Abstract
BACKGROUND
The continued growth in the uses of umbilical cord blood (UCB) will require the development of meaningful post-thaw quality assays. This study examines both conventional and new measures for assessing UCB quality after long-term storage.
STUDY DESIGN AND METHODS
The first arm of the study involved thawing UCB in storage for short (~1 year) and long period of time (> 11 years). Conventional post thaw measures (CFU, TNC, CD34+45+) were quantified in addition to apoptosis. The second arm of the study involved taking units stored in liquid nitrogen and imposing a storage lesion by storing the units in −80°C for various periods of time. After storage lesion, the units were thawed and assessed.
RESULTS
In the first arm of the study, there was little difference in the post thaw measures between UCB stored for short and long periods of time. There was a slight increase in the percentage of CD34+45+ cells with time in storage and a reduction in the number of cells expressing apoptosis markers. When moved from liquid nitrogen to −80°C storage, the nucleated cell count varied little but there was a distinct drop in frequency of CFU and increase in percentage of cells expressing both early and late markers of apoptosis.
CONCLUSION
Nucleated cell counts do not reflect damage to hematopoietic progenitors during long-term storage. Expression of caspases and other markers of apoptosis provide an early biomarker of damage during storage, which is consistent with other measures such as CFU and percentage of CD34+45+ cells.
Keywords: flow cytometry, colony forming unit, caspase assay, α-fucosidase, storage temperature, cord blood
Introduction
Umbilical cord blood (UCB) is an increasingly important source of hematopoietic stem cells (HSCs). Since the first clinical use of UCB to treat a patient with Fanconi anemia in 19881, the use of UCB has grown. Currently 28% of HSC transplants performed in the US annually are from UCB.2 UCB is also being considered as a source of immune cells (lymphocytes and monocytes) for different immunotherapies3 and mesenchymal stem cells for regenerative medicine applications. The potential applications for UCB continue to grow.4
UCB is collected at birth and as a result, is typically collected and cryopreserved for later use. The first UCB bank was developed at the New York Blood Center in 19925 and since that time, UCB banking has grown considerably. Currently, more than 780,000 UCB units are stored in private UCB banks and over 400,000 units are stored in public UCB banks.6 The rapid growth in the number of UCB units that have been stored > 10 years provides an important opportunity to determine shelf life. Theoretically, storage of cryopreserved cells in liquid nitrogen should result in a shelf life of thousands of years.7 In practice, temperature excursions commonly experienced in liquid nitrogen storage units when the tank is accessed to insert or remove samples can lead to degradation of affected units.8
The stability of UCB in long-term storage (> 5 yrs.) has been studied by a variety of investigators. Yamamoto, et al. found that after being preserved for 10 years, UCB had a reduced number of hematopoetic progenitor cells and some units had the inability to form colonies.9 In contrast, Broxmeyer, et al. determined that even after storage periods of 21-23.5 years, hematopoietic progenitors from thawed UCB units engrafted readily in immune deficient mice for a majority of units thawed.10 Mugishima et al. tested the frequency of colony forming units (CFU) in UCB as a function of time in storage.11 They observed a decrease frequency of CFU for UCB stored on liquid nitrogen over a 12 yr period.11 The stability of other hematopoietic stem cells products (bone marrow or peripheral blood progenitor cells) in long-term storage has also been studied. Spurr and colleagues observed maintenance of CFU capacity and CD34+ content for between 5-14 years in storage although content was not correlated to duration in storage.12 However, Fernyhough and colleagues observed a change in colony formation and CD34+ content with time in storage for units stored between 11 and 19 years.13
The continued growth in the uses of UCB will require the development of meaningful post-thaw quality assays/information suitable for use at the transplant site as well as the UCB bank. Recently, an UCB Apgar score was developed as a measure for engraftment potential for UCB.14 This score combines a precryopreservation score, a postthaw score and a composite score (a combination of precryopreservation and postthaw scores) to predict the engraftment potential of a given UCB unit. This scoring system can then be used for screening of UCB donors for transplantation. Transplant studies of UCB suggest that both CD34+ and colony forming unit (CFU) content correlate with engraftment and long-term survival.15,16 There are significant differences from site to site with regard to these assays (specifically CD34+ enumeration and CFU assays) when performed post thaw, and these differences are felt to influence the reliability of the assay for clinical use.17 In addition, CFU assays require days/weeks for completion making its use at the transplant site difficult. Finally, both enumeration of CD34+ cells and CFU of assays do not provide insight as to the mechanisms of damage during long-term storage.
Studies have demonstrated that hematopoietic cells exhibit significant cell losses post-thaw resulting from post-thaw apoptosis.18-20 These studies demonstrated that damage to the mitochondria resulted in release of caspases and ultimately, to the loss of the cell due to apoptosis. A similar outcome was observed by Cosentino and colleagues who demonstrated that improper storage (specifically, cycling of temperature in storage) resulted in higher levels of post thaw apoptosis for peripheral blood mononuclear cells stored on liquid nitrogen.8 Similarly, a study of UCB in long-term storage suggests that hematopoietic stem cells (HSCs) expressing signs of apoptosis (Annexin V staining) did not engraft when transplanted into an animal transplant model.21 These studies suggest that damage to the mitochondria during long-term storage may be an important mechanism of damage/cell loss. Assaying caspase production or other metrics of damage to the mitochondria may be an assay for quality that can be used to monitor stability of UCB in storage either by UCB banks or at the site of use.
The objective of this investigation is to characterize damage during long-term storage and evaluate potential markers for improper storage. To this end, UCB units that have been frozen for varying levels of time (~ 1 year versus > 11 years) were thawed and post thaw metrics compared. Additional UCB units were stored intentionally in improper conditions (at −80°C) to impose a storage lesion. Standard metrics for UCB were determined (total nucleated cell count, fraction of cells expressing CD34, colony formation after culture in methylcellulose and viability using 7-AAD). In addition, caspase expression as a marker of apoptosis (and damage during storage) was also determined.
Methods
All studies were performed on frozen umbilical cord blood (UCB) units from three different sources: National Heart, Lung and Blood Institute (NHLBI), St. Louis Cord Blood Bank and archival units from the defunct American Red Cross Cord Blood Bank at the University of Minnesota. All units were red blood cell reduced to obtain mononuclear cell concentrates and cryopreserved with dimethylsulfoxide (DMSO) added to achieve a final concentration of 10% and frozen in a controlled rate freezer with a validated protocol. Additional details on the processing of units obtained from NHLBI are detailed by Fraser et al. 22 These units remained in storage 11.5 to 16.1 years (average 13.6±1.3 years). The protocol for processing of UCB units from the St. Louis Cord Blood Bank is reviewed by Alonso et al. 23 These units remained in storage 0.58 to 1.4 years (average 1.0±0.26 years). Chrysler et al describe the details on the processing of units obtained from the American Red Cross.24 These units remained in storage 11.3 to 13.7 years (average 12.7+0.63 years). All UCB units were stored in the vapor phase of liquid nitrogen and temperatures during storage and shipment did not exceed −150°C.
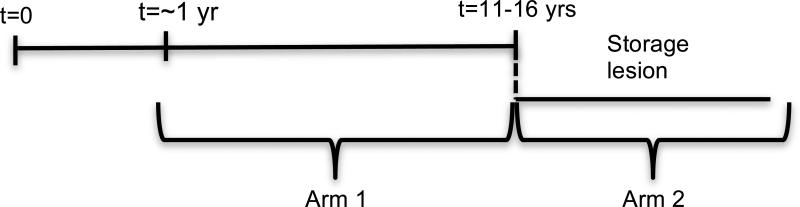
The studies performed were divided into two arms: (1) a comparison of units stored for a short period of time (~1 year) and units in long-term storage (> 11 years); (2) units stored long-term on liquid nitrogen, then transferred to a −80°C freezer, thereby imposing a storage lesion. UCB units were placed in lesion for 1 week, 1 month, 3 months, or 6 months (Fig 1).
Fig 1.
Schematic of study organization. The first arm represents CBU that have been stored on LN2 for different periods of time. The second arm of the study represents CBU that have been placed in a storage lesion (−80°C) for different periods of time.
Post thaw processing and assays
UCB units were thawed in a 37°C water bath and washed in 60mL of a 1:1 mixture of 10% Dextran40 (Hospira) and 5% Human Serum Albumin, or HSA (Grifols). A post-thaw cell count was taken at this time using a cell counter (Beckman Coulter). Samples were then centrifuged at 500 g for 15 minutes, the supernatant removed and the cells resuspended in the Dextran 40/HSA solution to a concentration of 1×107 cells/ml. Samples of the thawed UCB unit were tested using the following assays.
Total Nucleated Cell (TNC) Counts
The TNCs in the thawed UCB units was determined using a Beckman Coulter Z1 Particle Counter (Beckman Coulter). An aliquot of UCB cells was diluted 500-fold in Coulter IsotonDiluent (Beckman Coulter), Zap-Oglobin II Lytic Reagent (Beckman Coulter) was added to lyse red blood cells, and three replicate counts of the diluted sample were obtained. The average of the three counts was reported.
Colony Forming Unit (CFU) Counts
Quantification of CFU was performed following the protocol described for the MethoCult H4435 Enriched Media (Stem Cell Technologies). Briefly, UCB cells were combined with Iscove's MDM (StemCell Technologies) containing 2% Fetal Bovine Serum (FBS)(Stem Cell Technologies) to a concentration of 2.5 × 105 cells/mL. This cell suspension was then added to the MethoCult H4435 Enriched Media (Stem Cell Technologies), dispensed into a 35mm petri dish and incubated for 14-16 days. The total number of colonies were counted and recorded.
Flow Cytometry
All flow cytometry was performed on a BD FACSCalibur machine using ISHAGE gating strategies.25 In both the CD34 enumeration and caspase assays the CD34 cells were defined as a subset of the CD45+, or white blood cell, population. The numbers of cells in each category was determined by multiplying the ratio of desired events to the total CD45+ events and multiplied by the total nucleated cell count post thaw to determine the total number of cells expressing (or not) caspase.
CD34 Enumeration
Samples are tested for CD34 expression using the BD Biosciences CD45/CD34 combination kit as per manufacturer's instructions (BD Biosciences). Briefly, staining reagents were added to the cell suspension, mixed and incubated at room temperature in the dark. Subsequently, red blood cells were lysed and the sample was incubated on ice and taken for flow cytometry.
Caspase Assay for Analysis of Mitochondrial Damage
UCB unit cells were stained for the presence of caspase as per manufacturers instruction (Vybrant FAM Poly Caspases Assay Kit, Life Technologies). Briefly, cells were incubated with FLICA reagent (BD Biosciences) in a FACS tube and then incubated for 60 minutes at 37°C in the dark. The sample was washed twice, stained with CD45APC (BD Biosciences) and CD34PE (BD Biosciences) and incubated for 30 min at 4°C in the dark. After a final wash step, the cells were stained with ViaProbe Cell Viability Solution (7-AAD, BD Biosciences) and the solution was incubated at room temperature. The fractions of viable, early and late apoptotic, and necrotic CD34+ cells were determined. Each tube was spiked with fluorescent beads (PKH, Sigma) to determine the cell losses due to washing. Early apoptotic cells stained positive for caspases but negative for 7AAD. Late apoptotic cells stained positive for both caspases and 7AAD. Necrotic cells were negative for caspases but stain positive for 7AAD and viable cells stain negative for both caspases and 7AAD.
Statistical methods
Changes in post thaw measures were analyzed by the two-sample t-test assuming unequal variances. Changes in post thaw measures with time in lesion were analyzed using linear regression, which estimated the rate of change over time. P-values less than 0.05 are considered statistically significant. The analysis was conducted using SAS version 9.3 (SAS Institute, Inc., Cary NC).
Results
Influence of time in storage on liquid nitrogen on viability
The first arm of the investigation determined changes in standard post thaw measures (enumeration of CD34+ cells using flow cytometry, CFU, TNC) for CBU stored on liquid nitrogen for short and long periods of time when stored in the vapor phase of liquid nitrogen. There was no decline in the TNC or frequency of CFUs with time in storage but there was a statistically significant increase in the percentage of cells expressing CD34+ surface markers with time in storage (Table 1). Beyond conventional measures of post thaw assessment, the fraction of cells that are apoptotic was also of interest. There was a decline in the fraction of CD34+45+ cells that were early apoptotic with time in storage as well as an increase in the fraction of viable cells with time in storage.
Table 1.
Variation in TNC, CD34+ enumeration, frequency of CFU and expression of apoptosis markers in CD34+45+ cells for Arm 1 of study. The results of each method are shown as a mean and standard deviation (SD) and the number of repetitions (n).
| Measure | Short term storage Mean (SD) n | Long term storage Mean (SD) n | P-value |
|---|---|---|---|
| Pre-freeze minus post-thaw CD34+ fraction* | 0.000215 (0.000940) n=16 | 0.000868 (0.000992), n=21 | 0.049 |
| Pre-freeze minus post-thaw CFU fraction† | 0.001150 (0.000418), n=16 | 0.000998 (0.000826), n=21 | 0.469 |
| Pre-freeze minus post-thaw TNC fraction‡ | 0.283 (0.055) n=16 | 0.364 (0.181) n=21 | 0.065 |
| Expression of apoptosis markers for CD34+ cells | |||
| Late apoptotic§ | 38.13 (18.69) n=16 | 25.77 (17.30) n=17 | 0.058 |
| Early apoptotic∥ | 10.06 (2.07) n=16 | 7.44 (4.11) n=17 | 0.006 |
| Necrotic cells¶ | 4.00 (2.35) n=16 | 5.00 (2.86) n=17 | 0.159 |
| Viable cells** | 47.67 (16.99) n=16 | 60.99 (18.27) n=17 | 0.006 |
Total number of CD34+ cells divided by the total number of nucleated cells
Total number of colony forming units divided by the total number of nucleated cells
1-(Total number of nucleated cells post thaw divided by the total number of nucleated cells pre-freeze)
Early apoptotic cells stain positive for caspase but negative for 7AAD
Late apoptotic cells stain positive for both caspase and 7AAD
Necrotic cells are negative for caspase but stain positive for 7AAD
Viable cells stain negative for both caspase and 7AAD.
Influence of duration in lesion storage conditions on viability
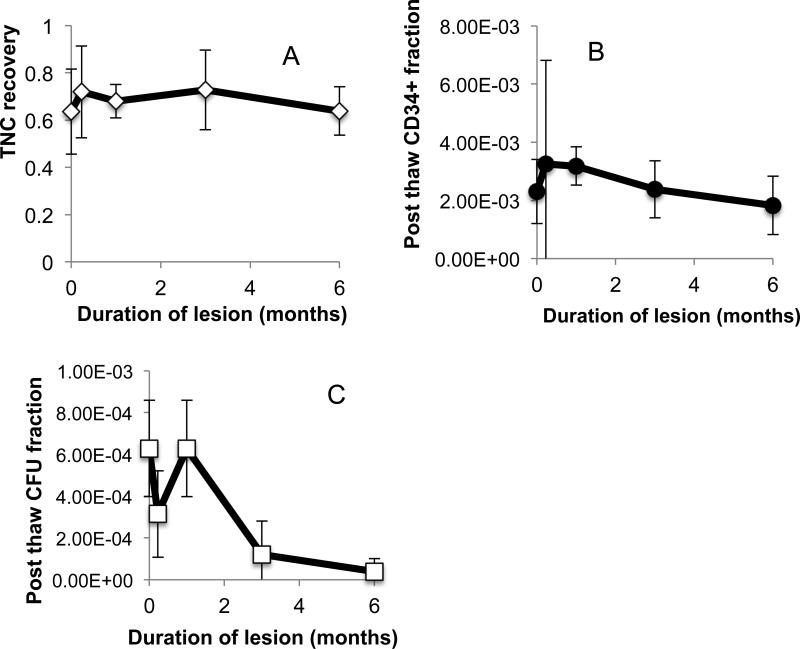
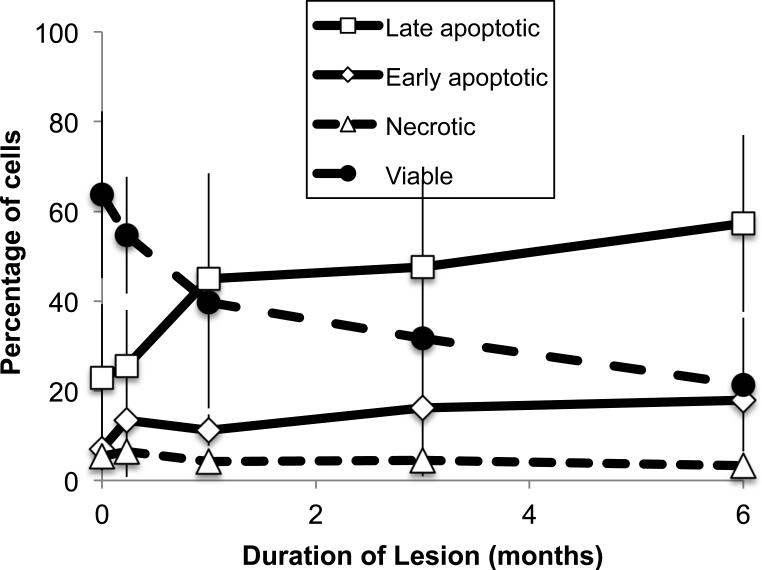
The first arm of the study demonstrated that there were little differences in conventional measures with duration in storage for samples stored in the vapor phase of liquid nitrogen. The second arm of the study involved imposing a storage lesion on UCB units and measuring trends in conventional measures (CFU, TNC and expression of CD34+ expression) as well as caspase expression. UCB units stored in liquid nitrogen > 11 years were transferred into an −80°C freezer to induce a storage lesion. Units were thawed after 1 week, 1 month, 3 months and 6 months. Loss in viability was so significant after 6 months that longer storage periods were not pursued. The variation in TNCs, frequency of CFUs and enumeration of CD34+ cells with time in lesion is shown in Fig. 2. It is noteworthy that there is little variation in TNC counts with time in lesion. However, both CFUs and the fraction of cells CD34+ cells declined with time in storage and the decline could be modeled using linear regression (Table 2). The fraction of cells expressing early markers for apoptosis increases significantly with time in storage when UCB units are stored under lesion and the fraction of viable cells (as defined by 1- (early apoptotic+late apoptotic+necrotic)) declined significantly (Fig. 2 and Table 2)
Fig 2.
Variation in (a) TNC, (b) expression of CD34+ cells and (c) CFU with time in lesion for CBU stored at – 80°C.
Table 2.
Variation in TNC, CD34+ enumeration, CFU and expression of apoptosis markers in CD34+45+ cells as a function of time in lesion. Data was analyzed using linear regression
| Measure | Rate of change per month Mean (SE) | P-value | Corr (r) |
|---|---|---|---|
| Pre-freeze minus post-thaw CD34+ fraction (n=38) | 0.00000810 (0.00005689) | 0.887 | 0.02 |
| Pre-freeze minus post-thaw CFU fraction (n=34) | −0.00007460 (0.00006157) | 0.231 | −0.16 |
| Pre-freeze minus post-thaw TNC fraction (n=38) | −0.001(0.010) | 0.895 | −0.02 |
| Expression of apoptosis markers for CD34+ cells | |||
| Late apoptotic CD34+ cells %(n=34) | 5.357 (1.110) | <0.001 | 0.57 |
| Early apoptotic CD34+ cells % (log scale) (n=34) | 0.114 (0.036) | 0.003 | 0.41 |
| Necrotic CD34+ cells % (log scale) (n=34) | −0.091 (0.035) | 0.013 | −0.35 |
Discussion
There has been significant interest in quality metrics for UCB. Recently, a cord blood Apgar score was developed as a measure for engraftment potential for UCB. 14 The focus of this study is to characterize potential metrics or biomarkers for damage to UCB units in long-term storage. In the first arm of this study, post thaw measures as a function of storage time were quantified. UCB and most hematopoietic stem cells (HSC) are stored on liquid nitrogen (see ref 26 review) and the stability of different HSC products as a function of storage time have been studied for storage periods of up to 20 years. In the first arm of the study, a variety of measures (nucleated cell count, frequency of CFU and percentage of necrotic cells) did not exhibit changes with duration in storage. These results of this study were consistent with previous studies. 10,27 The apparent increase in percentage of CD34+45+ cells with time in storage is consistent with a study by Flores and colleagues.17 The authors established that the measured increase resulted not from improved recovery of CD34+ cells but the inherent challenges in quantifying CD34+ and variations from institution to institution. As with the Flores study, the UCB units used in this investigation came from 3 different institutions and it is likely that the variation on recovery of CD34+45+ cell reflects differences in quantifying CD34+ content rather than the influence of duration in storage. The results of this investigation suggest that there is little degradation in the quality of the UCB unit with time in storage for the periods studied (~1 year to 11-16 years).
It is not clear whether conventional measures of post thaw assessment (TNC, CFU, etc) are adequate or if additional biomarkers are needed to characterize damage during long term storage of UCB units. Our hypothesis was that apoptosis could be used as a potential early marker for degradation of UCB units during long-term storage. Costentino and colleagues8 demonstrated improper temperature storage conditions resulted in increased apoptosis for peripheral blood mononuclear cells and Shim and colleagues demonstrated that frozen and thawed CD34+ cells isolated from thawed UCB units expressing Annexin V did not engraft in animals.21 In order to test whether apoptosis could be used as a biomarker for long-term damage, UCB units were moved from liquid nitrogen to −80°C storage to impose a well-characterized temperature lesion. It is noteworthy that the TNC count, which is commonly performed post thaw, did not reflect damage to the cells due to improper storage. The lack of correlation between nucleated cell counts and other post thaw measures has been observed in other studies. Goodwin and colleagues observed that trypan blue staining of UCB cells post thaw did not correlate with the frequency of CFU detected in the same sample.28 These studies suggest that TNC counts post thaw should not be used to assess the suitability of a UCB unit post thaw.
In contrast, frequency of CFU and percentage of CD34+ cells decreased rapidly with time in storage lesion. These measures are used routinely to characterize UCB units prefreeze and as a part of the UCB Apgar score14. The fraction of both early and late apoptotic cells as a function of time in storage lesion increased at short periods of lesion suggesting that it can be an early marker for storage damage. The most common method of quantifying apoptosis for UCB involves staining for Annexin V expression on the surface of a cell21. In this investigation, the expression of caspases was used as a marker for apoptosis. Caspase expression is upstream in the apoptosis cascade of expression of phosphatidyserine on the surface of the cell membrane, and therefore it is an earlier marker of damage29 and has been associated directly with freeze-thaw damage.30 These results suggest that apoptosis is an early marker for damage during long-term storage and could be used to augment the Apgar score for UCB for characterizing UCB quality. Additional studies would need to be performed to confirm apoptosis as an early marker and its influence of engraftment.
Fig 3.
Variation in the fraction of CD34+cells that are early and late apoptotic, necrotic and viable as a function of time in lesion.
Acknowledgements
These studies were funded in part by 1R21HL112653 and NIH P30 CA77598 utilizing the following Masonic Cancer Center, University of Minnesota shared resource: Translational Therapy Laboratory.
Footnotes
There is no conflict of interest associated with this paper.
References
- 1.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.CIBMTR Available from: http://www.nmdpresearch.org.
- 3.McKenna DH, Brunstein CG. Umbilical cord blood: current status and future directions. Vox Sang. 2011;100:150–62. doi: 10.1111/j.1423-0410.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 4.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–92. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679–90. [PubMed] [Google Scholar]
- 6.Butler MG, Menitove JE. Umbilical cord blood banking: an update. J Assist Reprod Genet. 2011;28:669–76. doi: 10.1007/s10815-011-9577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meryman H. Review of biological freezing. In: Meryman H, editor. Cryobiology. Academic Press; New York, NY: 1966. pp. 1–114. [Google Scholar]
- 8.Cosentino M, Corwin W, Baust JG, Diaz-Mayoral N, Cooley H, Shao W, Van Buskirk R, Baust JG. Preliminary Report: Evaluation of Storage Conditions and Cryococktails during Peripheral Blood Mononuclear Cell Cryopreservation. Cell Preserv. Tech. 2007;4:189–204. [Google Scholar]
- 9.Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, Suzuki M, Tanaka Y, Watanabe T, Fujimoto Y, Hosaki I, Nishihira H, Isoyama K. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. International Journal of Hematology. 2011;93:99–105. doi: 10.1007/s12185-010-0755-x. [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, Mallett C, Ye Z, Witting S, Cornetta K, Cheng L, Yoder MC. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–7. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S, Sato K, Klein JP, Gale RP. Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant. 1999;23:395–6. doi: 10.1038/sj.bmt.1701580. [DOI] [PubMed] [Google Scholar]
- 12.Spurr EE, Wiggins NE, Marsden KA, Lowenthal RM, Ragg SJ. Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5-14 years) cryostorage. Cryobiology. 2002;44:210–7. doi: 10.1016/s0011-2240(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 13.Fernyhough L, Buchan V, McArthur L, Hock B. Relative recovery of haematopoietic stem cell products after cryogenic storage of up to 19 years. Bone Marrow Transplantation. 2012;48:32–5. doi: 10.1038/bmt.2012.97. [DOI] [PubMed] [Google Scholar]
- 14.Page KM, Zhang L, Mendizabal A, Wease S, Carter S, Shoulars K, Gentry T, Balber AE, Kurtzberg J. The Cord Blood Apgar: a novel scoring system to optimize selection of banked cord blood grafts for transplantation (CME). Transfusion. 2012;52:272–83. doi: 10.1111/j.1537-2995.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 16.Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S, Kurtzberg J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–89. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores AI, McKenna DH, Montalban MA, De la Cruz J, Wagner JE, Bornstein R. Consistency of the initial cell acquisition procedure is critical to the standardization of CD34+ cell enumeration by flow cytometry: results of a pairwise analysis of umbilical cord blood units and cryopreserved aliquots. Transfusion. 2009;49:636–47. doi: 10.1111/j.1537-2995.2008.02035.x. [DOI] [PubMed] [Google Scholar]
- 18.de Boer F, Drager AM, Pinedo HM, Kessler FL, Monnee-van Muijen M, Weijers G, Westra G, van der Wall E, Netelenbos T, Oberink JW, Huijgens PC, Schuurhuis GJ. Early apoptosis largely accounts for functional impairment of CD34+ cells in frozen- thawed stem cell grafts. J Hematother Stem Cell Res. 2002;11:951–63. doi: 10.1089/152581602321080619. [DOI] [PubMed] [Google Scholar]
- 19.de Boer F, Drager AM, Pinedo HM, Kessler FL, van der Wall E, Jonkhoff AR, van der Lelie J, Huijgens PC, Ossenkoppele GJ, Schuurhuis GJ. Extensive early apoptosis in frozen-thawed CD34-positive stem cells decreases threshold doses for haematological recovery after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 2002;29:249–55. doi: 10.1038/sj.bmt.1703357. [DOI] [PubMed] [Google Scholar]
- 20.Xiao M, Dooley DC. Assessment of cell viability and apoptosis in human umbilical cord blood following storage. J Hematother Stem Cell Res. 2003;12:115–22. doi: 10.1089/152581603321210190. [DOI] [PubMed] [Google Scholar]
- 21.Shim JS, Cho B, Kim M, Park GS, Shin JC, Hwang HK, Kim TG, Oh IH. Early apoptosis in CD34+ cells as a potential heterogeneity in quality of cryopreserved umbilical cord blood. Br J Haematol. 2006;135:210–3. doi: 10.1111/j.1365-2141.2006.06270.x. [DOI] [PubMed] [Google Scholar]
- 22.Fraser JK, Cairo MS, Wagner EL, McCurdy PR, Baxter-Lowe LA, Carter SL, Kernan NA, Lill MC, Slone V, Wagner JE, Wallas CH, Kurtzberg J. Cord Blood Transplantation Study (COBLT): cord blood bank standard operating procedures. J Hematother. 1998;7:521–61. doi: 10.1089/scd.1.1998.7.521. [DOI] [PubMed] [Google Scholar]
- 23.Alonso JM, 3rd, Regan DM, Johnson CE, Oliver DA, Fegan R, Lasky LC, Wall DA. A simple and reliable procedure for cord blood banking, processing, and freezing: St Louis and Ohio Cord Blood Bank experiences. Cytotherapy. 2001;3:429–33. doi: 10.1080/146532401317248036. [DOI] [PubMed] [Google Scholar]
- 24.Chrysler G, McKenna D, Schierman T, Kadidlo D, Askari S, Miller J, Clay M, McCullough J. Cellular characteristics of cord blood and cord blood transplantation. In: Broxmeyer H, editor. Cord Blood: biology, immunology, banking and clinical transplantation. AABB; Bethesda, MD: 2004. pp. 219–58. [Google Scholar]
- 25.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 26.Hubel A, Spindler R, Skubitz AP. Storage of human biospecimens: selection of the optimal storage temperature. Biopreserv Biobank. 2014;12:165–75. doi: 10.1089/bio.2013.0084. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, Suzuki M, Tanaka Y, Watanabe T, Fujimoto Y, Hosaki I, Nishihira H, Isoyama K. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. Int J Hematol. 2011;93:99–105. doi: 10.1007/s12185-010-0755-x. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin HS, Grunzinger LM, Regan DM, McCormick KA, Johnson CE, Oliver DA, Mueckl KA, Alonso JM, Wall DA. Long term cryostorage of UC blood units: ability of the integral segment to confirm both identity and hematopoietic potential. Cytotherapy. 2003;5:80–6. [PubMed] [Google Scholar]
- 29.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Stroh C, Cassens U, Samraj AK, Sibrowski W, Schulze-Osthoff K, Los M. The role of caspases in cryoinjury: caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002;16:1651–3. doi: 10.1096/fj.02-0034fje. [DOI] [PubMed] [Google Scholar]