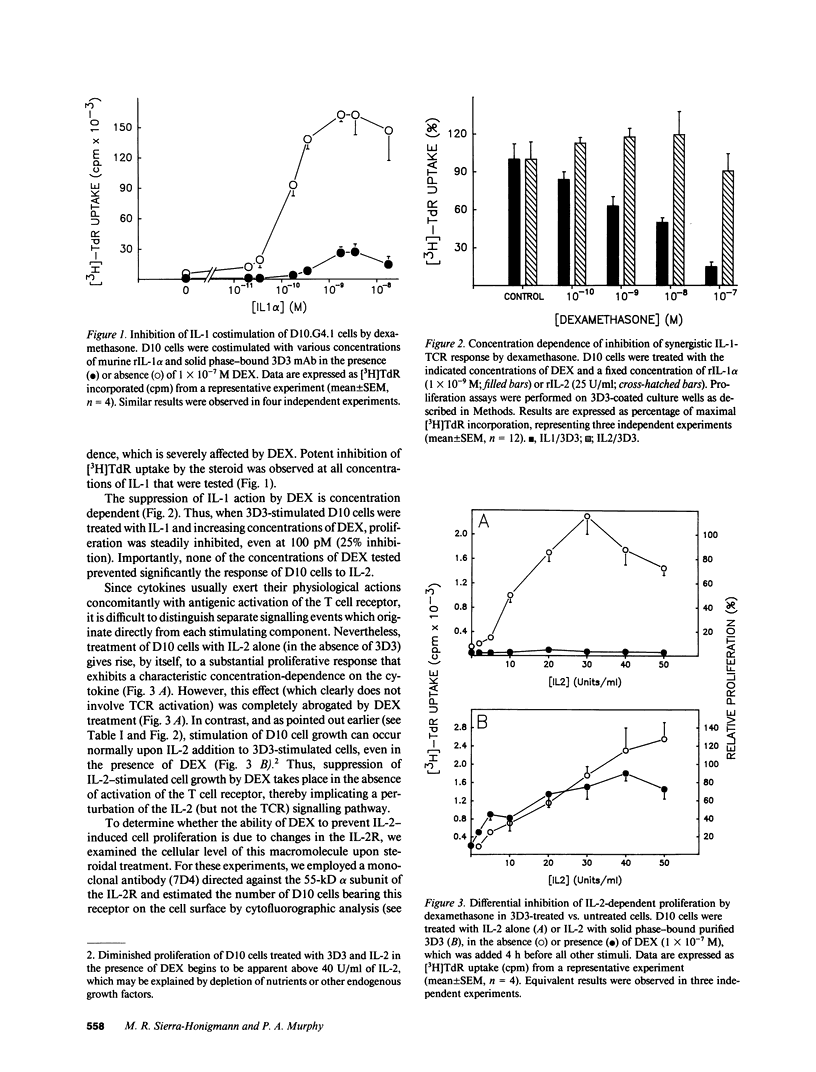
Abstract
The immune and inflammatory responses are largely inhibited by glucocorticosteroids. In thymocytes, for example, glucocorticosteroids cause apoptosis, whereas they suppress the activity of phospholipase A2 and the production of eicosanoids in tissues actively engaged in inflammation. The immunosuppressive action of dexamethasone (DEX) was studied in vitro by employing a model cell system, namely the murine Th2 clone D10.G4.1 (D10) and its clonotypic anti-T cell receptor (TCR) mAb 3D3. Although the proliferative response of D10 cells to 3D3 stimulation was not affected by DEX, the costimulation provided by IL-1 was dramatically inhibited. Substitution of 3D3 by exogenous IL-4 (as the IL-1 costimulant) failed to prevent the inhibition of proliferation caused by DEX. Yet, when 3D3-mediated stimulation of TCR was supplemented with IL-2, D10 cells were capable of proliferating, even in the presence of DEX. Thus, TCR stimulation on D10 cells remained intact and their resulting propagation was not compromised by DEX treatment. These results provide evidence that immunosuppression caused by DEX is TCR independent and involves an early cytokine-signalling event.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Robb R. J., Malek T. R. Proliferation of T lymphocytes in response to interleukin 2 varies with their state of activation. J Immunol. 1986 Oct 15;137(8):2572–2578. [PubMed] [Google Scholar]
- Bertoglio J. H., Leroux E. Differential effects of glucocorticoids on the proliferation of a murine helper and a cytolytic T cell clone in response to IL-2 and IL-4. J Immunol. 1988 Aug 15;141(4):1191–1196. [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. II. The effect on the in vitro generation of cytolytic T cells. J Immunol. 1979 Oct;123(4):1632–1638. [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980 Jun;124(6):2828–2833. [PubMed] [Google Scholar]
- Lew W., Oppenheim J. J., Matsushima K. Analysis of the suppression of IL-1 alpha and IL-1 beta production in human peripheral blood mononuclear adherent cells by a glucocorticoid hormone. J Immunol. 1988 Mar 15;140(6):1895–1902. [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M. Glucocorticoid physiology, pharmacology and stress. Adv Exp Med Biol. 1986;196:81–96. doi: 10.1007/978-1-4684-5101-6_6. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., López-Rivas A. IL-2 protects T lymphocytes from glucocorticoid-induced DNA fragmentation and cell death. J Immunol. 1989 Dec 15;143(12):4166–4170. [PubMed] [Google Scholar]
- Ortega G., Robb R. J., Shevach E. M., Malek T. R. The murine IL 2 receptor. I. Monoclonal antibodies that define distinct functional epitopes on activated T cells and react with activated B cells. J Immunol. 1984 Oct;133(4):1970–1975. [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Schleimer R. P., Jacques A., Shin H. S., Lichtenstein L. M., Plaut M. Inhibition of T cell-mediated cytotoxicity by anti-inflammatory steroids. J Immunol. 1984 Jan;132(1):266–271. [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]


