Abstract
Aims
Mice are increasingly being used as models to investigate aspects of urinary dysfunction that humans with lower urinary tract symptoms (LUTS) experience. One method used to examine voiding function is the spontaneous void spot assay. The purpose of this study was to characterize and identify animal husbandry conditions that might confound results of the spontaneous void spot assay in male C57Bl/6J mice.
Methods
Mice were placed in cages lined with filter paper for four hours and urine was visualized with UV transillumination. Voiding parameters including urine spot number, spot size, total urine area, primary void area, corner and center voiding were quantified.
Results
Adult male mice void more frequently with advancing age and a subpopulation (5–10%) display a frequent spotting pattern at 6–9 weeks of age. Voiding was not significantly different in male mice weaned to group housing (4–6 per cage) versus single housing, and was not altered when they were used as breeders. Voiding was changed upon transferring group housed adult males to single density cages, which decreased total urine area. Repeated assays of male voiding behavior over three consecutive days increased primary void area by the third day of monitoring and revealed that voiding behavior is impacted by routine cage changes and time of day.
Conclusions
Together these results identify housing and husbandry practices that influence male voiding behaviors in the spontaneous void spot assay and will inform voiding behavior analyses conducted with male C57Bl/6J mice.
Keywords: lower urinary tract, void spot assay, prostate, bladder, filter paper
Introduction
Lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) pose a significant burden to the aging population [1]. There is a growing need to identify the underlying basis of LUTS to improve diagnosis and develop new noninvasive therapies.
Mice are frequently used to model aspects of urinary dysfunction experienced by humans, including incontinence, overactive bladder, and bladder outlet obstruction [2–5]. However, mice are anatomically and behaviorally distinct from humans, and cannot be assessed by the same urodynamic tests. As the number of mouse models of urinary dysfunction expands, so does the need for assays that quantify physiologically relevant aspects of voiding function while minimizing variables associated with mouse voiding behavior.
Many assays are available for measuring mouse voiding function, including: cystometry for measuring intravesicular pressures associated with bladder filling and emptying, bladder organ baths for characterizing in vitro contractile responses to various stimuli, electromyography for assessing neuromuscular function, and metabolic cages for examining uroflow [2, 4, 5]. Many of these methods are invasive, labor intensive, require specialized equipment, or do not allow for repeated analysis of urinary function. To complement these approaches, the void spot assay has been used to study urinary function [6–14]. This method is inexpensive, noninvasive and can be used to track individual mice over time. Mice are housed for a fixed period of time in a cage fitted with filter paper, the paper is removed and urine visualized under UV light. Urinary patterns are graded by blinded reviewers [12] or analyzed by imaging software [6].
The void spot assay, like other urinary function assays in mice, is confounded by social and behavioral influences. Mice void to scent mark territory, manifest social behaviors and establish dominance [14, 15]. Yu et al., 2014 validated the spontaneous void spot assay in female mice. They examined testing time of day, repeated days of testing, mouse age and strain on several voiding parameters (spot number, total urine area, primary void area and percentage of total urine area within primary void, corner voids and center voids). They found minimal individual and within strain variability. However, variability was observed between strains, revealing a genetic component to micturition.
Though male mice may be uniquely susceptible to behavioral influences on voiding function [14, 15] there has been no study to identify husbandry practices (i.e. caging density) or experimental factors (i.e. animal age, time of day conducted) that influence male voiding behavior using the void spot assay. There is a particular need to understand whether and how husbandry influences voiding in male mice so they can also be used to investigate outcomes of prostate biology and function on voiding behavior. This study utilized the C57Bl/6J male mouse line to identify animal husbandry conditions that impact spontaneous void spot assay outcomes. We found mouse age profoundly influences voiding frequency and 5–10% of male mice develop a frequent spotting pattern after 6 weeks of age. Voiding was stable across repeated daily measures, not influenced by mating or caging density at weaning. Voiding was altered by time of day, routine cage changes and when caging density was changed in adulthood. Together these results identify common practices that influence male voiding behaviors in the spontaneous void spot assay. These results should be considered when voiding behavior analysis is conducted with male C57Bl/6J mice.
Materials and Methods
Animals
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred to produce mice used in this study. Mice were housed in polysulfone cages containing corn cob bedding and maintained on a 12 hour light and dark cycle at 25±5°C and 20–50% relative humidity. Feed (Diet 2019 for males and Diet 7002 for pregnant females, Harlan Teklad, Madison, WI) and water were available ad libitum except during the testing period, when only feed was available. All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Spontaneous Void Spot Assay
Void assays were conducted and analyzed as described previously [6]. Single or group housed animals (4–6 per cage) were moved to a clean, empty cage lined with 3MM Whatman filter paper (Fisher Scientific #057163W) with one animal per cage. Assays were conducted over four hours with access to food but not water in the same quiet location and time of day unless indicated otherwise. Filter papers were imaged using UV light and analyzed using Image J Software (Version 1.46r). Images were analyzed by an individual blinded to treatment conditions. Brightness and contrast were optimized to uniformly visualize urine. Thresholding of images was done in Image J to make urine spots black. Image J particle analysis (Image J 1.46r) was performed on spots greater than 0.02cm2 (corresponding to ~0.75μl urine), reducing non-specific marks potentially deposited by paws and tails that pass through urine spots (Supplemental Figure 1). Overlapping urine spots were defined by the presence of a definitive border outlining each spot in the raw images, these spots were then counted by hand and area determined individually using the freehand selection tool (Supplemental Figure 1). These values were incorporated into the Image J analysis. Similarly if the primary void was in proximity to another distinct void, the primary void area was calculated by hand using the freehand selection tool to only incorporate a single spot.
Statistical analyses
All data are reported as mean ± standard error of the mean unless indicated otherwise. Statistical analysis was performed using R version 2.13.1. Homogeneity of variance was determined using Levene’s test, p ≤0.05 indicating unequal variance and use of nonparametric analysis. For parametric data, student’s T-test, or one way ANOVA followed by Tukey’s Honest Significant Difference (HSD) test were used to identify significant differences (p ≤ 0.05) between or among treatment groups. For nonparametric data Welch’s T-test or Kruskal Wallis Test followed by Wilcoxon tests with bonferroni correction were used to indentify significant difference (p≤0.05) between or among treatment groups. For assays conducted on the same animals, repeated measures ANOVA followed by pairwise T-test with Holm correction were used to identify significant differences between or among treatment groups.
Results
Male mouse age influences voiding
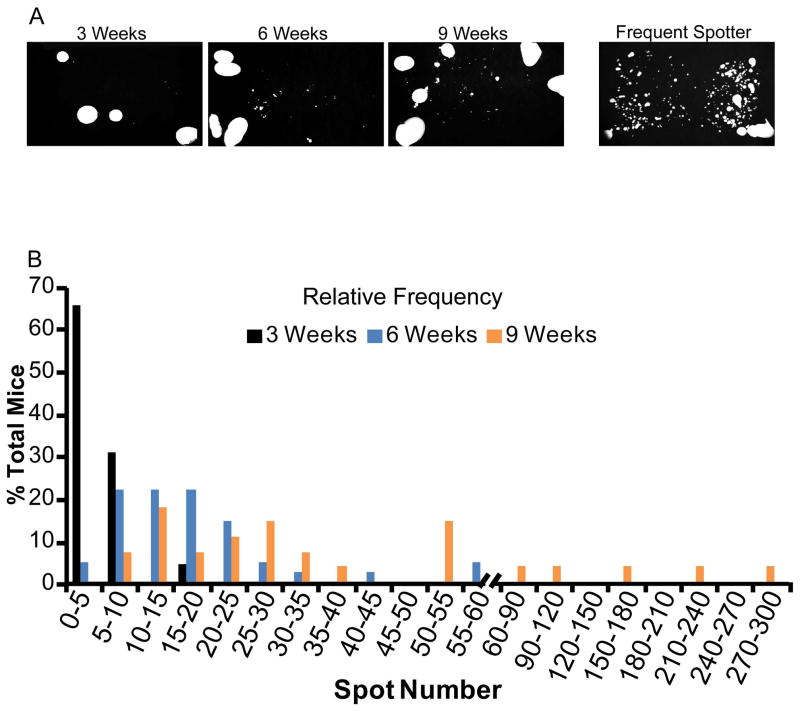
Void spot assays can be used to evaluate voiding function over time. Baseline analysis often begins during puberty or shortly after sexual maturation, and experimental cohorts are matched within a defined age range. We examined whether age influences voiding behavior in young mice, which are most likely to be used to initiate a study. Three, six and nine week old male mice were evaluated by the void spot assay (Figure 1A) and relative frequency of spot number determined (Figure 1B). Three week old males had a median void spot number of 4, with 86% voiding less than 8 times within the testing period. Six week old mice had a median void spot number of 15, with 90% voiding less than 30 times within the testing period. Nine week old mice had a median void spot number of 28, with 82% voiding less than 60 times within the testing period (Figure 1B). A subpopulation of six and nine week old male mice display frequent spotting, defined here as 50 and 100+ spots at six and nine weeks respectively, and represent 5 and 10% of the population at these ages.
Figure 1. Aging increases spot number in young adult male mice.
Void assays were conducted and urine visualized under UV transillumination to quantify spot number. (A) Representative images of spontaneous void spot assay testing in adult virgin C57Bl/6J male mice. (B) Relative frequency of urine spot number in 3–9 week old male C57Bl/6J mice. Results represent n=23, 41 and 28 mice at 3, 6 and 9 weeks of age respectively.
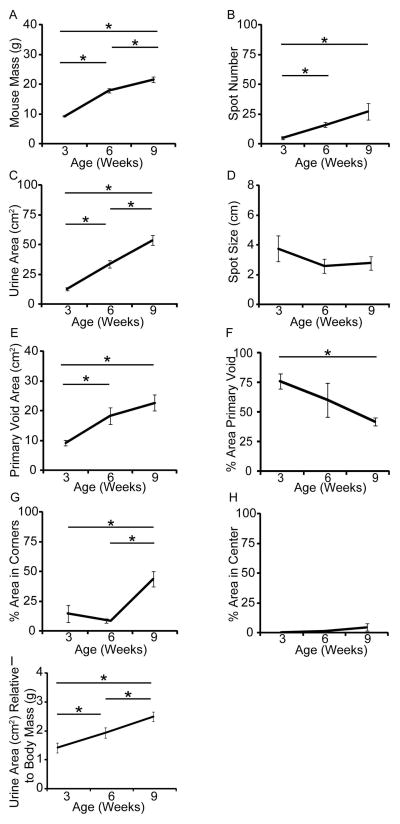
Mouse urine output and frequency increase with advanced age [6, 12]. Whether voiding parameters also change over time in young male mice, and how quickly these changes occur, is unknown. To test whether aging influences voiding parameters, the same cohort of mice was tested at three, six, and nine weeks of age. Body mass significantly and progressively increased with age (Figure 2A). Urine spot number (index of urinary frequency) significantly increased from three to six or nine weeks of age (Figure 2B). Total urine area (index of total urine output) significantly and progressively increased from three to six and nine weeks (Figure 2C), while individual urine spot size did not change with age (Figure 2D). Primary void area (largest void during the testing period) significantly increased from three to six or nine weeks of age (Figure 2E). Percent total urine contained in the primary void decreased between three and nine weeks of age (Figure 2F). Percent total urine area within corners (each corner defined as 1% of paper area) significantly increased from three to nine and six to nine weeks but not from three to six weeks (Figure 2G) while percentage of total urine within the center (center defined as 9% of paper area) was unchanged at all time points (Figure 2H). These results reveal that voiding parameters rapidly change as C57BL/6J adolescent male mice undergo sexual maturation, particularly during the three to six week age interval.
Figure 2. Age influences several voiding parameters in young adult male mice.
Void assays were conducted on the same cohort of mice at 3, 6 and 9 weeks of age. Quantification of (A) mouse mass, (B) spot number, (C) total urine area, (D) spot size, (E) primary void area, (F) percent area within primary void, (G) percent area within corners and (H) percent area within the center. (I) Total urine area normalized to mouse mass at three, six and nine weeks. Results are mean±SE, n=11. Asterisk indicates significant differences p≤0.05.
We next tested whether mouse body mass, which increases with age (Figure 2A), correlates with voiding parameters. There was a significant positive correlation between mouse body mass and urine spot number, total urine area, primary void area and percent total urine area within corners (Supplemental Figure 2). There was no significant correlation between mouse mass and individual urine spot size, percent total urine area in primary void and percent total urine within center (Supplemental Table I). Therefore, it is appropriate to normalize urine spot number, total and primary void area and percent urine within corners to body mass. When this was done on voiding data from mice at three, six and nine weeks of age, total urine area remained significantly different between all three time points (Figure 2I). Together these results indicate that tightly matched age cohorts should help minimize variability between groups. Initiating studies with baseline assays on six+ week old mice will identify frequent spotting mice to ensure they are equally distributed among experimental groups or excluded from studies when appropriate.
Conventional housing does not alter voiding behavior in young adult mice
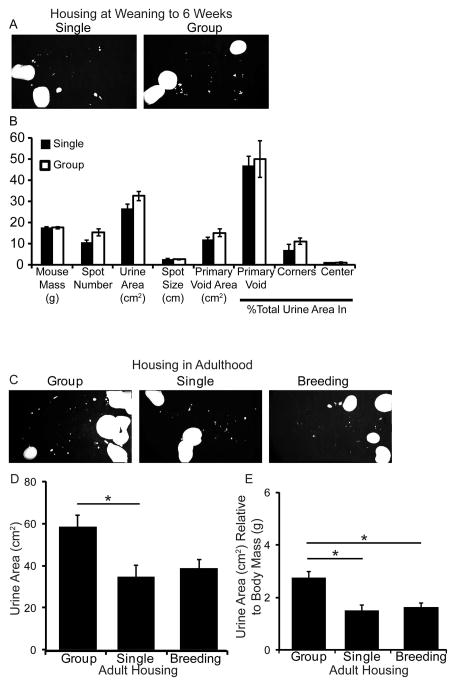
If male mice are to be housed with other male mice, it is recommended that males be group housed from time of weaning to reduce fighting and cage costs (Jackson Labs, www.jax.org). We examined if spontaneous voiding is influenced by group versus single housing. Male littermates were void tested prior to weaning and exhibited no significant differences in voiding parameters listed above (results not shown). Mice were weaned with littermates to group housing (up to six mice/cage) or transferred to single housing. Void spot assays were repeated at six weeks of age. There were no significant differences in body mass or measured void spot assay parameters between group or singly housed males (Figure 3A–B). Therefore, male littermates can be group housed up to at least six weeks of age without altering voiding behavior relative to mice that were singly housed from weaning to six weeks of age.
Figure 3. Group vs single housing has no appreciable impact on voiding behavior from weaning to 6 weeks of age but housing changes after 6 weeks of age decreases urine area.
(A) Representative images of spontaneous void assay testing in adult virgin C57Bl/6J male mice singly or group housed from time of weaning to 6 weeks of age. (B) Quantification of mouse mass, spot number, total urine area, spot size (diameter), primary void area, percent area in primary void, corners and center. Results are mean±SE, n=9 single, n=19 (5 cages) group. (C) Representative images of spontaneous void assay testing in adult virgin C57Bl/6J male mice. Mice group housed from weaning to 8 weeks of age were void tested, separated into single housing for one week and void tested again, then paired with a multiparous female for one week followed by a void assay. (D) Quantification of urine area. (E) Quantification of urine area relative to mouse body mass. Results are mean±SE, n=6. Asterisk indicates significant differences p≤0.05.
Group to single housing decreases urine area in mature mice
It is often necessary to place group housed animals into single cages once they reach adulthood (i.e. recovery after surgery, monitoring food and water consumption, etc). A change in caging density can be stressful. It can influence hormone levels, which in turn are capable of influencing voiding [16, 17]. We therefore tested whether changes from group to single housing after six weeks of age alters voiding. Adult virgin males group housed at weaning were void tested at eight weeks of age, individually housed for one week, and void tested again (Figure 3C–D). There was a significant reduction in urine area following the move to single versus group housing (Figure 3D). There were no significant differences in spot number, spot size, primary void area, percent urine area within primary void, corners or center (Supplemental Table II). This suggests a change in caging density after mice reach adulthood alters voiding behavior. Therefore, maintaining animals in the same housing conditions throughout an experiment will decrease variability in void spot assays.
We also examined if single housed males can be used for breeding without influencing voiding behavior. The same singly housed virgin male mice used above were housed with a multiparous female mouse for one week and then void tested (Figure 3C–D). There were no significant differences in void spot assay parameters after males had been housed with female mice versus when males were singly housed (Figure 3D, Supplemental Table II). Body mass significantly increased over the three week testing period; body mass did not alter parameters affected by housing or breeding. However, normalizing to body mass significantly reduced the difference in total urine area between group to singly housed males and between group and breeding males (Figure 3E).
Voiding exhibits stable daily patterns
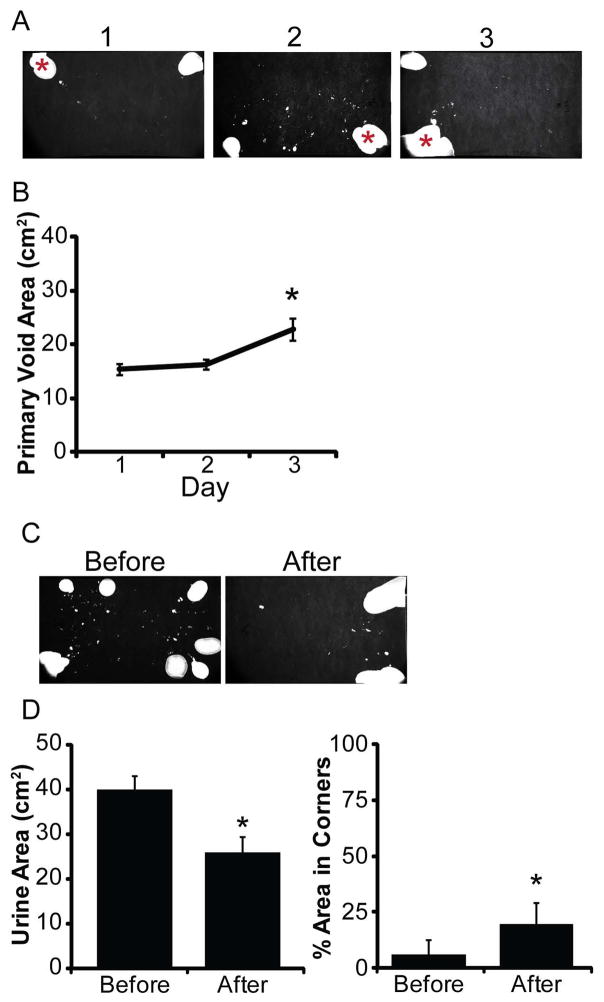
Female mice display reproducible day to day void spot assay patterns, suggesting a single void assay is representative of their voiding and acclimation to the cage fitted with filter paper does not alter voiding on consecutive days [6]. To determine whether this is also true for males, nine week old male mice were tested at the same time of day over three consecutive days (Figure 4A). There were no significant differences in body mass, spot number, urine area, spot size, percent area in primary void, percent urine area in corners and percent area in center (Supplemental Table III). There was a significant increase in average urine area in the primary void on day 3 (22.8 cm2) compared to days 1 (15.3 cm2) and 2 (16.2 cm2) (Figure 4B). These results suggest that void assays are relatively stable day to day but male mice exhibit more variation in day to day voiding than females.
Figure 4. Consecutive daily void testing of mice increases primary void area.
(A) Representative images of spontaneous void assay testing in adult virgin C57Bl/6J male mice over three consecutive days. Asterisk indicates the primary void. Mice were tested on three consecutive days at the same time of day. (B) Quantification of primary void area. Results are mean±SE. Asterisk indicates a significant difference p≤0.05. (C) Representative images of spontaneous void assay testing in adult virgin C57Bl/6J male mice before and after routine cage changes. (D) Quantification of urine area and percent area in corners. Results are mean±SE, n=10. Asterisk indicates significant differences p≤0.05.
Routine cage changes affect voiding parameters
Routine cage changes increase anxiety behaviors in C57Bl/6J mice [16]. Whether this effects voiding behavior is unknown. Male mice (nine weeks old) were void tested one day prior to cage changes, the following day cages were changed per normal animal husbandry and void assays conducted that same day (Figure 4C). There was no significant change in body mass, spot number, spot size, primary void area or percent area in primary void and center before versus after cage changes (Supplemental Table IV). There was a significant decrease in total urine area and increase in percent urine area in corners after cage changes versus before (Figure 4D), indicating cage changes alone can alter voiding parameters. These results in conjunction with those observed during testing over three consecutive days suggests day to day changes exist in the male mouse population. Conducting repeated measurements of voiding not on the day of routine cage changes may help reduce variability.
Urine output is affected by time of day
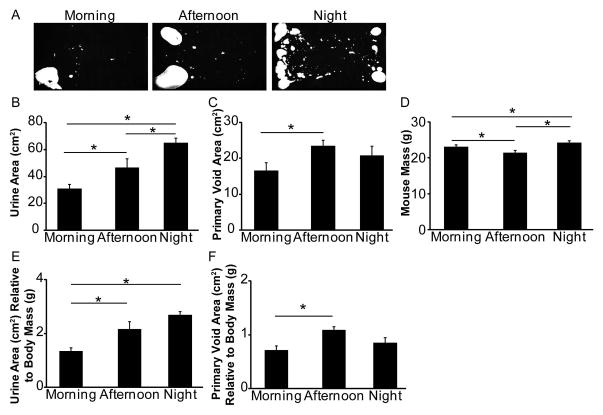
Mice are typically maintained on a 12 hour light/dark cycle and are most active during the dark cycle. Mouse and human voiding behavior change with activity (ie. mice urinate less frequently during the light cycle) and mice are known to have diurnal variation in urine production [18]. We next tested if time of day when voiding assays are conducted influences voiding patterns as assessed by the void spot assay. The same cohort of virgin male mice was tested either in the morning (9am-1pm), afternoon (2pm-6pm) or night (10pm-2am) during a one week period (Figure 5A, representative images from same animal). Total urine area was greater at night compared to morning and afternoon, and in afternoon compared to morning (Figure 5B). The primary void area was also greater in afternoon compared to morning (Figure 5C). There were no significant differences in spot number, spot size, percent area in primary void, corners or center (Supplemental Table V). We also observed a significant difference in body mass, lowest in the afternoon and highest at midnight (Figure 5D). The same voiding parameters (urine area and primary void area) were affected when voiding was normalized to body mass (Figure 5E–F, Supplemental Table V). Normalized values show an increase in total urine area from morning to afternoon or night, but not between afternoon and night (Figure 5E) and an increase in primary void area from morning to afternoon (Figure 5F). These results indicate that time of day influences male mouse urine output and conducting voiding assays at the same time of day should minimize variability.
Figure 5. Time of day influences voiding behavior in adult male mice.
(A) Representative images of spontaneous void assay testing in the same adult virgin male mice. Mice were tested in the morning (9am–1pm), afternoon (2pm–6pm) or night (10pm–2am) over a one week period. Quantification of (B) urine area, (C) primary void area, (D) body mass, (E) urine area normalized to body mass and (F) primary void area normalized to body mass. Results are mean±SE, n=8. Asterisk indicates significant differences p≤0.05.
Discussion
This study addressed animal husbandry and experimental design influences on spontaneous void spot assays for male C57Bl/6J mice. Isolating functional voiding parameters from confounding variables is crucial to accurately assess physiological mechanisms responsible for voiding function and dysfunction.
Our results indicate heterogeneity of voiding in relation to spot number as young adult male C57Bl/6J mice mature. This is likely due to multiple factors including increasing body mass and fluid requirements, establishing dominance and/or age associated changes of the LUT [6, 13–15]. Based on these findings, mice six weeks of age or older should be used to initiate a study. Our results also reveal a subpopulation of young adult male mice which display frequent spotting. These mice can be identified around six to nine weeks of age (coinciding with sexual maturity). Conducting baseline voiding assays prior to experimentation is essential for controlling the mouse population’s natural heterogeneity in voiding behavior. The underlying cause for the appearance of frequent spotters within a population is unknown and, in the inbred C57BL/6J mice used in this study, is not genetic. This pattern is typical of dominant male voiding behavior [14, 15]. Whether these characteristics are innate, potentially derived from an epigenetic origin, triggered by housing, stress or simply from some mice being messier than others in regard to urine placement and/or tracking through their urine or underlying inflammation/infection is an unknown mechanism that requires further study.
Our results indicate that male C57Bl/6J mice display more variability in day to day voiding compared to that of females [6]. We observed an increase in primary void area on the third consecutive void assay compared to the previous two days. No other voiding parameters were significantly altered. This variation could be due to habituation to the cage/filter paper environment and surroundings, both of which can influence behavior [19–20]. In an effort to reduce these variables, our assays were conducted in the same style of cage used for routine housing and the same rooms where mice are normally housed. These results suggest that void assays collected over multiple days may vary and one or two days of testing may be more accurate than three or more days of consecutive testing. We also found that routine cage changes and time of day are significant factors that need to be controlled in voiding studies using male mice.
Even though male mice scent mark their territory with urine in response to males of a different strain, they do not typically mark in response to their own urine or that of genetically identical mice [15,19–22]. Since the C57Bl/6J mice used here are genetically similar it is not surprising that our results indicate male mice can be group housed at weaning without altering voiding parameters by 6 weeks of age-thus saving on housing costs. However, our results did reveal moving from group to single housing once mice reached six weeks of age was sufficient to decrease urine area. These results are consistent with the fact that changes in caging density alone are sufficient to alter testosterone levels, which may subsequently influence voiding frequency in male mice [17]. Therefore, for all experiments requiring single housing for any portion of the experiment, singly housing mice for at least one week prior to and throughout the testing period should reduce variability in the void spot assay.
There is a strong hormonal influence on male mouse marking behavior. Castration decreases marking while addition of testosterone propionate and estradiol benzoate restore marking behavior in castrated males [14, 23, 24]. Postnatally androgenized female mice also exhibit increased marking behavior [23]. Therefore, chemical or genetic studies that alter mouse hormone levels could potentially change void spot assay endpoints independent of physiological changes of the prostate, bladder, urethra or other anatomical sites. Additionally our studies were conducted on wild type mice. Studies using animal models of bladder inflammation, obstruction, diabetes etc. in which voiding is altered may require additional analysis (i.e categorizing spot number by size) [25] or optimization (i.e animal models of diabetes which void large volumes of urine may not require a four hour testing period).
As with any method there are both advantages and limitations to the void spot assay. Advantages include its ease, requiring just cages and filter papers and a four hour time period. One of the biggest advantages is that it can produce repeated measurements on the same animal. Limitations are that it is a semi-quantitative analysis. Void spots can be overlapped on the paper making quantitation more difficult and mice are able to walk freely raising the possibility that they can leave spots on the paper from their paws or tails. Similarly it is difficult to truly acclimatize mice to filter papers since changing out filter paper introduces a new surface to mark on which to mark. Adaptations to the void spot assay using wire mesh to suspend mice over the filter paper or video recording of voiding events can be used as complementary approaches to minimize some of these variables. Overall the void spot assay is a valuable tool that can be used in combination with other complementary methods to analyze voiding function in mice.
Conclusion
Several steps can be taken to reduce variability when conducting void spot assays on C57Bl/6J male mice. Animals can be weaned with male littermates to group housing (two to six mice/cage) until at least six weeks of age. Baseline assays around six weeks of age allow for the identification and distribution of frequent spotters into the experimental design. During the study, conducting void spot assays on the same day of the week, at the same time of day, not on the day of a cage change and reducing changes in housing density should reduce variability. These practices will help minimize variability due to handling, housing and behavior and allow for a better representation of physiologically relevant changes in voiding function.
Supplementary Material
(A) Raw image of filter paper under UV illumination, asterisks indicate overlapping spots. (B) The same image after thresholding. (C) Same image after Image J particle analysis on threshold image reveals overlapping spots as one large spot. (D–F) Same image showing correction for overlapping spots. Some overlapping spots can be identified in the raw image and their area calculated by hand using the freehand selection tool in Image J. Yellow line indicates manual outline of separate spots calculated independently.
Body mass was measured and void assays conducted on a cohort of mice age 3–9 weeks of age. Pearson’s correlation coefficient was determined for body mass and (A) spot number (r=0.6, p=0.0001), (B) total urine area (r=0.8, p=1.21×10−8), (C) primary void area (r=0.6, p=2.22×10−5) and (D) percent total urine area within corners (r=0.4, p=0.029). n=32.
Acknowledgments
The authors wish to thank Joan Jorgensen for her technical assistance and National Institutes of Health Grants DK099328 (CMV), DK097826 (WB), DK093690 and ESO18764 (WAR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173:1309–13. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Lysiak JJ, Steers WD. Bladder and urethral function in a mouse model of cavernous nerve injury. Neurourol Urodyn. 2013;32:1038–1043. doi: 10.1002/nau.22354. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Daneshgari F, Liu G. Successful induction of stress urinary incontinence in mice by vaginal distension does not depend on the estrous cycle. Urology. 2014;83:958.e1–6. doi: 10.1016/j.urology.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 2012;153:5556–5565. doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 2013;27:1950–1961. doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol. 2014;306:F1296–307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundbeck F, Oussoren Y, Stewart FA. Early and late damage in the mouse bladder after radiation combined with cyclophosphamide or cisplatinum, evaluated by two different functional assays. Acta Oncol. 1993;32:679–687. doi: 10.3109/02841869309092452. [DOI] [PubMed] [Google Scholar]
- 8.Stewart FA, Lundbeck F, Oussoren Y, Luts A. Acute and late radiation damage in mouse bladder: a comparison of urination frequency and cystometry. Int J Radiat Oncol Biol Phys. 1991;21:1211–1219. doi: 10.1016/0360-3016(91)90278-c. [DOI] [PubMed] [Google Scholar]
- 9.Stevens G, Joiner M, Joiner B, Johns H, Denekamp J. Early detection of damage following bilateral renal irradiation in the mouse. Radiother Oncol. 1991;20:124–131. doi: 10.1016/0167-8140(91)90146-8. [DOI] [PubMed] [Google Scholar]
- 10.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn. 2008;27:548–552. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 11.Hodges SJ, Zhou G, Deng F, Aboushwareb T, Turner C, Andersson K, Santago P, Case D, Sun T, Christ GJ. Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin ii knockout mice. J Urol. 2008;179:2046–2051. doi: 10.1016/j.juro.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate. 2013;73:1123–1133. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol (Lond) 2014;592:537–549. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 15.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen S, Miller MM, Filipski SB, Tolwani RJ. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci. 2011;50:479–483. [PMC free article] [PubMed] [Google Scholar]
- 17.Mucignat-Caretta C, Cavaggioni A, Redaelli M, Da Dalt L, Zagotto G, Gabai G. Age and isolation influence steroids release and chemical signaling in male mice. Steroids. 2014;83:10–16. doi: 10.1016/j.steroids.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Negoro H, Kanematsu A, Matsuo M, Okamura H, Tabata Y, Ogawa O. Development of diurnal micturition pattern in mice after weaning. J Urol. 2013;189:740–746. doi: 10.1016/j.juro.2012.07.140. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in c57bl/6j mice. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevison CM, Barnard CJ, Beynon RJ, Hurst JL. The consequences of inbreeding for recognizing competitors. Proc Biol Sci. 2000;267:687–694. doi: 10.1098/rspb.2000.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Natl Acad Sci US A. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Horm Behav. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 24.Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson TM, Moses M, Uchtmann K, Keil KP, Bjorling DE, Vezina CM, Wood RW, Ricke WA. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol. 2014 doi: 10.1016/j.juro.2014.08.093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Raw image of filter paper under UV illumination, asterisks indicate overlapping spots. (B) The same image after thresholding. (C) Same image after Image J particle analysis on threshold image reveals overlapping spots as one large spot. (D–F) Same image showing correction for overlapping spots. Some overlapping spots can be identified in the raw image and their area calculated by hand using the freehand selection tool in Image J. Yellow line indicates manual outline of separate spots calculated independently.
Body mass was measured and void assays conducted on a cohort of mice age 3–9 weeks of age. Pearson’s correlation coefficient was determined for body mass and (A) spot number (r=0.6, p=0.0001), (B) total urine area (r=0.8, p=1.21×10−8), (C) primary void area (r=0.6, p=2.22×10−5) and (D) percent total urine area within corners (r=0.4, p=0.029). n=32.