cells, defined by lymphocytes having both T cell and NK cell surface markers, from HCV-infected individuals showed dysregulated expression of killer immunoglobulin-like receptors [40]. Golden-Mason at al. indicated that inhibitory NKG2A is upregulated in CD56+CD3+ lymphocytes in acute HCV infection, and showed a positive correlation of inhibitory NKG2A expression on CD56+CD3+ lymphocytes to HCV viral load from month 0 and month 6 after infection [41]. Werner et al. showed increased NKG2D expression on peripheral CD1d-restricted NKT cells as part of the innate immune response to acute HCV exposure in healthcare workers who did not develop acute hepatitis [42]. Our current study reveals a not-yet-told correlation of genotype-associated chronicity of HCV persistence with NKG2D expression on CD56+CD3+ lymphocytes. This is the first to specify a single group of immune cells influenced by HCV genotype difference, and may provide an explanation to the natural history of HCV infection.
We also emphasized that the CD56+CD3+ lymphocyte cell subpopulation may also influence treatment response. CD8+ NKT cells exist in humans but are rare in mice [43]. In a previous study, Lin et al. revealed that human peripheral blood CD8+CD56+CD3+ lymphocytes from healthy donors may up-regulate cytotoxic-related surface antigens such as CD16 and NKG2D upon stimulation [44]. Kuylenstierna et al. showed that human peripheral NKT cells express NKG2D almost exclusively on the CD4 negative counterpart, and NKG2D-expressing CD4 negative NKT cells can be activated through NKG2D in a CD1d-independent fashion [11]. On the other hand, it has also been noticed that conventional CD8+ T cells with variant TCRs express NK cell markers upon activation, especially during chronic viral infections such as HIV or CMV [12]. Altogether, though the origin of NKG2D-expessing CD8+CD56+CD3+ lymphocytes might of some dispute, their crucial role in chronic HCV infection is evident for the first time by the current study.
Regulation of NKG2D expression might be multifactorial and both influenced by the virus and the host. HCV core amino acid mutation at aa70 or aa91, a non-double wild type, is known to be predictive to treatment failure in genotype 1 infection [45]. In this study, NKG2D expression on CD56+CD3+ lymphocytes is significantly decreased in non-double wild type cases (Fig 3). We hypothesized that the HCV with different structure proteins may play roles in reduction of NKG2D expression, and this hypothesis was supported by the result of co-incubation of different HCV strains (Fig 4). The co-incubation design has limitations that both direct interaction and humeral factors contained in the supernatants could be possible. HCV infection has been reported to inhibit NK cell function through interaction with CD81 [46, 47] or by the imbalance of cytokines [21]. On the other hand, although we emphasized the genotype-associated difference in NKG2D expression, we also noticed a differential expression of NKG2D within HCV genotype 1 (Fig 1D). Our result didn’t support significant correlation with IL28B polymorphisms, however, we cannot rule out the possibility of correlation to other host factors, such as IFNL4 polymorphisms, which has been reported by Jouvin-Marche et al. about the correlation of favorable genotype with higher early HCV viral load reduction during PEG-IFN/ ribavirin treatment [48]. Recently, Fernandez-Sanchez et al. showed epigenetic modification of histone acetylation determines NKG2D gene expression in NK cells and CTLs in vitro [49], and Quan et al. revealed that HCV core protein may influence this process [50]. Sato et al. showed that inhibition of histone deacetylase suppresses HCV replication [51]. López-Rodríguez et al. found that histone deacetylase polymorphism helps predict IFN-based HCV treatment response [52]. Thus, epigenetic modification of NKG2D expression by HCV proteins may be one possible mechanism. Whether these viral or host factors work on CD56+CD3+ lymphocytes is still unknown, and needs to be further studied.
Type I interferon response are impaired in chronic HCV infection [2, 4], however, exogenous administration of interferon-α during treatment may help eradicate HCV through enforcement of CD56+CD3+ lymphocyte function. Though we observed changes of NKG2D expression in CD56+CD3+ lymphocytes during and after PEG-IFN/ ribavirin treatment (unpublished data), the mechanism how exogenous interferon-α work on NKG2D expression still needs further investigation.
In conclusion, Decreased NKG2D expression on CD56+CD3+ lymphocytes in chronic HCV genotype 1 infection predicts inferior treatment response to PEG-IFN/ribavirin therapy compared to genotype 2. Treatment of chronic hepatitis C progresses rapidly, and IFN-free therapies are becoming new standards in clinical settings. However, analysis of NKG2D expression on peripheral CD56+CD3+ lymphocytes may provide better stratification of treatable patients with genotype 1 infection who may not be suitable for direct-antiviral agents. Furthermore, the better understanding of the mechanisms how HCV interacts with host immunity may provide some hints for possible treatment to HCV infections and its related complications.
Supporting Information
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by grants-in-aid for Scientific Research from the Japan Ministry of Education, Culture, Sports, Science and Technology and Keio Gijuku Academic Development Funds. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. The Journal of experimental medicine. 2005;201(11):1709–14. Epub 2005/06/09. 10.1084/jem.20050808 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosen HR. Clinical practice. Chronic hepatitis C infection. The New England journal of medicine. 2011;364(25):2429–38. Epub 2011/06/24. 10.1056/NEJMcp1006613 PubMed . [DOI] [PubMed] [Google Scholar]
- 3. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31. Epub 2008/06/20. 10.1002/hep.22375 PubMed . [DOI] [PubMed] [Google Scholar]
- 4. Rosen HR. Emerging concepts in immunity to hepatitis C virus infection. The Journal of clinical investigation. 2013;123(10):4121–30. Epub 2013/10/03. 10.1172/JCI67714 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. Journal of virology. 2008;82(10):5043–53. Epub 2008/03/14. 10.1128/JVI.01548-07 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134(7):1927–37, 37 e1-2. Epub 2008/06/14. 10.1053/j.gastro.2008.02.033 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature reviews Immunology. 2003;3(10):781–90. Epub 2003/10/03. 10.1038/nri1199 PubMed . [DOI] [PubMed] [Google Scholar]
- 8. Yamagiwa S, Kamimura H, Ichida T. Natural killer cell receptors and their ligands in liver diseases. Medical molecular morphology. 2009;42(1):1–8. Epub 2009/03/19. 10.1007/s00795-008-0434-7 PubMed . [DOI] [PubMed] [Google Scholar]
- 9. Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunological reviews. 2010;235(1):267–85. Epub 2010/06/12. 10.1111/j.0105-2896.2010.00893.x PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad A, Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. Journal of leukocyte biology. 2004;76(4):743–59. Epub 2004/06/26. 10.1189/jlb.0304197 PubMed . [DOI] [PubMed] [Google Scholar]
- 11. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. European journal of immunology. 2011;41(7):1913–23. Epub 2011/05/19. 10.1002/eji.200940278 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+ T cells. Current opinion in immunology. 2001;13(4):465–70. Epub 2001/08/11. PubMed . [DOI] [PubMed] [Google Scholar]
- 13. Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. The Journal of experimental medicine. 2005;202(12):1623–6. Epub 2005/12/21. 10.1084/jem.20051600 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163(4):2314–21. Epub 1999/08/10. PubMed . [PubMed] [Google Scholar]
- 15. Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunological reviews. 2000;174:5–20. Epub 2000/05/12. PubMed . [DOI] [PubMed] [Google Scholar]
- 16. Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Human immunology. 1999;60(1):20–31. Epub 1999/02/10. PubMed . [DOI] [PubMed] [Google Scholar]
- 17. Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, et al. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168(4):1519–23. Epub 2002/02/02. PubMed . [DOI] [PubMed] [Google Scholar]
- 18. Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3294–8. Epub 2001/03/15. 10.1073/pnas.051604498 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annual review of immunology. 2005;23:877–900. Epub 2005/03/18. 10.1146/annurev.immunol.23.021704.115742 PubMed . [DOI] [PubMed] [Google Scholar]
- 20. Alter G, Jost S, Rihn S, Reyor LL, Nolan BE, Ghebremichael M, et al. Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. Journal of hepatology. 2011;55(2):278–88. Epub 2010/12/21. 10.1016/j.jhep.2010.11.030 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sene D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS pathogens. 2010;6(11):e1001184 Epub 2010/11/19. 10.1371/journal.ppat.1001184 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hadziyannis SJ, Sette H Jr., Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Annals of internal medicine. 2004;140(5):346–55. Epub 2004/03/05. PubMed . [DOI] [PubMed] [Google Scholar]
- 23. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. The New England journal of medicine. 2002;347(13):975–82. Epub 2002/09/27. 10.1056/NEJMoa020047 PubMed . [DOI] [PubMed] [Google Scholar]
- 24. Chayama K, Hayes CN. Hepatitis C virus: How genetic variability affects pathobiology of disease. Journal of gastroenterology and hepatology. 2011;26 Suppl 1:83–95. Epub 2011/01/14. 10.1111/j.1440-1746.2010.06550.x PubMed . [DOI] [PubMed] [Google Scholar]
- 25. Kumada H, Okanoue T, Onji M, Moriwaki H, Izumi N, Tanaka E, et al. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatology research: the official journal of the Japan Society of Hepatology. 2010;40(1):8–13. Epub 2010/02/17. 10.1111/j.1872-034X.2009.00634.x PubMed . [DOI] [PubMed] [Google Scholar]
- 26. Tsukiyama-Kohara K, Kohara M, Yamaguchi K, Maki N, Toyoshima A, Miki K, et al. A second group of hepatitis C viruses. Virus genes. 1991;5(3):243–54. Epub 1991/07/01. PubMed . [DOI] [PubMed] [Google Scholar]
- 27. Simmonds P, Smith DB, McOmish F, Yap PL, Kolberg J, Urdea MS, et al. Identification of genotypes of hepatitis C virus by sequence comparisons in the core, E1 and NS-5 regions. The Journal of general virology. 1994;75 (Pt 5):1053–61. Epub 1994/05/01. PubMed . [DOI] [PubMed] [Google Scholar]
- 28. Toyoda H, Kumada T, Takaguchi K, Shimada N, Tanaka J. Changes in hepatitis C virus genotype distribution in Japan. Epidemiology and infection. 2014:1–5. Epub 2014/03/07. 10.1017/S0950268814000478 PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nature genetics. 2009;41(10):1105–9. Epub 2009/09/15. 10.1038/ng.449 PubMed . [DOI] [PubMed] [Google Scholar]
- 30. Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, et al. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139(2):499–509. Epub 2010/05/04. 10.1053/j.gastro.2010.04.049 PubMed . [DOI] [PubMed] [Google Scholar]
- 31. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. Epub 2009/08/18. 10.1038/nature08309 PubMed . [DOI] [PubMed] [Google Scholar]
- 32. Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, et al. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. The Journal of clinical investigation. 1995;96(1):224–30. Epub 1995/07/01. 10.1172/JCI118025 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Someya T, et al. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon-ribavirin combination therapy. Intervirology. 2005;48(6):372–80. Epub 2005/07/19. 10.1159/000086064 PubMed . [DOI] [PubMed] [Google Scholar]
- 34. Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature medicine. 2005;11(7):791–6. Epub 2005/06/14. 10.1038/nm1268 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sugiyama K, Suzuki K, Nakazawa T, Funami K, Hishiki T, Ogawa K, et al. Genetic analysis of hepatitis C virus with defective genome and its infectivity in vitro. Journal of virology. 2009;83(13):6922–8. Epub 2009/04/17. 10.1128/JVI.02674-08 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurosaki M, Enomoto N, Murakami T, Sakuma I, Asahina Y, Yamamoto C, et al. Analysis of genotypes and amino acid residues 2209 to 2248 of the NS5A region of hepatitis C virus in relation to the response to interferon-beta therapy. Hepatology. 1997;25(3):750–3. Epub 1997/03/01. 10.1002/hep.510250343 PubMed . [DOI] [PubMed] [Google Scholar]
- 37. Ye L, Wang X, Wang S, Wang Y, Song L, Hou W, et al. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology. 2009;49(3):753–62. Epub 2008/12/17. 10.1002/hep.22715 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly-Rogers J, Madrigal-Estebas L, O'Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Human immunology. 2006;67(11):863–73. Epub 2006/12/06. 10.1016/j.humimm.2006.08.292 PubMed . [DOI] [PubMed] [Google Scholar]
- 39. Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. The Journal of experimental medicine. 2002;195(5):625–36. Epub 2002/03/06. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szabo G, Chang S, Dolganiuc A. Altered innate immunity in chronic hepatitis C infection: cause or effect? Hepatology. 2007;46(4):1279–90. Epub 2007/09/22. 10.1002/hep.21938 PubMed . [DOI] [PubMed] [Google Scholar]
- 41. Golden-Mason L, Castelblanco N, O'Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. Journal of virology. 2007;81(17):9292–8. Epub 2007/06/08. 10.1128/JVI.00834-07 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Werner JM, Heller T, Gordon AM, Sheets A, Sherker AH, Kessler E, et al. Innate immune responses in hepatitis C virus exposed healthcare workers who do not develop acute infection. Hepatology. 2013. Epub 2013/03/07. 10.1002/hep.26353 PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Godfrey DI, Berzins SP. Control points in NKT-cell development. Nature reviews Immunology. 2007;7(7):505–18. Epub 2007/06/26. 10.1038/nri2116 PubMed . [DOI] [PubMed] [Google Scholar]
- 44. Lin H, Nieda M, Hutton JF, Rozenkov V, Nicol AJ. Comparative gene expression analysis of NKT cell subpopulations. Journal of leukocyte biology. 2006;80(1):164–73. Epub 2006/05/16. 10.1189/jlb.0705421 PubMed . [DOI] [PubMed] [Google Scholar]
- 45. Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, et al. Predictors of viral kinetics to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b. Journal of medical virology. 2007;79(11):1686–95. Epub 2007/09/15. 10.1002/jmv.20979 PubMed . [DOI] [PubMed] [Google Scholar]
- 46. Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. The Journal of experimental medicine. 2002;195(1):43–9. Epub 2002/01/10. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49(1):12–21. 10.1002/hep.22624 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jouvin-Marche E, Macek Jilkova Z, Thelu MA, Marche H, Fugier E, Van Campenhout N, et al. Lymphocytes degranulation in liver in hepatitis C virus carriers is associated with IFNL4 polymorphisms and ALT levels. The Journal of infectious diseases. 2014;209(12):1907–15. Epub 2014/01/15. 10.1093/infdis/jiu016 PubMed . [DOI] [PubMed] [Google Scholar]
- 49. Fernandez-Sanchez A, Baragano Raneros A, Carvajal Palao R, Sanz AB, Ortiz A, Ortega F, et al. DNA demethylation and histone H3K9 acetylation determine the active transcription of the NKG2D gene in human CD8+ T and NK cells. Epigenetics: official journal of the DNA Methylation Society. 2013;8(1):66–78. 10.4161/epi.23115 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition. Oncogene. 2014;33(22):2826–35. 10.1038/onc.2013.225 PubMed . [DOI] [PubMed] [Google Scholar]
- 51. Sato A, Saito Y, Sugiyama K, Sakasegawa N, Muramatsu T, Fukuda S, et al. Suppressive effect of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) on hepatitis C virus replication. Journal of cellular biochemistry. 2013;114(9):1987–96. 10.1002/jcb.24541 PubMed . [DOI] [PubMed] [Google Scholar]
- 52. Lopez-Rodriguez R, Hernandez-Bartolome A, Borque MJ, Rodriguez-Munoz Y, Martin-Vilchez S, Trapero-Marugan M, et al. Polymorphisms in histone deacetylases improve the predictive value of IL-28B for chronic hepatitis C therapy. Genes and immunity. 2013;14(5):317–24. 10.1038/gene.2013.24 PubMed . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.