Abstract
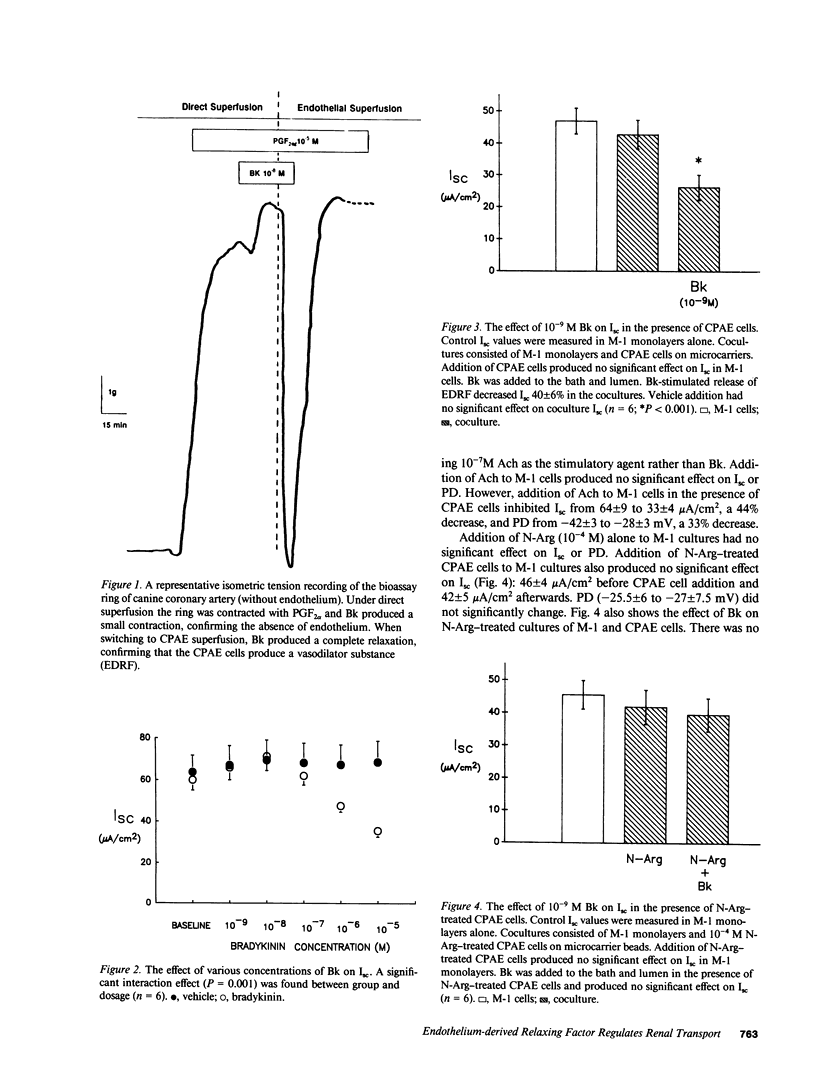
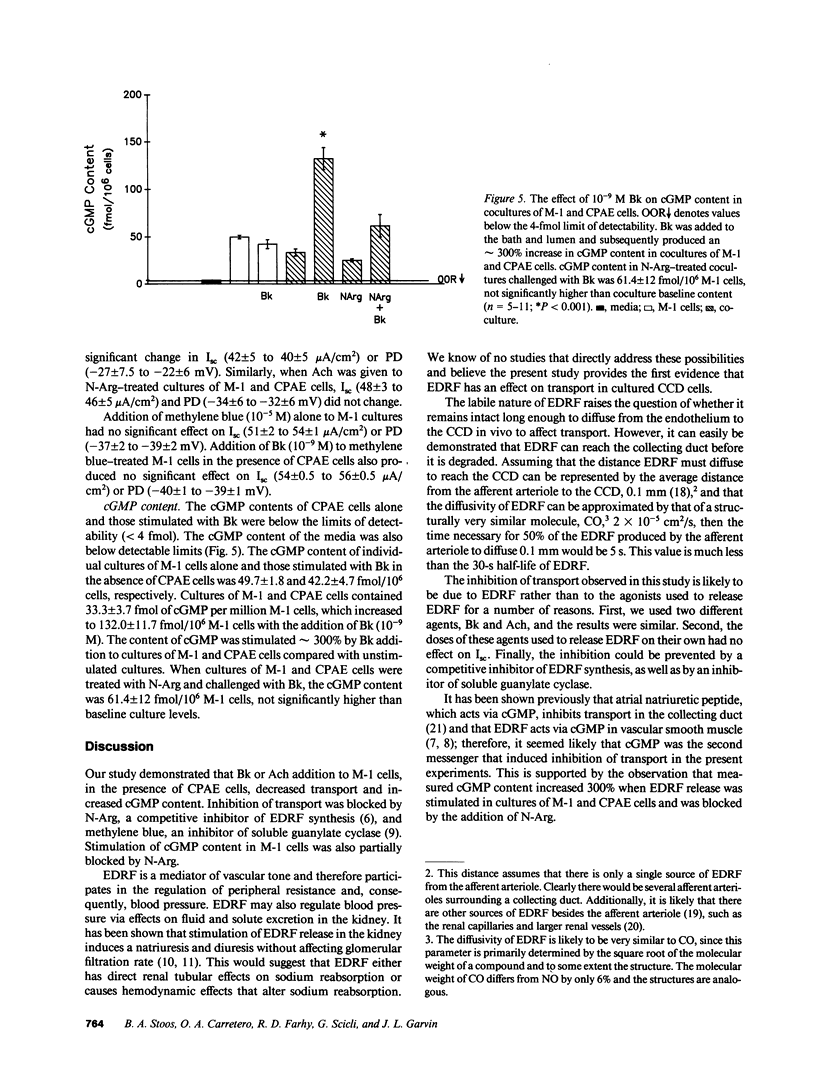
Stimulation of the release of endothelium-derived relaxing factor (EDRF) in the kidney has been shown to result in natriuresis without affecting glomerular filtration rate. This may be due to EDRF directly regulating solute transport in the cortical collecting duct (CCD). To test this hypothesis, we measured the effect of bradykinin (Bk) or acetylcholine (Ach) on short-circuit current (Isc; a measure of active transport) in a CCD cell line (M-1), in the presence or absence of cow pulmonary artery endothelial (CPAE) cells. 10(-9) M Bk or 10(-7) M Ach had no effect on M-1 Isc in which CPAE cells were absent. The addition of CPAE cells to M-1 cells also did not affect M-1 Isc. On the other hand, when 10(-9) M Bk or 10(-7) M Ach were added to M-1 cells in the presence of CPAE cells, Isc decreased from 43 +/- 4.5 to 26 +/- 4 and 64 +/- 9 to 33 +/- 4 microA/cm2, respectively (P less than 0.001). Nitroarginine (N-Arg, 10(-4) M), a competitive inhibitor of EDRF production, blocked the inhibition in M-1 Isc due to both agonists. Since cGMP is the second messenger of EDRF in vascular smooth muscle, we measured the effects of Bk on cGMP production in M-1 cells in the presence and absence of CPAE cells. Bk increased cGMP content in M-1 cells in the presence of CPAE cells from 33 +/- 3.4 to 132 +/- 11.7 fmol/10(6) M-1 cells (P less than 0.001). When cultures of M-1 and CPAE cells were treated with N-Arg and challenged with Bk, Bk's effect on cGMP was partially blocked (61.4 +/- 12 fmol/10(-6) M-1 cells; NS). These data suggest that EDRF inhibits transport and increases cGMP content in M-1 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Ito S., Johnson C. S., Carretero O. A. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991 May;87(5):1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon V., Harris R. C., Ichikawa I. A regulatory role for large vessels in organ circulation. Endothelial cells of the main renal artery modulate intrarenal hemodynamics in the rat. J Clin Invest. 1990 Jun;85(6):1728–1733. doi: 10.1172/JCI114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Raij L., Romero J. C. Effects of NG-monomethyl-L-arginine and L-arginine on acetylcholine renal response. Hypertension. 1990 Jun;15(6 Pt 1):659–663. doi: 10.1161/01.hyp.15.6.659. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Romero J. C. Mediatory role of endothelium-derived nitric oxide in renal vasodilatory and excretory effects of bradykinin. Am J Hypertens. 1991 Mar;4(3 Pt 1):260–262. doi: 10.1093/ajh/4.3.260. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Sands J. M., Knepper M. A. ANF inhibits NaCl and fluid absorption in cortical collecting duct of rat kidney. Am J Physiol. 1989 Jan;256(1 Pt 2):F179–F186. doi: 10.1152/ajprenal.1989.256.1.F179. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Sands J. M., Knepper M. A. Atrial natriuretic factor inhibits vasopressin-stimulated osmotic water permeability in rat inner medullary collecting duct. J Clin Invest. 1988 Oct;82(4):1383–1390. doi: 10.1172/JCI113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kudo L. H. Atrial peptide and cGMP effects on NaCl transport in inner medullary collecting duct. Am J Physiol. 1990 Aug;259(2 Pt 2):F258–F268. doi: 10.1152/ajprenal.1990.259.2.F258. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Schorer A. E., Raij L. Effects of endothelium-derived relaxing factor and nitric oxide on rat mesangial cells. Am J Physiol. 1990 Jan;258(1 Pt 2):F162–F167. doi: 10.1152/ajprenal.1990.258.1.F162. [DOI] [PubMed] [Google Scholar]
- Stoos B. A., Náray-Fejes-Tóth A., Carretero O. A., Ito S., Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991 Jun;39(6):1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Endothelium and control of vascular function. State of the Art lecture. Hypertension. 1989 Jun;13(6 Pt 2):658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension. 1990 Aug;16(2):162–169. doi: 10.1161/01.hyp.16.2.162. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Kikeri D., Silva P., Burrowes M., Brenner B. M. Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Invest. 1988 Sep;82(3):1067–1074. doi: 10.1172/JCI113663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Brenner B. M., Seifter J. L. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol. 1987 Mar;252(3 Pt 2):F551–F559. doi: 10.1152/ajprenal.1987.252.3.F551. [DOI] [PubMed] [Google Scholar]


