Abstract
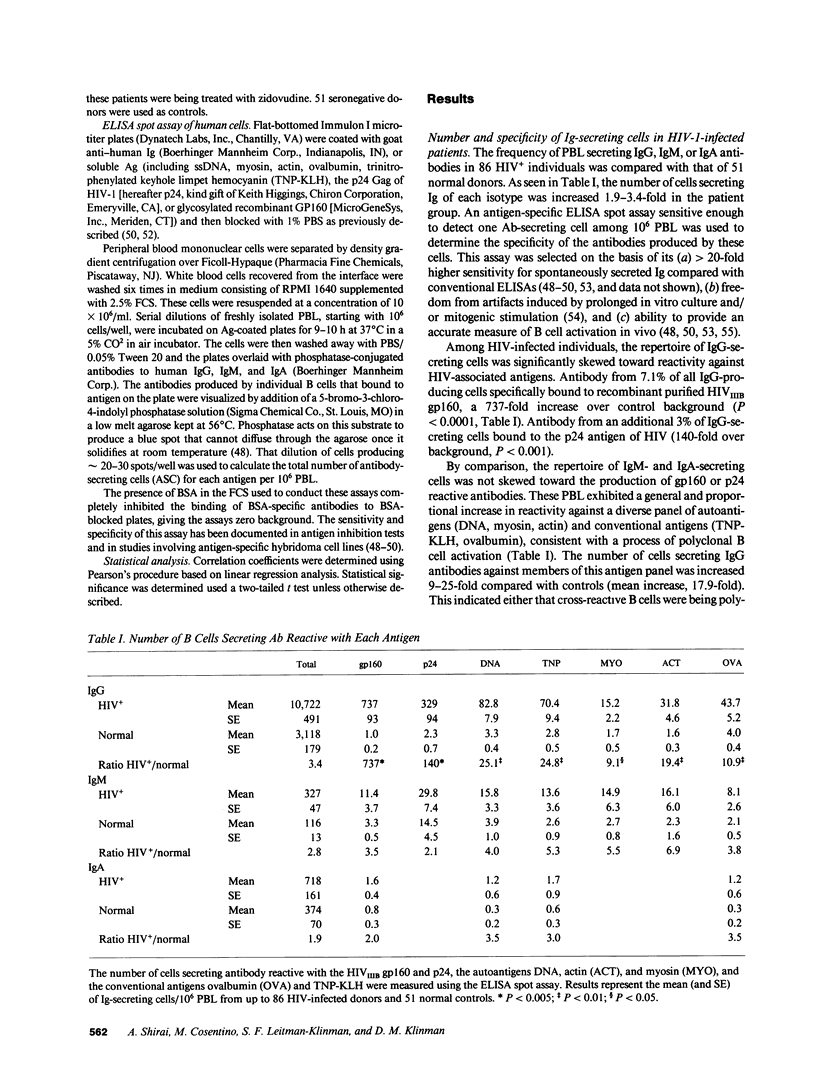
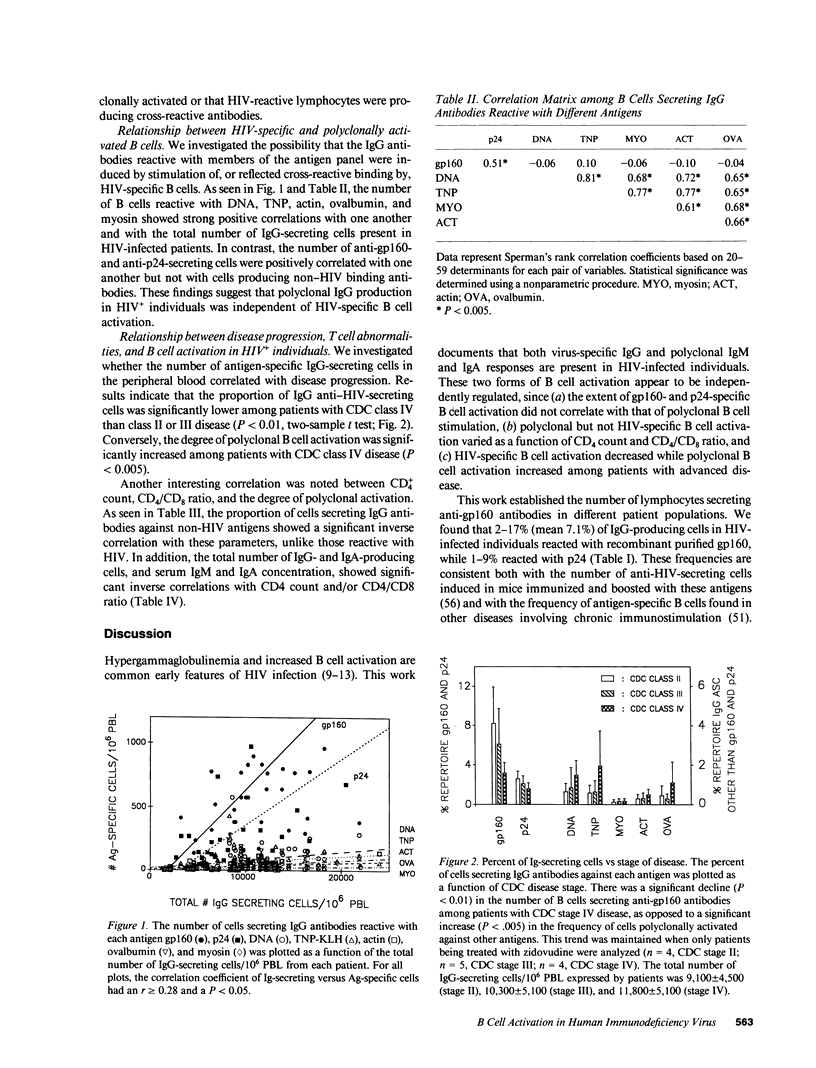
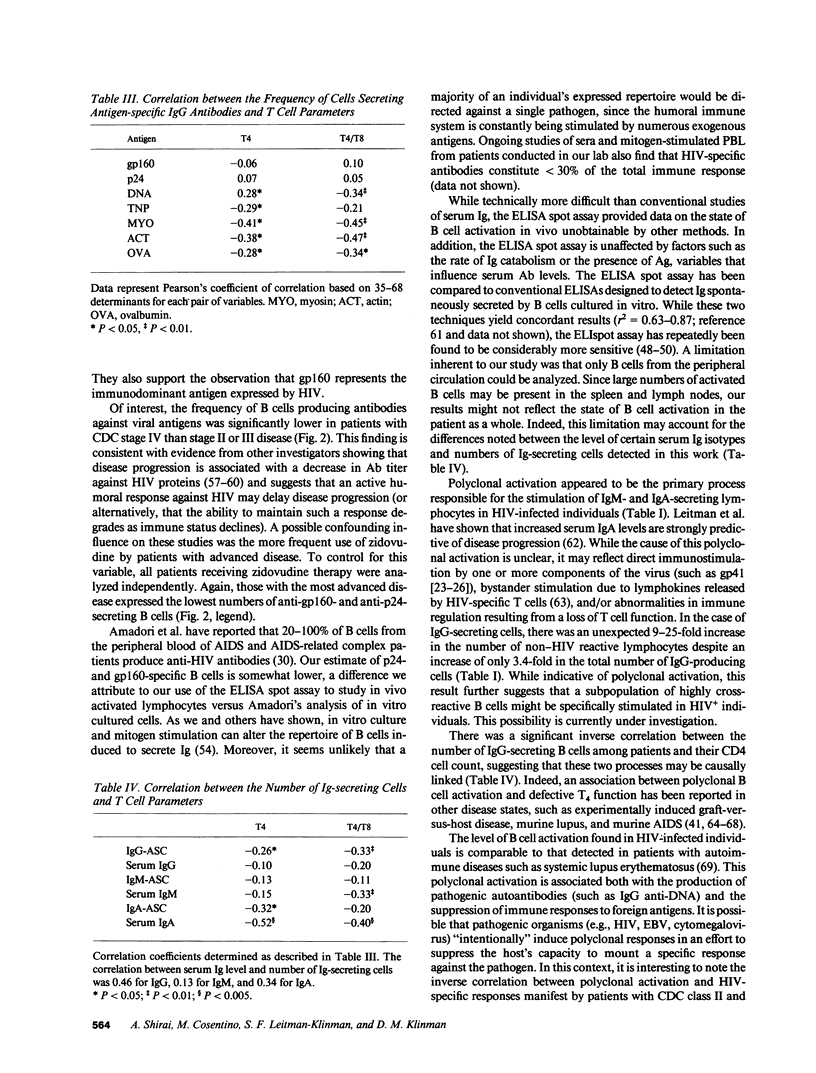
Peripheral blood lymphocytes (PBL) were obtained from HIV-1-infected patients at different stages of disease. The absolute number of IgM-, IgG-, and IgA-producing lymphocytes per 10(6) PBL was increased 2.8-, 3.4-, and 1.9-fold, respectively, compared with normal controls. 2-17% of IgG-secreting patient cells reacted with the gp160 envelope glycoprotein of HIV-1 (a 737-fold increase over background), while 1-9% reacted with p24 (140-fold over background). In addition to this HIV-specific B cell activation, the number of lymphocytes reactive with nonviral antigens such as DNA, myosin, actin, trinitrophenylated keyhole limpet hemocyanin, and ovalbumin was increased by a mean of 17.9-fold. Evidence suggests that the latter changes reflect an HIV-induced polyclonal B cell activation unrelated to the production of anti-HIV antibodies. For example, the proportion of IgG anti-gp160- and anti-p24-secreting lymphocytes declined in patients with advanced disease, whereas the number of B cells producing antibodies to non-HIV antigens rose. Moreover, CD4 cell count and T4/T8 ratio showed a significant inverse correlation with the degree of polyclonal activation but not with anti-HIV responsiveness. These observations demonstrate that both quantitative and qualitative changes in B cell activation accompany (and may be predictive of) disease progression in HIV-infected individuals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Amadori A., Chieco-Bianchi L. B-cell activation and HIV-1 infection: deeds and misdeeds. Immunol Today. 1990 Oct;11(10):374–379. doi: 10.1016/0167-5699(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Amadori A., De Rossi A., Faulkner-Valle G. P., Chieco-Bianchi L. Spontaneous in vitro production of virus-specific antibody by lymphocytes from HIV-infected subjects. Clin Immunol Immunopathol. 1988 Mar;46(3):342–351. doi: 10.1016/0090-1229(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Amadori A., Zamarchi R., Ciminale V., Del Mistro A., Siervo S., Alberti A., Colombatti M., Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989 Oct 1;143(7):2146–2152. [PubMed] [Google Scholar]
- Amadori A., de Rossi A., Giaquinto C., Faulkner-Valle G., Zacchello F., Chieco-Bianchi L. In-vitro production of HIV-specific antibody in children at risk of AIDS. Lancet. 1988 Apr 16;1(8590):852–854. doi: 10.1016/s0140-6736(88)91603-0. [DOI] [PubMed] [Google Scholar]
- Ammann A. J., Schiffman G., Abrams D., Volberding P., Ziegler J., Conant M. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA. 1984 Mar 16;251(11):1447–1449. [PubMed] [Google Scholar]
- Ando D. G., Ebling F. M., Hahn B. H. Detection of native and denatured DNA antibody forming cells by the enzyme-linked immunospot assay. A clinical study of (New Zealand black x New Zealand white)F1 mice. Arthritis Rheum. 1986 Sep;29(9):1139–1146. doi: 10.1002/art.1780290912. [DOI] [PubMed] [Google Scholar]
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Brun-Vezinet F., Rouzioux C., Barre-Sinoussi F., Klatzmann D., Saimot A. G., Rozenbaum W., Christol D., Gluckmann J. C., Montagnier L., Chermann J. C. Detection of IgG antibodies to lymphadenopathy-associated virus in patients with AIDS or lymphadenopathy syndrome. Lancet. 1984 Jun 9;1(8389):1253–1256. doi: 10.1016/s0140-6736(84)92444-9. [DOI] [PubMed] [Google Scholar]
- Chirmule N., Kalyanaraman V. S., Saxinger C., Wong-Staal F., Ghrayeb J., Pahwa S. Localization of B-cell stimulatory activity of HIV-1 to the carboxyl terminus of gp41. AIDS Res Hum Retroviruses. 1990 Mar;6(3):299–305. doi: 10.1089/aid.1990.6.299. [DOI] [PubMed] [Google Scholar]
- Dar O., Salaman M. R., Seifert M. H., Isenberg D. A. Spontaneous antibody-secreting cells against DNA and common environmental antigens in systemic lupus erythematosus. J Autoimmun. 1990 Oct;3(5):523–530. doi: 10.1016/s0896-8411(05)80018-6. [DOI] [PubMed] [Google Scholar]
- Dorsett B., Cronin W., Chuma V., Ioachim H. L. Anti-lymphocyte antibodies in patients with the acquired immune deficiency syndrome. Am J Med. 1985 Apr;78(4):621–626. doi: 10.1016/0002-9343(85)90405-x. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Freitas A. A., Guilbert B., Holmberg D., Wennerström G., Coutinho A., Avrameas S. Analysis of autoantibody reactivities in hybridoma collections derived from normal adult BALB/c mice. Ann Inst Pasteur Immunol. 1986 Jul-Aug;137D(1):33–45. [PubMed] [Google Scholar]
- Friedman S. M., Principato M. A., Thompson G. S., Teichman F. Antigen-specific and polyclonal immunoglobulin production induced by a cloned tetanus toxoid-specific T cell line. J Immunol. 1983 Mar;130(3):1164–1170. [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Grant M. D., Weaver M. S., Tsoukas C., Hoffmann G. W. Distribution of antibodies against denatured collagen in AIDS risk groups and homosexual AIDS patients suggests a link between autoimmunity and the immunopathogenesis of AIDS. J Immunol. 1990 Feb 15;144(4):1241–1250. [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigatsubo Y., Sakamoto H., Hagiwara E., Aoki A., Shirai A., Tani K., Okubo T., Klinman D. M. Quantitation of autoantibody-secreting B cells in systemic lupus erythematosus. Autoimmunity. 1989;5(1-2):71–78. doi: 10.3109/08916938909029144. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Eisenberg R. A., Steinberg A. D. Development of the autoimmune B cell repertoire in MRL-lpr/lpr mice. J Immunol. 1990 Jan 15;144(2):506–511. [PubMed] [Google Scholar]
- Klinman D. M., Higgins K. W., Conover J. Sequential immunizations with rgp120s from independent isolates of human immunodeficiency virus type 1 induce the preferential expansion of broadly crossreactive B cells. J Exp Med. 1991 Apr 1;173(4):881–887. doi: 10.1084/jem.173.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Morse H. C., 3rd Characteristics of B cell proliferation and activation in murine AIDS. J Immunol. 1989 Feb 15;142(4):1144–1149. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Novel ELISA and ELISA-spot assays used to quantitate B cells and serum antibodies specific for T cell and bromelated mouse red blood cell autoantigens. J Immunol Methods. 1987 Sep 24;102(2):157–164. doi: 10.1016/0022-1759(87)90072-x. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa T., Louis J., Izui S., Lambert P. H. Autoimmune response to DNA, red blood cells, and thymocyte antigens in association with polyclonal antibody synthesis during experimental African trypanosomiasis. J Immunol. 1979 Jan;122(1):296–301. [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Laurence J., Brun-Vezinet F., Schutzer S. E., Rouzioux C., Klatzmann D., Barré-Sinoussi F., Chermann J. C., Montagnier L. Lymphadenopathy-associated viral antibody in AIDS. Immune correlations and definition of a carrier state. N Engl J Med. 1984 Nov 15;311(20):1269–1273. doi: 10.1056/NEJM198411153112001. [DOI] [PubMed] [Google Scholar]
- Leitman S. F., Klein H. G., Melpolder J. J., Read E. J., Esteban J. I., Leonard E. M., Harvath L., Shih J. W., Nealon R., Foy J. Clinical implications of positive tests for antibodies to human immunodeficiency virus type 1 in asymptomatic blood donors. N Engl J Med. 1989 Oct 5;321(14):917–924. doi: 10.1056/NEJM198910053211401. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Nicholson J. K., Spira T. J., Jaffe H. W., Kaplan J. E., Fishbein D. B., O'Malley P., Aloisio C. H., Black C. M. Antibody response to human immunodeficiency virus in homosexual men. Relation of antibody specificity, titer, and isotype to clinical status, severity of immunodeficiency, and disease progression. J Clin Invest. 1987 Aug;80(2):316–324. doi: 10.1172/JCI113075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildvan D., Mathur U., Enlow R. W., Romain P. L., Winchester R. J., Colp C., Singman H., Adelsberg B. R., Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Mizuma H., Litwin S., Zolla-Pazner S. B-cell activation in HIV infection: relationship of spontaneous immunoglobulin secretion to various immunological parameters. Clin Exp Immunol. 1988 Mar;71(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Monestier M., Manheimer-Lory A., Bellon B., Painter C., Dang H., Talal N., Zanetti M., Schwartz R., Pisetsky D., Kuppers R. Shared idiotypes and restricted immunoglobulin variable region heavy chain genes characterize murine autoantibodies of various specificities. J Clin Invest. 1986 Sep;78(3):753–759. doi: 10.1172/JCI112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Mizuochi T., Sharrow S. O., Singer A., Shearer G. M. Graft-vs-host reaction limited to a class II MHC difference results in a selective deficiency in L3T4+ but not in Lyt-2+ T helper cell function. J Immunol. 1987 Mar 1;138(5):1355–1362. [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Spira T. J., Cross G. D., Jones B. M., Reinherz E. L. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J Clin Invest. 1984 Jan;73(1):191–201. doi: 10.1172/JCI111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S., Chirmule N., Leombruno C., Lim W., Harper R., Bhalla R., Pahwa R., Nelson R. P., Good R. A. In vitro synthesis of human immunodeficiency virus-specific antibodies in peripheral blood lymphocytes of infants. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7532–7536. doi: 10.1073/pnas.86.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Good R. A., Gallo R. C., Saxinger C. Stimulatory and inhibitory influences of human immunodeficiency virus on normal B lymphocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9124–9128. doi: 10.1073/pnas.83.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Saxinger C., Gallo R. C., Good R. A. Influence of the human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8198–8202. doi: 10.1073/pnas.82.23.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyclonal activation of B cells in homosexual men. N Engl J Med. 1984 Aug 23;311(8):536–537. doi: 10.1056/NEJM198408233110814. [DOI] [PubMed] [Google Scholar]
- Prince H. E., Kermani-Arab V., Fahey J. L. Depressed interleukin 2 receptor expression in acquired immune deficiency and lymphadenopathy syndromes. J Immunol. 1984 Sep;133(3):1313–1317. [PubMed] [Google Scholar]
- Pruzanski W., Jacobs H., Laing L. P. Lymphocytotoxic antibodies against peripheral blood B and T lymphocytes in homosexuals with AIDS and ARC. AIDS Res. 1983 1984;1(3):211–220. doi: 10.1089/aid.1.1983.1.211. [DOI] [PubMed] [Google Scholar]
- Ranki A., Weiss S. H., Valle S. L., Antonen J., Krohn K. J. Neutralizing antibodies in HIV (HTLV-III) infection: correlation with clinical outcome and antibody response against different viral proteins. Clin Exp Immunol. 1987 Aug;69(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P., Poli G., Kehrl J. H., Fauci A. S. Activated B lymphocytes from human immunodeficiency virus-infected individuals induce virus expression in infected T cells and a promonocytic cell line, U1. J Exp Med. 1991 Jan 1;173(1):1–5. doi: 10.1084/jem.173.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Safai B., Sarngadharan M. G., Groopman J. E., Arnett K., Popovic M., Sliski A., Schüpbach J., Gallo R. C. Seroepidemiological studies of human T-lymphotropic retrovirus type III in acquired immunodeficiency syndrome. Lancet. 1984 Jun 30;1(8392):1438–1440. doi: 10.1016/s0140-6736(84)91933-0. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Higgins S. E., Folks T., Fauci A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science. 1986 Sep 5;233(4768):1084–1086. doi: 10.1126/science.3016902. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Haller O., Vogt M., Lüthy R., Joller H., Oelz O., Popovic M., Sarngadharan M. G., Gallo R. C. Antibodies to HTLV-III in Swiss patients with AIDS and pre-AIDS and in groups at risk for AIDS. N Engl J Med. 1985 Jan 31;312(5):265–270. doi: 10.1056/NEJM198501313120502. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Sei Y., Tsang P. H., Petrella R. J., Bekesi J. G. Multiple dysfunctions in developmental and activational stages of T lymphocytes, B lymphocytes and monocytes in ARC and AIDS patients. Immunol Lett. 1987 Nov;16(2):157–162. doi: 10.1016/0165-2478(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Shearer G. M. Allogeneic leukocytes as a possible factor in induction of aids in homosexual men. N Engl J Med. 1983 Jan 27;308(4):223–224. doi: 10.1056/NEJM198301273080415. [DOI] [PubMed] [Google Scholar]
- Sonnabend J., Witkin S. S., Purtilo D. T. Acquired immunodeficiency syndrome, opportunistic infections, and malignancies in male homosexuals. A hypothesis of etiologic factors in pathogenesis. JAMA. 1983 May 6;249(17):2370–2374. [PubMed] [Google Scholar]
- Spickett G., Beattie R., Farrant J., Bryant A., Dalgleish A., Webster D. Assessment of responses of normal human B lymphocytes to different isolates of human immunodeficiency virus: role of normal donor and of cell line used to prepare viral isolate. AIDS Res Hum Retroviruses. 1989 Jun;5(3):355–366. doi: 10.1089/aid.1989.5.355. [DOI] [PubMed] [Google Scholar]
- Stahl R. E., Friedman-Kien A., Dubin R., Marmor M., Zolla-Pazner S. Immunologic abnormalities in homosexual men. Relationship to Kaposi's sarcoma. Am J Med. 1982 Aug;73(2):171–178. doi: 10.1016/0002-9343(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Engleman E. G. Anti-DNA antibody production by CD5+ and CD5- B cells of patients with systemic lupus erythematosus. J Clin Invest. 1990 Jan;85(1):238–247. doi: 10.1172/JCI114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via C. S., Morse H. C., 3rd, Shearer G. M. Altered immunoregulation and autoimmune aspects of HIV infection: relevant murine models. Immunol Today. 1990 Jul;11(7):250–255. doi: 10.1016/0167-5699(90)90099-u. [DOI] [PubMed] [Google Scholar]
- Via C. S., Sharrow S. O., Shearer G. M. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987 Sep 15;139(6):1840–1849. [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. Functional heterogeneity of L3T4+ T cells in MRL-lpr/lpr mice. L3T4+ T cells suppress major histocompatibility complex-self-restricted L3T4+ T helper cell function in association with autoimmunity. J Exp Med. 1988 Dec 1;168(6):2165–2181. doi: 10.1084/jem.168.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. Q., Johnson E. A., Donnelly R. P., Lavia M. F., Tsang K. Y. Specificity of anti-lymphocyte antibodies in sera from patients with AIDS-related complex (ARC) and healthy homosexuals. Clin Exp Immunol. 1988 Aug;73(2):168–173. [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Redfield R. R., Broder S. Mechanisms of B cell activation in patients with acquired immunodeficiency syndrome and related disorders. Contribution of antibody-producing B cells, of Epstein-Barr virus-infected B cells, and of immunoglobulin production induced by human T cell lymphotropic virus, type III/lymphadenopathy-associated virus. J Clin Invest. 1986 Aug;78(2):439–447. doi: 10.1172/JCI112595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. L., Stites D. P. Hypothesis: AIDS is an autoimmune disease directed at the immune system and triggered by a lymphotropic retrovirus. Clin Immunol Immunopathol. 1986 Dec;41(3):305–313. doi: 10.1016/0090-1229(86)90001-2. [DOI] [PubMed] [Google Scholar]


