Abstract
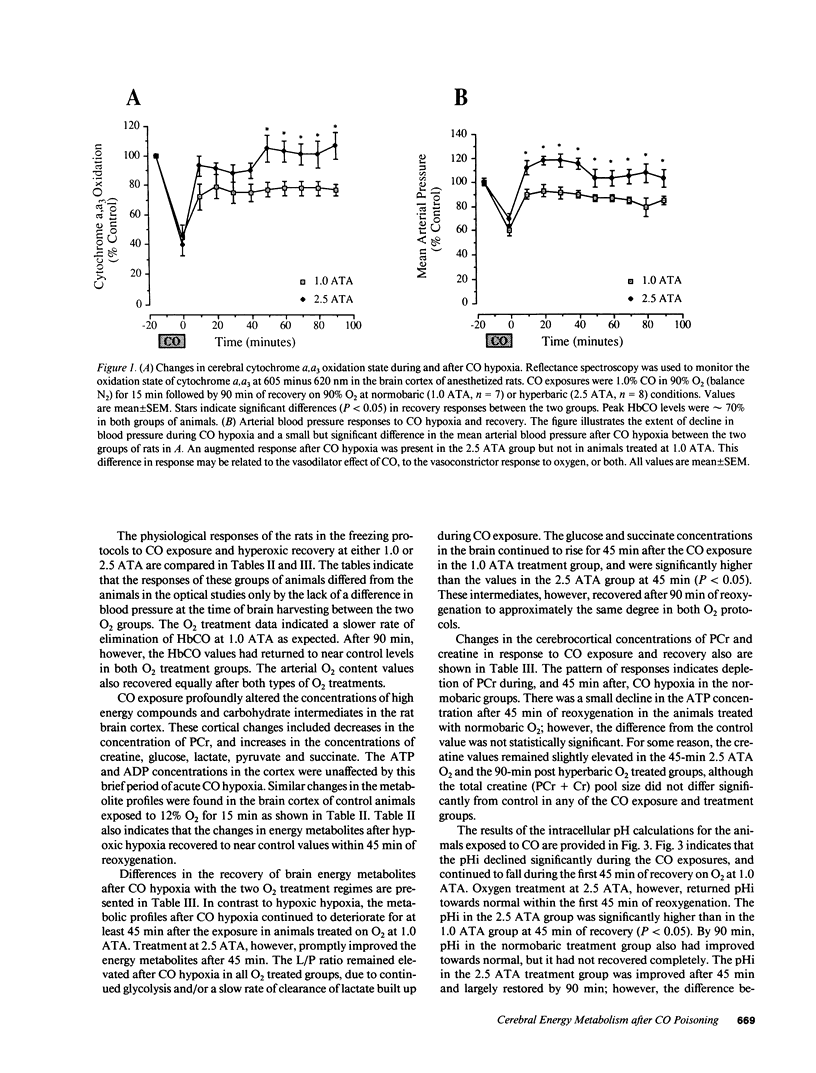
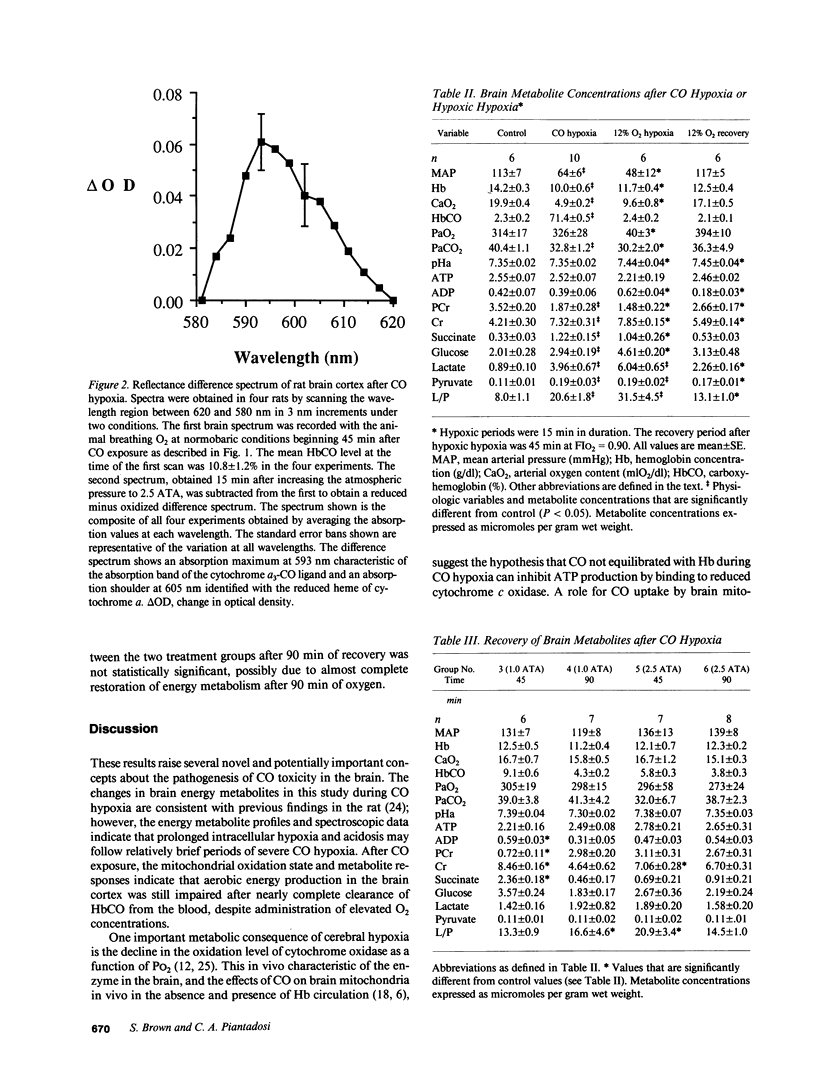
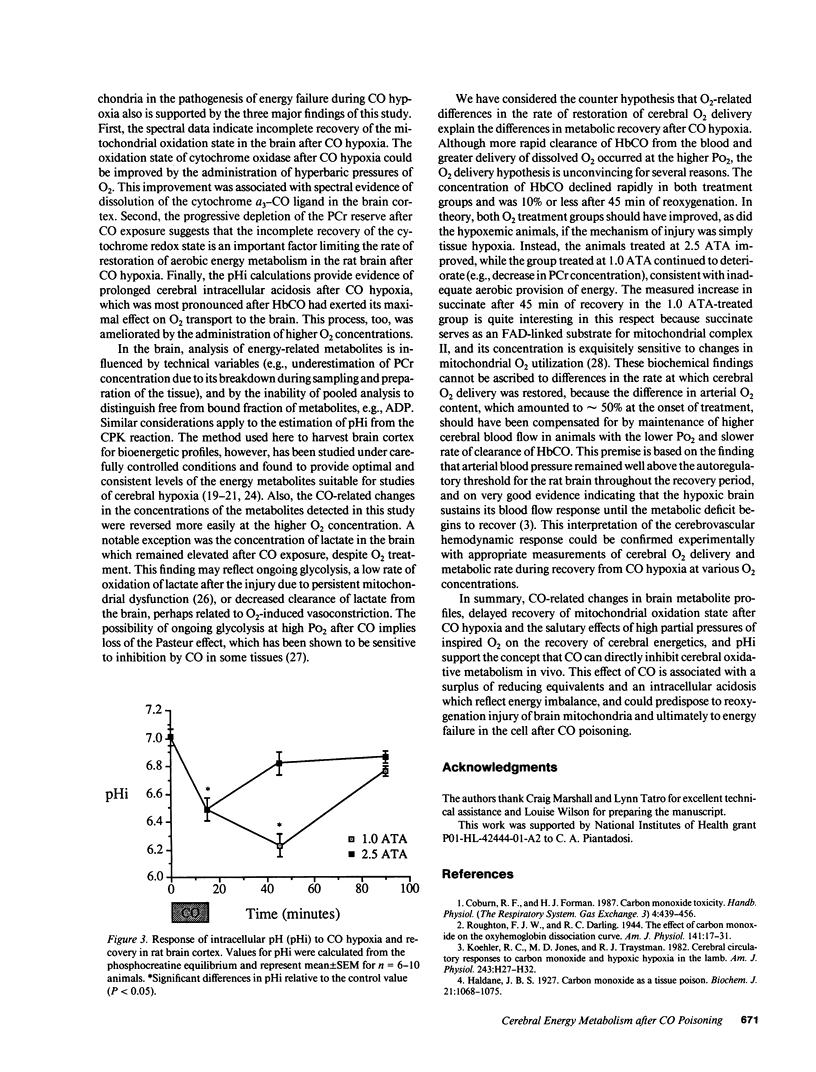
Carbon monoxide (CO) may inhibit mitochondrial electron transport in the brain and increase the toxic effects of the gas. This hypothesis was investigated in anesthetized rats during CO exposure and recovery at either normobaric or hyperbaric O2 concentrations. During exposure and recovery, we measured the oxidation level of cerebrocortical cytochrome c oxidase by differential spectroscopy and biochemical metabolites known to reflect aerobic energy provision in the brain. CO exposure (HbCO = 71 +/- 1%) significantly decreased blood pressure and cytochrome oxidation level. Cerebral ATP was maintained while lactate/pyruvate, glucose, and succinate rose, and phosphocreatine (PCr) fell, relative to control (P less than 0.05). Intracellular pH (pHi) calculated from the PCr equilibrium also declined during the exposures. During recovery, HbCO fell more rapidly at hyperbaric than at normobaric O2 levels, but returned to 10% or less in both groups by 45 min. Cytochrome oxidation state improved to 80% of control after 90 min at normobaric O2, but recovered completely after hyperbaric O2 (P less than 0.05). In normobaric O2, PCr and pHi continued to fall for 45 min after CO exposure and did not recover completely by 90 min. PCr and pHi in animals after hyperbaric O2 improved within 45 min, but also remained below control at 90 min. These data indicate that intracellular uptake of CO can impair cerebral energy metabolism, despite the elimination of HbCO from the blood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. D., Piantadosi C. A. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol (1985) 1990 Feb;68(2):604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- Chance B., Erecinska M., Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann N Y Acad Sci. 1970 Oct 5;174(1):193–204. doi: 10.1111/j.1749-6632.1970.tb49786.x. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Mayers L. B. Myoglobin O2 tension determined from measurement of carboxymyoglobin in skeletal muscle. Am J Physiol. 1971 Jan;220(1):66–74. doi: 10.1152/ajplegacy.1971.220.1.66. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., MacMillan V., Siesjö B. K. The effect of hypercapnic acidosis upon some glycolytic and Krebs cycle-associated intermediates in the rat brain. J Neurochem. 1972 Nov;19(11):2507–2517. doi: 10.1111/j.1471-4159.1972.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Haldane J. B. Carbon Monoxide as a Tissue Poison. Biochem J. 1927;21(5):1068–1075. doi: 10.1042/bj0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Keizer J. H., LaManna J. C., Rosenthal M. Reflectance spectrophotometry of cytochrome aa3 in vivo. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):858–872. doi: 10.1152/jappl.1977.43.5.858. [DOI] [PubMed] [Google Scholar]
- Koehler R. C., Jones M. D., Jr, Traystman R. J. Cerebral circulatory response to carbon monoxide and hypoxic hypoxia in the lamb. Am J Physiol. 1982 Jul;243(1):H27–H32. doi: 10.1152/ajpheart.1982.243.1.H27. [DOI] [PubMed] [Google Scholar]
- Kreisman N. R., Sick T. J., LaManna J. C., Rosenthal M. Local tissue oxygen tension-cytochrome a,a3 redox relationships in rat cerebral cortex in vivo. Brain Res. 1981 Aug 10;218(1-2):161–174. doi: 10.1016/0006-8993(81)91298-1. [DOI] [PubMed] [Google Scholar]
- Laser H. Tissue metabolism under the influence of carbon monoxide. Biochem J. 1937 Sep;31(9):1677–1682. doi: 10.1042/bj0311677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan V. The effects of acute carbon monoxide intoxication on the cerebral energy metabolism of the rat. Can J Physiol Pharmacol. 1975 Jun;53(3):354–362. doi: 10.1139/y75-051. [DOI] [PubMed] [Google Scholar]
- Piantadosi C. A., Jöbsis-Vandervliet F. F. Spectrophotometry of cerebral cytochrome a, a3 in bloodless rats. Brain Res. 1984 Jul 2;305(1):89–94. doi: 10.1016/0006-8993(84)91122-3. [DOI] [PubMed] [Google Scholar]
- Piantadosi C. A., Lee P. A., Sylvia A. L. Direct effects of CO on cerebral energy metabolism in bloodless rats. J Appl Physiol (1985) 1988 Aug;65(2):878–887. doi: 10.1152/jappl.1988.65.2.878. [DOI] [PubMed] [Google Scholar]
- Piantadosi C. A., Sylvia A. L., Jöbsis F. F. Cyanide-induced cytochrome a,a3 oxidation-reduction responses in rat brain in vivo. J Clin Invest. 1983 Oct;72(4):1224–1233. doi: 10.1172/JCI111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén U., Ratcheson R. A., Salford L. G., Siesjö B. K. Optimal freezing conditions for cerebral metabolites in rats. J Neurochem. 1973 Nov;21(5):1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Tamura M., Oshino N., Chance B., Silver I. A. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys. 1978 Nov;191(1):8–22. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- Yoshikawa S., Caughey W. S. Heart cytochrome c oxidase. An infrared study of effects of oxidation state on carbon monoxide binding. J Biol Chem. 1982 Jan 10;257(1):412–420. [PubMed] [Google Scholar]


