Abstract
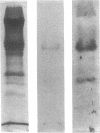
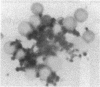
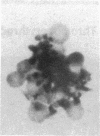
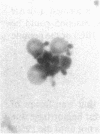
In an attempt to overcome the limitations and drawbacks of using fresh platelets for transfusion therapy of thrombocytopenic patients, we have performed in vitro experiments on an autologous, semi-artificial alternative to platelet transfusions. Based on our previous studies of the interactions of unactivated and activated platelets with beads coated with peptides of various lengths, all of which contained the arginine-glycine-aspartic acid (RGD) cell recognition sequence, the peptide Ac-CGGRGDF-NH2 was chosen for covalent coupling to erythrocytes. A heterobifunctional crosslinking reagent (N-maleimido-6-aminocaproyl ester of 1-hydroxy-2-nitrobenzene-4-sulfonic acid) was used to crosslink via the peptide's free sulfhydryl group and the erythrocyte's surface amino groups. Approximately 0.5-1.5 x 10(6) peptide molecules bound per erythrocyte after 2 h of incubation, and most of the peptides appeared to crosslink to glycophorin A. The resulting cells, termed thromboerythrocytes, interacted selectively with activated platelets to form mixed aggregates. Studies with fluid phase RGD peptides and monoclonal antibodies indicated that the RGD peptides on the thromboerythrocytes interacted with the GPIIb/IIIa receptors on activated platelets. Thromboerythrocytes could also bind to platelets adherent to collagen. There was minimal erythrocyte hemolysis during the formation of thromboerythrocytes and studies of thromboerythrocyte osmotic fragility and cellular deformability showed no significant changes from control erythrocytes. Whereas there is a 20:1 ratio of erythrocytes to platelets in the circulation of normal individuals, the erythrocytes from as little as 50 ml of blood could be transformed into the equivalent of 2 U of platelets by numbers (equivalent to 18 U of platelets by mass), and reinfused into the same individual within several hours. These data encourage us to proceed to in vivo studies to assess the hemostatic efficacy of thromboerythrocytes in thrombocytopenic animals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agam G., Livne A. Passive participation of fixed platelets in aggregation facilitated by covalently bound fibrinogen. Blood. 1983 Jan;61(1):186–191. [PubMed] [Google Scholar]
- Agam G., Livne A. Platelet-platelet recognition during aggregation: distinct mechanisms determined by the release reaction. Thromb Haemost. 1984 Apr 30;51(2):145–149. [PubMed] [Google Scholar]
- Agam G., Livne A. Resolution and reconstitution of interplatelet recognition during aggregation. Thromb Haemost. 1988 Jun 16;59(3):504–506. [PubMed] [Google Scholar]
- Aldwin L., Nitecki D. E. A water-soluble, monitorable peptide and protein crosslinking agent. Anal Biochem. 1987 Aug 1;164(2):494–501. doi: 10.1016/0003-2697(87)90524-0. [DOI] [PubMed] [Google Scholar]
- Anstee D. J. Blood group-active surface molecules of the human red blood cell. Vox Sang. 1990;58(1):1–20. doi: 10.1111/j.1423-0410.1990.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Kahn R. A., Knipp M. A., Newman P. J. Reconstruction of platelet proteins into phospholipid vesicles. Functional proteoliposomes. J Clin Invest. 1985 Jan;75(1):35–39. doi: 10.1172/JCI111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee W. L., Langlois R. G., Vanderlaan M., Jensen R. H. Binding specificities of eight monoclonal antibodies to human glycophorin A--studies with McM, and MkEn(UK) variant human erythrocytes and M- and MNV-type chimpanzee erythrocytes. J Immunol. 1984 Dec;133(6):3149–3155. [PubMed] [Google Scholar]
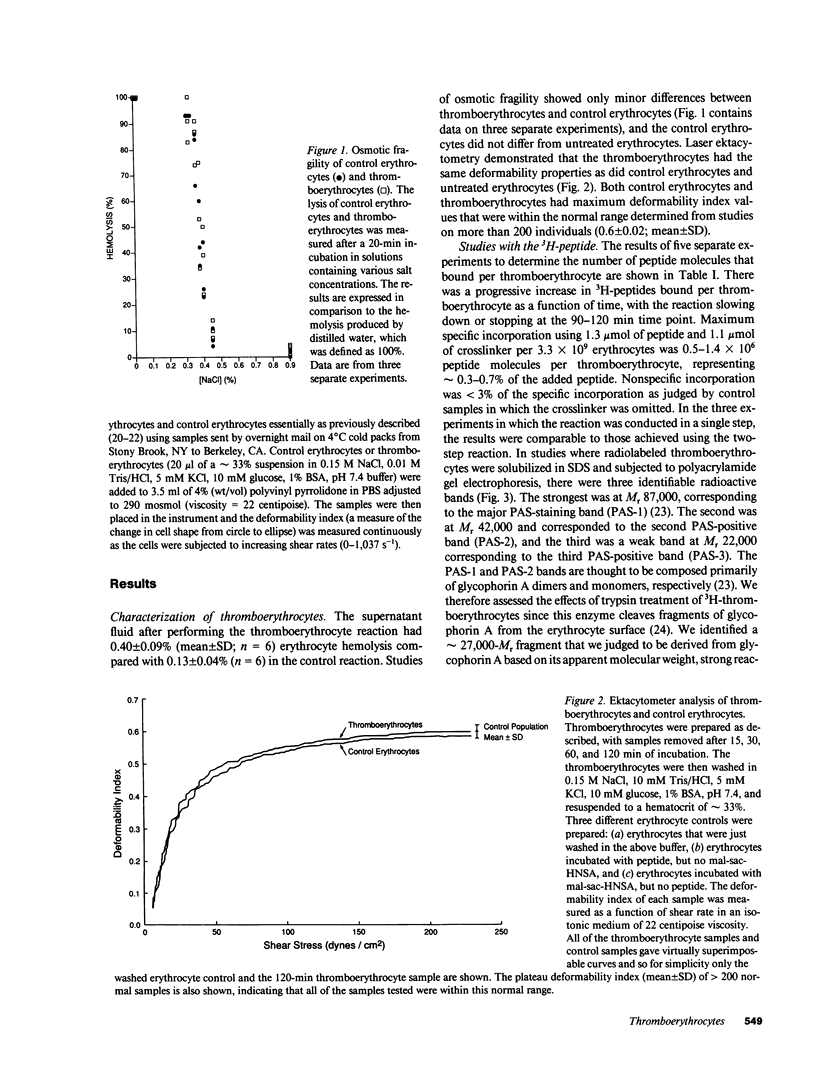
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
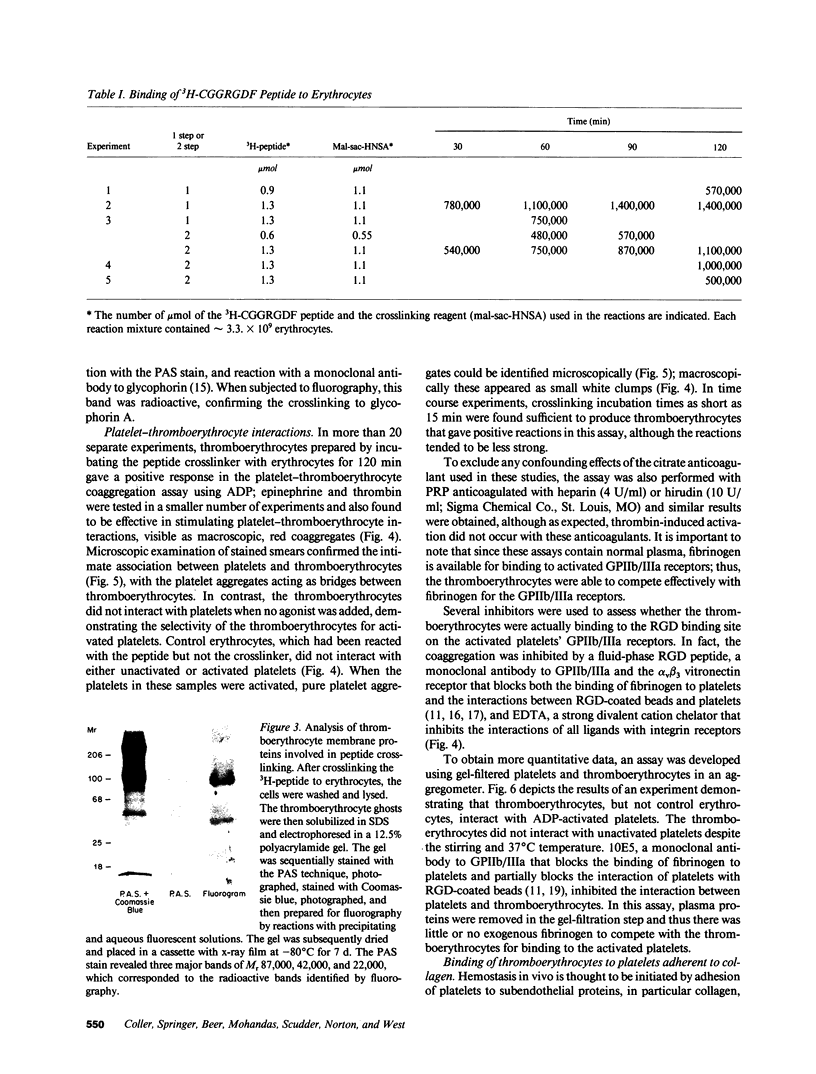
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
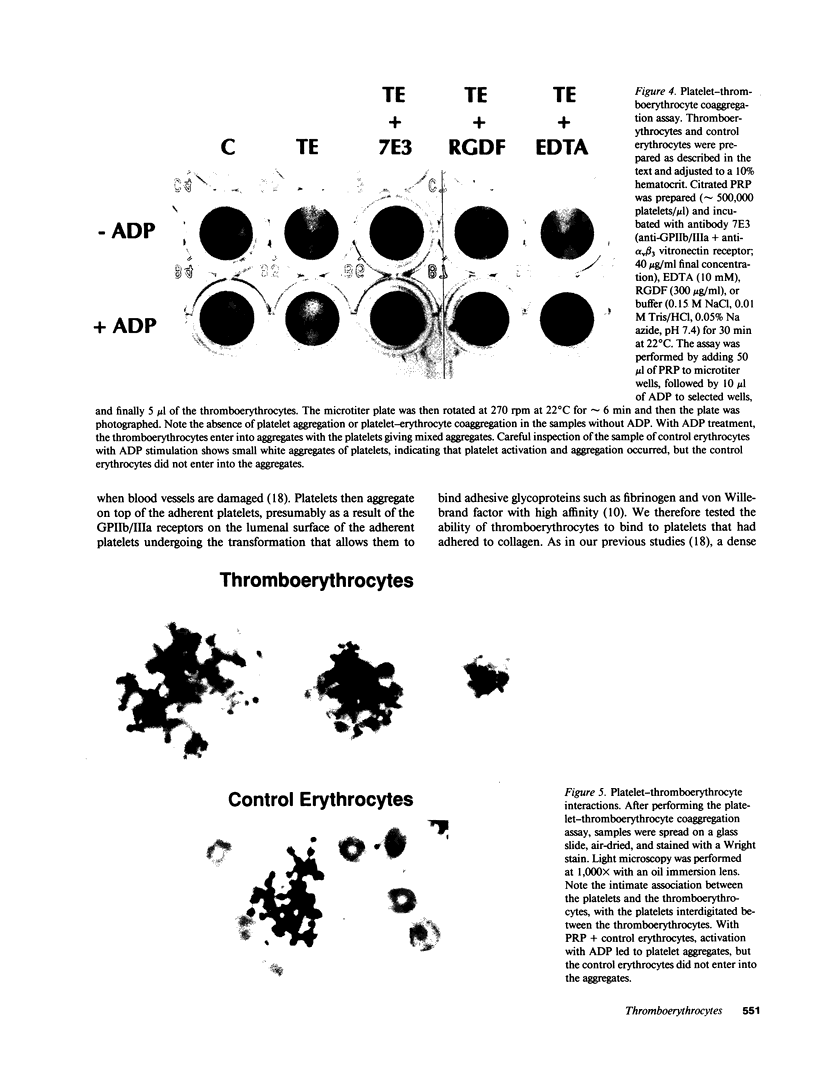
- Coller B. S., Cheresh D. A., Asch E., Seligsohn U. Platelet vitronectin receptor expression differentiates Iraqi-Jewish from Arab patients with Glanzmann thrombasthenia in Israel. Blood. 1991 Jan 1;77(1):75–83. [PubMed] [Google Scholar]
- Coller B. S. Interaction of normal, thrombasthenic, and Bernard-Soulier platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia. Blood. 1980 Feb;55(2):169–178. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Til H. P., de Vrijer F., Woutersen R. A., Cassee F. R., van Bladeren P. J. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res. 1991 Mar-Apr;259(3-4):363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- HJORT P. F., PERMAN V., CRONKITE E. P. Fresh, disintegrated platelets in radiation thrombocytopenia: correction of prothrombin consumption without correction of bleeding. Proc Soc Exp Biol Med. 1959 Oct;102:31–35. doi: 10.3181/00379727-102-25132. [DOI] [PubMed] [Google Scholar]
- Houston D. S., Robinson P., Gerrard J. M. Inhibition of intravascular platelet aggregation by endothelium-derived relaxing factor: reversal by red blood cells. Blood. 1990 Sep 1;76(5):953–958. [PubMed] [Google Scholar]
- JACKSON D. P., SORENSEN D. K., CRONKITE E. P., BOND V. P., FLIEDNER T. M. Effectiveness of transfusions of fresh and lyophilized platelets in controlling bleeding due to thrombocytopenia. J Clin Invest. 1959 Oct;38:1689–1697. doi: 10.1172/JCI103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R. A., Allen R. W., Baldassare J. Alternate sources and substitutes for therapeutic blood components. Blood. 1985 Jul;66(1):1–12. [PubMed] [Google Scholar]
- Kiefel V., Santoso S., Katzmann B., Mueller-Eckhardt C. The Bra/Brb alloantigen system on human platelets. Blood. 1989 Jun;73(8):2219–2223. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J. Platelet GPIIb-IIIa: molecular variations and alloantigens. Thromb Haemost. 1991 Jul 12;66(1):111–118. [PubMed] [Google Scholar]
- Parise L. V., Phillips D. R. Platelet membrane glycoprotein IIb-IIIa complex incorporated into phospholipid vesicles. Preparation and morphology. J Biol Chem. 1985 Feb 10;260(3):1750–1756. [PubMed] [Google Scholar]
- Pasvol G., Chasis J. A., Mohandas N., Anstee D. J., Tanner M. J., Merry A. H. Inhibition of malarial parasite invasion by monoclonal antibodies against glycophorin A correlates with reduction in red cell membrane deformability. Blood. 1989 Oct;74(5):1836–1843. [PubMed] [Google Scholar]
- Roy S. N., Procyk R., Kudryk B. J., Redman C. M. Assembly and secretion of recombinant human fibrinogen. J Biol Chem. 1991 Mar 15;266(8):4758–4763. [PubMed] [Google Scholar]
- Rybak M. E. Glycoproteins IIb and IIIa and platelet thrombospondin in a liposome model of platelet aggregation. Thromb Haemost. 1986 Apr 30;55(2):240–245. [PubMed] [Google Scholar]
- SCHULMAN I., CURRIMBHOY Z., SMITH C. H., ERLANDSON M. E., SCHORR J. B., FORT E., WEHMAN J. Phosphatides as platelet substitutes in blood coagulation. Ann N Y Acad Sci. 1958 Oct 13;75(1):195–202. doi: 10.1111/j.1749-6632.1958.tb36866.x. [DOI] [PubMed] [Google Scholar]
- Santos M. T., Valles J., Marcus A. J., Safier L. B., Broekman M. J., Islam N., Ullman H. L., Eiroa A. M., Aznar J. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991 Feb;87(2):571–580. doi: 10.1172/JCI115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C. A., Aisner J., Wiernik P. H. Frozen autologous platelet transfusion for patients with leukemia. N Engl J Med. 1978 Jul 6;299(1):7–12. doi: 10.1056/NEJM197807062990102. [DOI] [PubMed] [Google Scholar]
- Surgenor D. M., Wallace E. L., Hao S. H., Chapman R. H. Collection and transfusion of blood in the United States, 1982-1988. N Engl J Med. 1990 Jun 7;322(23):1646–1651. doi: 10.1056/NEJM199006073222306. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Dale G. L. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987 Sep;70(3):791–795. [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Bevers E. M., Comfurius P., Rosing J., Tilly R. H., Verhallen P. F. Loss of membrane phospholipid asymmetry during activation of blood platelets and sickled red cells; mechanisms and physiological significance. 1989 Nov 23-Dec 19Mol Cell Biochem. 91(1-2):23–31. doi: 10.1007/BF00228075. [DOI] [PubMed] [Google Scholar]