Abstract
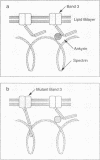
Hereditary ovalocytic red cells are characterized by a marked increase in membrane rigidity and resistance to invasion by malarial parasites. The underlying molecular defect in ovalocytes remained a mystery until Liu and colleagues (N. Engl. J. Med. 1990. 323:1530-38) made the surprising observation that the ovalocytic phenotype was linked to a structural polymorphism in band 3, the anion transporter. We have now defined the mutation in band 3 gene and established the biophysical sequelae of this mutation. This mutation involves the deletion of amino-acids 400-408 in the boundary between the cytoplasmic and the first transmembrane domains of band 3. The biophysical consequences of this mutation are a marked decrease in lateral mobility of band 3 and an increase in membrane rigidity. Based on these findings, we propose the following model for increased membrane rigidity. The mutation induces a conformational change in the cytoplasmic domain of band 3, leading to its entanglement in the skeletal protein network. This entanglement inhibits the normal unwinding and stretching of the spectrin tetramers necessary for membrane extension, leading to increased rigidity. These findings imply that the cytoplasmic domain of an integral membrane protein can have profound effects on membrane material behavior.
Full text
PDF

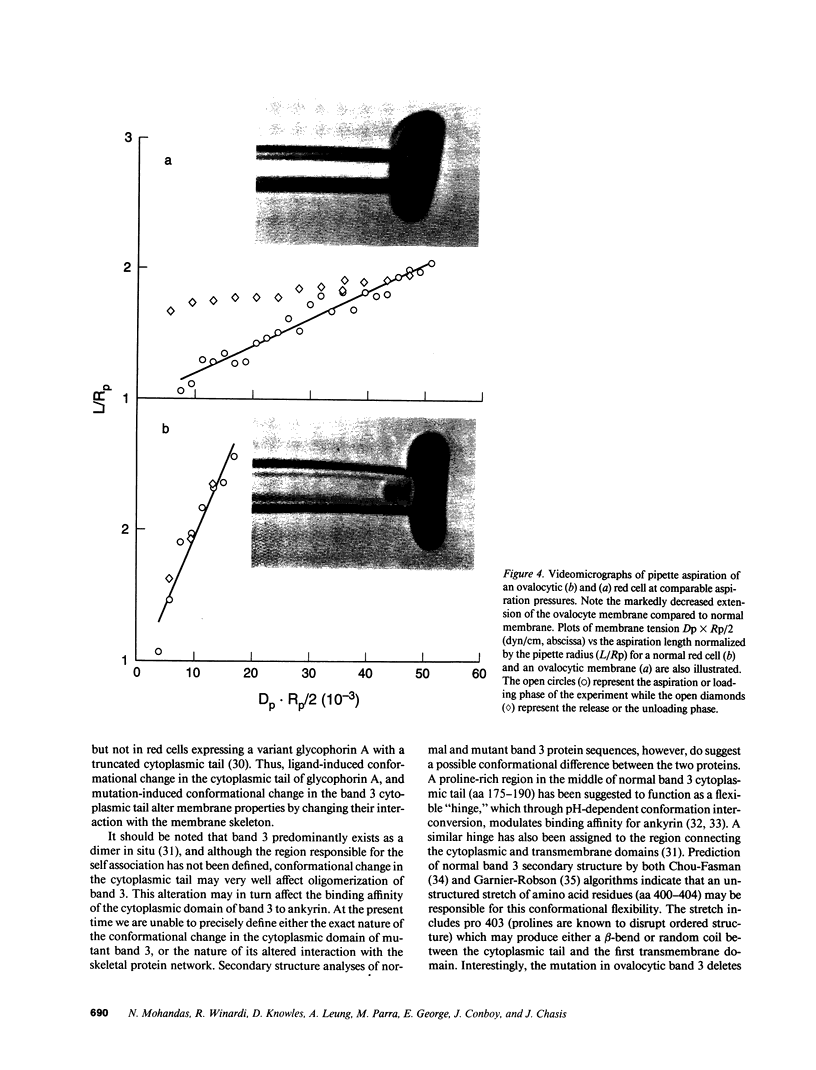


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato D., Booth P. B. Hereditary ovalocytosis in Melanesians. P N G Med J. 1977 Mar;20(1):26–32. [PubMed] [Google Scholar]
- Baer A., Lie-Injo L. E., Welch Q. B., Lewis A. N. Genetic factors and malaria in the Temuan. Am J Hum Genet. 1976 Mar;28(2):179–188. [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N., Shohet S. B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985 Jun;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Reid M. E., Jensen R. H., Mohandas N. Signal transduction by glycophorin A: role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol. 1988 Oct;107(4):1351–1357. doi: 10.1083/jcb.107.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Davis L., Lux S. E., Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J Biol Chem. 1989 Jun 5;264(16):9665–9672. [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Evans E., Mohandas N., Leung A. Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J Clin Invest. 1984 Feb;73(2):477–488. doi: 10.1172/JCI111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- George E., Mohandas N., Duraisamy G., Adeeb N., Zainuddin Z. A., Teng M. S., Vimala R. Hereditary ovalocytosis in Malays. Med J Malaysia. 1988 Dec;43(4):327–331. [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley T., Saul A., Lamont G., Hudson D. E., Miller L. H., Kidson C. Resistance of Melanesian elliptocytes (ovalocytes) to invasion by Plasmodium knowlesi and Plasmodium falciparum malaria parasites in vitro. J Clin Invest. 1983 Mar;71(3):780–782. doi: 10.1172/JCI110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Kidson C., Lamont G., Saul A., Nurse G. T. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5829–5832. doi: 10.1073/pnas.78.9.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M., Lodish H. F. Structure and organization of the murine band 3 gene. J Biol Chem. 1987 Jun 15;262(17):8035–8040. [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Zhai S., Palek J., Golan D. E., Amato D., Hassan K., Nurse G. T., Babona D., Coetzer T., Jarolim P. Molecular defect of the band 3 protein in southeast Asian ovalocytosis. N Engl J Med. 1990 Nov 29;323(22):1530–1538. doi: 10.1056/NEJM199011293232205. [DOI] [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A., Shohet S. B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983 Jul;20(3):225–242. [PubMed] [Google Scholar]
- Mohandas N., Lie-Injo L. E., Friedman M., Mak J. W. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984 Jun;63(6):1385–1392. [PubMed] [Google Scholar]
- Mueller T. J., Morrison M. Detection of a variant of protein 3, the major transmembrane protein of the human erythrocyte. J Biol Chem. 1977 Oct 10;252(19):6573–6576. [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Ranney H. M., Rosenberg G. H., Morrison M., Mueller T. J. Frequencies of Band 3 variants of human red cell membranes in some different populations. Br J Haematol. 1990 Jun;75(2):262–267. doi: 10.1111/j.1365-2141.1990.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Saul A., Lamont G., Sawyer W. H., Kidson C. Decreased membrane deformability in Melanesian ovalocytes from Papua New Guinea. J Cell Biol. 1984 Apr;98(4):1348–1354. doi: 10.1083/jcb.98.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Temperature dependence of the yield shear resultant and the plastic viscosity coefficient of erythrocyte membrane. Implications about molecular events during membrane failure. Biophys J. 1982 Sep;39(3):273–278. doi: 10.1016/S0006-3495(82)84517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoukakos D., Vasseur C., Driancourt C., Blouquit Y., Delaunay J., Wajcman H., Bursaux E. Human erythrocyte band 3 polymorphism (band 3 Memphis): characterization of the structural modification (Lys 56----Glu) by protein chemistry methods. Blood. 1991 Aug 15;78(4):1117–1120. [PubMed] [Google Scholar]