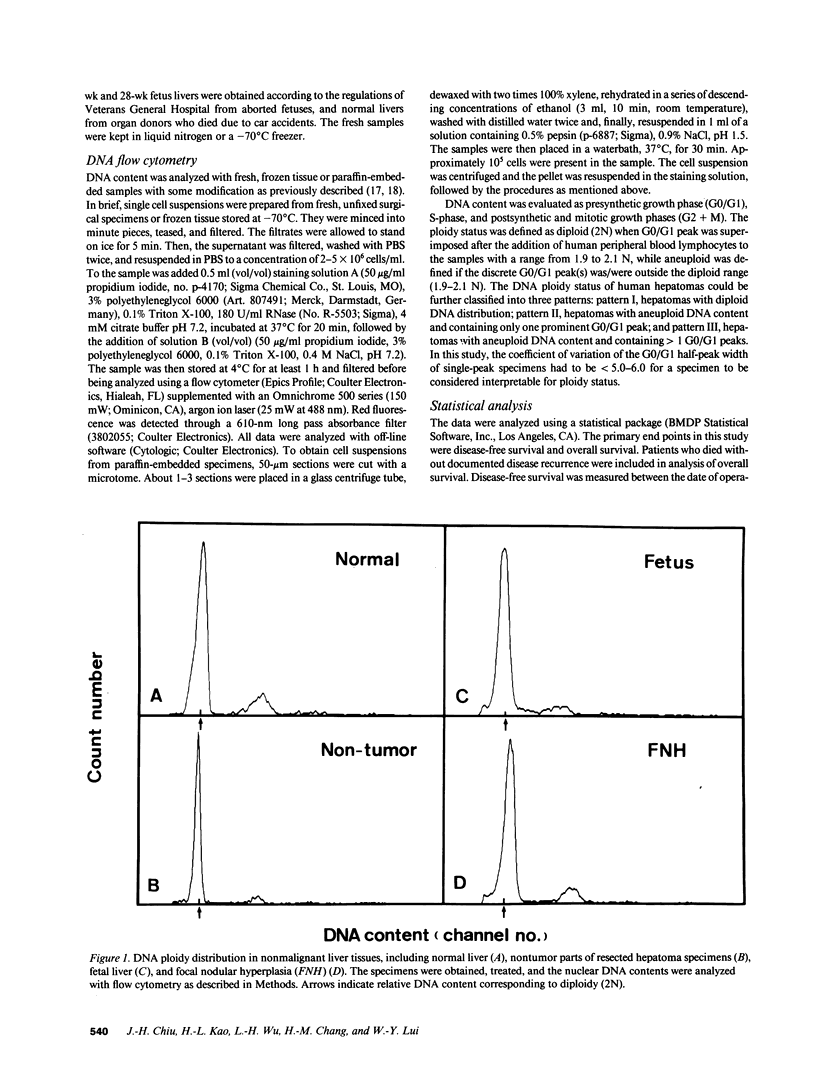
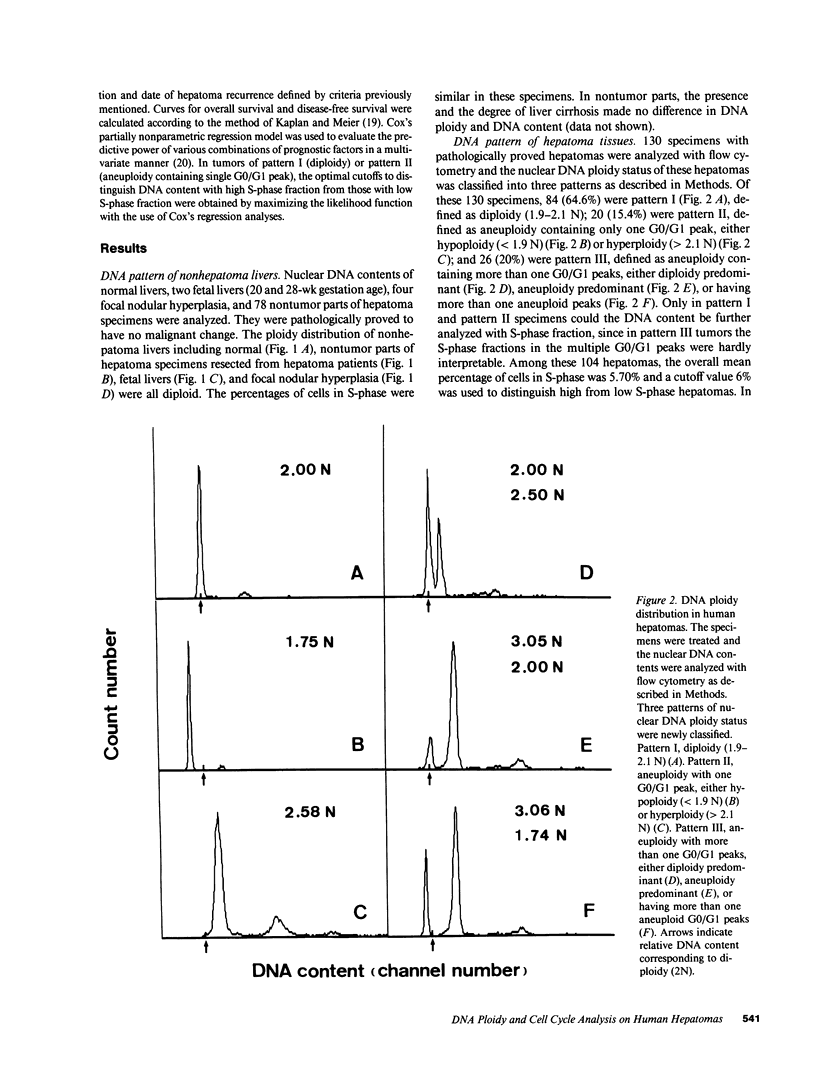
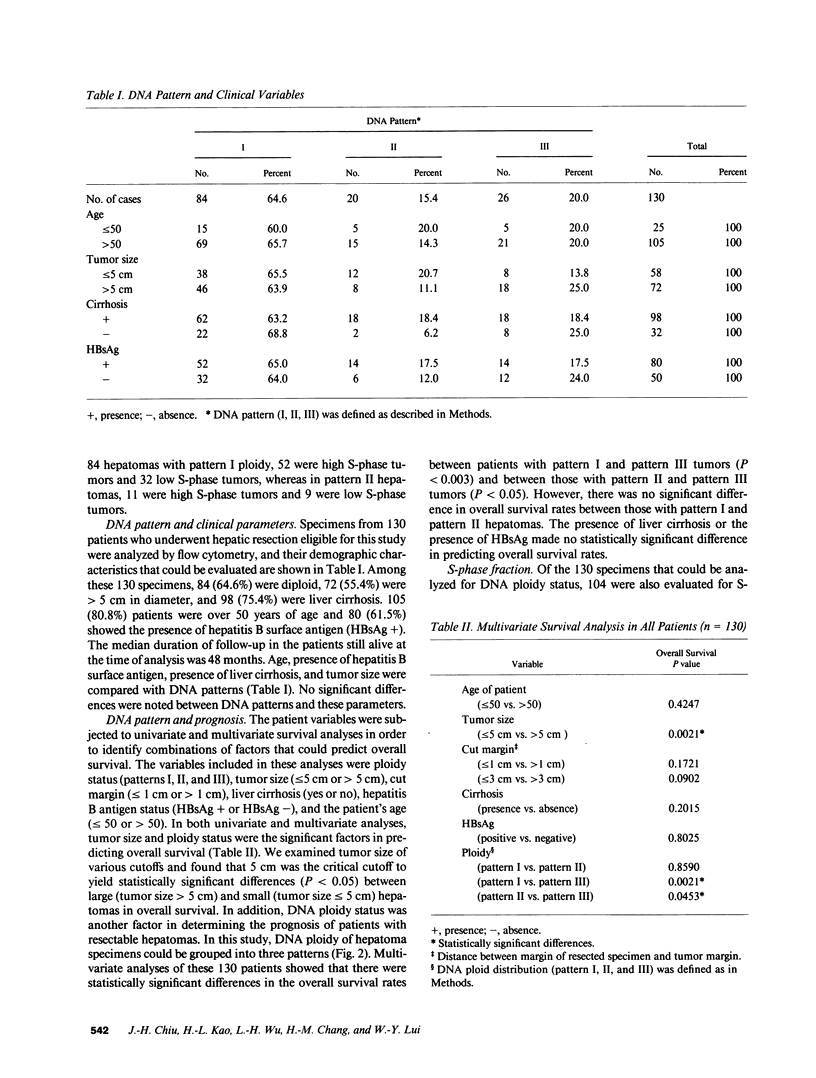
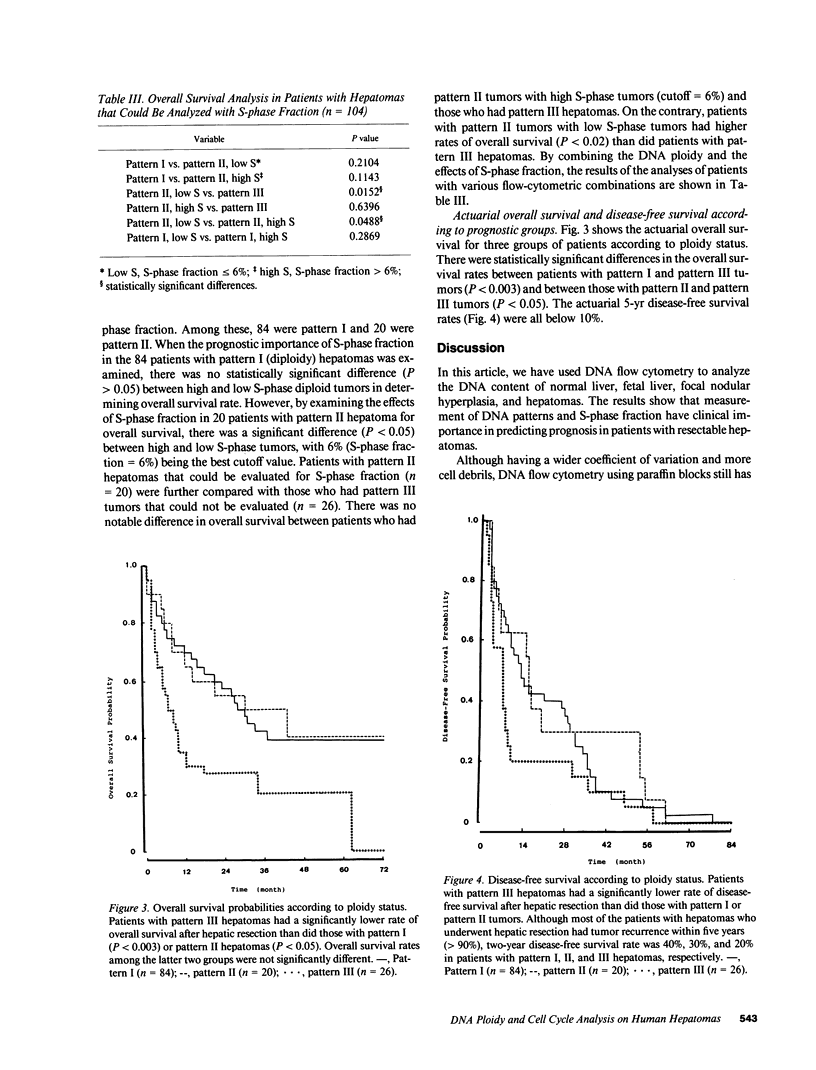
Abstract
To investigate the change of DNA content and the effect of synthetic phase (S-phase) fraction on hepatocytes and hepatomas, DNA content and S-phase fraction were measured by flow cytometry in human livers and hepatoma tissues. The ploidy status of nontumor parts of resected hepatoma, fetal liver, and focal nodular hyperplasia were diploid, similar to that of the normal liver. Three patterns of DNA ploidy in human hepatoma cells were newly classified, namely, pattern I, diploid tumors; pattern II, aneuploid tumors with single G0/G1 peak; and pattern III, aneuploid tumors with more than one G0/G1 peaks. Among the 130 resectable hepatomas measured for DNA ploidy status, 84 (64.6%) were pattern I, 20 (15.4%) pattern II, and 26 (20%) pattern III. Multivariate analyses for those 130 patients who underwent hepatic resection showed that, in addition to tumor size, DNA ploidy was another prognostic factor in predicting overall survival and disease-free survival. Patients with small tumors (less than 5 cm) had a significantly higher overall survival rate than those with large tumor (greater than 5 cm). Patients with pattern III hepatomas had a significantly lower overall survival rate and a higher recurrent rate than did those with pattern I or pattern II tumors. The S-phase fraction was a significant predictor of overall survival rate in patients with pattern II, but not with pattern I, tumors. We conclude that DNA flow-cytometric measurements of ploidy and S-phase fraction are potential important prognostic predictors in patients with resectable hepatomas.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonadonna G., Valagussa P., Tancini G., Rossi A., Brambilla C., Zambetti M., Bignami P., Di Fronzo G., Silvestrini R. Current status of Milan adjuvant chemotherapy trials for node-positive and node-negative breast cancer. NCI Monogr. 1986;(1):45–49. [PubMed] [Google Scholar]
- Clark G. M., Dressler L. G., Owens M. A., Pounds G., Oldaker T., McGuire W. L. Prediction of relapse or survival in patients with node-negative breast cancer by DNA flow cytometry. N Engl J Med. 1989 Mar 9;320(10):627–633. doi: 10.1056/NEJM198903093201003. [DOI] [PubMed] [Google Scholar]
- Coon J. S., Landay A. L., Weinstein R. S. Flow cytometric analysis of paraffin-embedded tumors: implications for diagnostic pathology. Hum Pathol. 1986 May;17(5):435–437. doi: 10.1016/s0046-8177(86)80029-6. [DOI] [PubMed] [Google Scholar]
- Deleener A., Castelain P., Preat V., de Gerlache J., Alexandre H., Kirsch-Volders M. Changes in nucleolar transcriptional activity and nuclear DNA content during the first steps of rat hepatocarcinogenesis. Carcinogenesis. 1987 Feb;8(2):195–201. doi: 10.1093/carcin/8.2.195. [DOI] [PubMed] [Google Scholar]
- Dressler L. G., Seamer L., Owens M. A., Clark G. M., McGuire W. L. Evaluation of a modeling system for S-phase estimation in breast cancer by flow cytometry. Cancer Res. 1987 Oct 15;47(20):5294–5302. [PubMed] [Google Scholar]
- Ewers S. B., Långström E., Baldetorp B., Killander D. Flow-cytometric DNA analysis in primary breast carcinomas and clinicopathological correlations. Cytometry. 1984 Jul;5(4):408–419. doi: 10.1002/cyto.990050419. [DOI] [PubMed] [Google Scholar]
- Ezaki T., Kanematsu T., Okamura T., Sonoda T., Sugimachi K. DNA analysis of hepatocellular carcinoma and clinicopathologic implications. Cancer. 1988 Jan 1;61(1):106–109. doi: 10.1002/1097-0142(19880101)61:1<106::aid-cncr2820610118>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W. Clinical and biological significance of aneuploidy in human tumours. J Clin Pathol. 1984 Sep;37(9):961–974. doi: 10.1136/jcp.37.9.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT D. D. Primary liver-cell carcinoma with a protracted clinical course. Report of a case. JAMA. 1963 Apr 13;184:146–148. doi: 10.1001/jama.1963.73700150014022. [DOI] [PubMed] [Google Scholar]
- Hedley D. W. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry. 1989 May;10(3):229–241. doi: 10.1002/cyto.990100302. [DOI] [PubMed] [Google Scholar]
- Mauro F., Teodori L., Schumann J., Göhde W. Flow cytometry as a tool for the prognostic assessment of human neoplasia. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):625–636. doi: 10.1016/0360-3016(86)90072-6. [DOI] [PubMed] [Google Scholar]
- Mori H., Tanaka T., Sugie S., Takahashi M., Williams G. M. DNA content of liver cell nuclei of N-2-fluorenylacetamide-induced altered foci and neoplasms in rats and human hyperplastic foci. J Natl Cancer Inst. 1982 Dec;69(6):1277–1282. [PubMed] [Google Scholar]
- Saeter G., Lee C. Z., Schwarze P. E., Ous S., Chen D. S., Sung J. L., Seglen P. O. Changes in ploidy distributions in human liver carcinogenesis. J Natl Cancer Inst. 1988 Nov 16;80(18):1480–1485. doi: 10.1093/jnci/80.18.1480. [DOI] [PubMed] [Google Scholar]
- Saeter G., Schwarze P. E., Nesland J. M., Juul N., Pettersen E. O., Seglen P. O. The polyploidizing growth pattern of normal rat liver is replaced by divisional, diploid growth in hepatocellular nodules and carcinomas. Carcinogenesis. 1988 Jun;9(6):939–945. doi: 10.1093/carcin/9.6.939. [DOI] [PubMed] [Google Scholar]
- Sarafoff M., Rabes H. M., Dörmer P. Correlations between ploidy and initiation probability determined by DNA cytophotometry in individual altered hepatic foci. Carcinogenesis. 1986 Jul;7(7):1191–1196. doi: 10.1093/carcin/7.7.1191. [DOI] [PubMed] [Google Scholar]
- Schwarze P. E., Pettersen E. O., Shoaib M. C., Seglen P. O. Emergence of a population of small, diploid hepatocytes during hepatocarcinogenesis. Carcinogenesis. 1984 Oct;5(10):1267–1275. doi: 10.1093/carcin/5.10.1267. [DOI] [PubMed] [Google Scholar]
- Stephenson R. A., Gay H., Fair W. R., Melamed M. R. Effect of section thickness on quality of flow cytometric DNA content determinations in paraffin-embedded tissues. Cytometry. 1986 Jan;7(1):41–44. doi: 10.1002/cyto.990070106. [DOI] [PubMed] [Google Scholar]
- Watson J. V. Oncogenes, cancer and analytical cytology. Cytometry. 1986 Sep;7(5):400–410. doi: 10.1002/cyto.990070503. [DOI] [PubMed] [Google Scholar]
- Wied G. L., Bartels P. H., Dytch H. E., Bibbo M. Rapid DNA evaluation in clinical diagnosis. Acta Cytol. 1983 Jan-Feb;27(1):33–37. [PubMed] [Google Scholar]
- Yoshida T., Okazaki N., Yoshino M., Kitaoka H., Hirohashi S., Shimozato Y. Minute hepatocellular carcinoma without appreciable change in size for seven years: a case report. Cancer. 1982 Apr 1;49(7):1491–1495. doi: 10.1002/1097-0142(19820401)49:7<1491::aid-cncr2820490730>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Young G. A., Hedley D. W., Rugg C. A., Iland H. J. The prognostic significance of proliferative activity in poor histology non-Hodgkin's lymphoma: a flow cytometry study using archival material. Eur J Cancer Clin Oncol. 1987 Oct;23(10):1497–1504. doi: 10.1016/0277-5379(87)90092-7. [DOI] [PubMed] [Google Scholar]


