Abstract
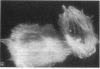
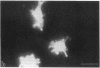
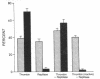
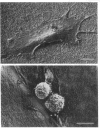
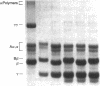
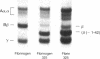
Adhesion and spreading of cultured human umbilical vein endothelial cells on fibrin surfaces of varying structure were characterized to understand better the interactions occurring between endothelium and fibrin at sites of vascular injury. Fibrin prepared with reptilase, which cleaves only fibrinopeptide A from fibrinogen, and fibrin prepared with thrombin, which cleaves both fibrinopeptide A and fibrinopeptide B, equally supported endothelial cell adhesion. In contrast, only fibrin made with thrombin mediated endothelial cell spreading, as assessed by fluorescence microscopy of cells stained with rhodamine phalloidin to identify actin stress fibers or by scanning electron microscopy. Fibrin prepared with reptilase failed to support cell spreading. To further investigate the role of the amino terminus of the fibrin beta chain after fibrinopeptide B cleavage in promoting cell spreading, protease III from Crotalus atrox venom was used to specifically cleave the amino-terminal 42 residues of the fibrinogen B beta chain. After clotting with thrombin, this fibrin derivative lacking B beta 1-42 failed to support significant cell spreading. Spreading on fibrin was unaffected by depletion of Weibel-Palade bodies from endothelial cells, indicating that the spreading was independent of stimulated von Willebrand factor release. We conclude that endothelial cell spreading on fibrin requires fibrinopeptide B cleavage and involves residues 15-42 of the fibrin beta chain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R. G., Saldeen K., Saldeen T. A fibrin(ogen) derived pentapeptide induces vasodilation, prostacyclin release and an increase in cyclic AMP. Thromb Res. 1983 May 1;30(3):213–218. doi: 10.1016/0049-3848(83)90074-9. [DOI] [PubMed] [Google Scholar]
- BAILEY K., BETTELHEIM F. R., LORAND L., MIDDLEBROOK W. R. Action of thrombin in the clotting of fibrinogen. Nature. 1951 Feb 10;167(4241):233–234. doi: 10.1038/167233a0. [DOI] [PubMed] [Google Scholar]
- BLOMBACK B., BLOMBACK M., NILSSON I. M. Coagulation studies on reptilase, an extract of the venom from Bothrops jararaca. Thromb Diath Haemorrh. 1958 Apr 15;1(1):76–86. [PubMed] [Google Scholar]
- Bar-Shavit R., Sabbah V., Lampugnani M. G., Marchisio P. C., Fenton J. W., 2nd, Vlodavsky I., Dejana E. An Arg-Gly-Asp sequence within thrombin promotes endothelial cell adhesion. J Cell Biol. 1991 Jan;112(2):335–344. doi: 10.1083/jcb.112.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha K., Müller-Peddinghaus R., Van Rooijen L. A. Bradykinin and thrombin effects on polyphosphoinositide hydrolysis and prostacyclin production in endothelial cells. Biochem J. 1989 Oct 1;263(1):149–155. doi: 10.1042/bj2630149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson C. T., Knowles W. J., Bell L., Albelda S. M., Castronovo V., Liotta L. A., Madri J. A. Spatiotemporal segregation of endothelial cell integrin and nonintegrin extracellular matrix-binding proteins during adhesion events. J Cell Biol. 1990 Mar;110(3):789–801. doi: 10.1083/jcb.110.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belew M., Gerdin B., Porath J., Saldeen T. Isolation of vasoactive peptides from human fibrin and fibrinogen degraded by plasmin. Thromb Res. 1978 Dec;13(6):983–994. doi: 10.1016/0049-3848(78)90227-x. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chen C. S., Chou S. H., Thiagarajan P. Fibrin(ogen) peptide B beta 15-42 inhibits platelet aggregation and fibrinogen binding to activated platelets. Biochemistry. 1988 Aug 9;27(16):6121–6126. doi: 10.1021/bi00416a044. [DOI] [PubMed] [Google Scholar]
- Cheng Y. F., Kramer R. H. Human microvascular endothelial cells express integrin-related complexes that mediate adhesion to the extracellular matrix. J Cell Physiol. 1989 May;139(2):275–286. doi: 10.1002/jcp.1041390209. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Berliner S. A., Vicente V., Ruggeri Z. M. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989 Sep 8;58(5):945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D., Matsuo O., Stassen J. M., Kettner C., Shaw E. In vivo studies of a synthetic inhibitor of thrombin. J Lab Clin Med. 1982 Jan;99(1):76–83. [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Lampugnani M. G., Giorgi M., Gaboli M., Federici A. B., Ruggeri Z. M., Marchisio P. C. Von Willebrand factor promotes endothelial cell adhesion via an Arg-Gly-Asp-dependent mechanism. J Cell Biol. 1989 Jul;109(1):367–375. doi: 10.1083/jcb.109.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Lampugnani M. G., Giorgi M., Gaboli M., Marchisio P. C. Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood. 1990 Apr 1;75(7):1509–1517. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Furie B., Bing D. H., Feldmann R. J., Robison D. J., Burnier J. P., Furie B. C. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J Biol Chem. 1982 Apr 10;257(7):3875–3882. [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Høglund A. S., Nilsen E. Actin pools and actin microfilament organization in cultured human endothelial cells after exposure to thrombin. Br J Haematol. 1984 Dec;58(4):617–625. doi: 10.1111/j.1365-2141.1984.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Gerdin B., Lindeberg G., Ragnarsson U., Saldeen T., Wallin R. Structural requirements for microvascular permeability-increasing ability of peptides. Studies on analogues of a fibrinogen pentapeptide fragment. Biochim Biophys Acta. 1983 Jun 9;757(3):366–370. doi: 10.1016/0304-4165(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Feld M., Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980 Feb;19(2):517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Hui K. Y., Haber E., Matsueda G. R. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983 Dec 9;222(4628):1129–1132. doi: 10.1126/science.6648524. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E., McDonagh J. Thrombin binding to the A alpha-, B beta-, and gamma-chains of fibrinogen and to their remnants contained in fragment E. J Biol Chem. 1988 Sep 25;263(27):13896–13900. [PubMed] [Google Scholar]
- Kadish J. L., Butterfield C. E., Folkman J. The effect of fibrin on cultured vascular endothelial cells. Tissue Cell. 1979;11(1):99–108. doi: 10.1016/0040-8166(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Inhibited thrombins. Interactions with fibrinogen and fibrin. Biochem J. 1987 Mar 15;242(3):881–887. doi: 10.1042/bj2420881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Cheng Y. F., Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990 Sep;111(3):1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryk B., Rohoza A., Ahadi M., Chin J., Wiebe M. E. Specificity of a monoclonal antibody for the NH2-terminal region of fibrin. Mol Immunol. 1984 Jan;21(1):89–94. doi: 10.1016/0161-5890(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Colotta F., Polentarutti N., Pedenovi M., Mantovani A., Dejana E. Thrombin induces c-fos expression in cultured human endothelial cells by a Ca2(+)-dependent mechanism. Blood. 1990 Sep 15;76(6):1173–1180. [PubMed] [Google Scholar]
- Languino L. R., Colella S., Zanetti A., Andrieux A., Ryckewaert J. J., Charon M. H., Marchisio P. C., Plow E. F., Ginsberg M. H., Marguerie G. Fibrinogen-endothelial cell interaction in vitro: a pathway mediated by an Arg-Gly-Asp recognition specificity. Blood. 1989 Feb 15;73(3):734–742. [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G., Marzec U., Anderson J., Harker L. A. Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 1984 Dec;74(6):1988–1995. doi: 10.1172/JCI111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G., Santell L. Stimulation and desensitization of tissue plasminogen activator release from human endothelial cells. J Biol Chem. 1988 Jul 5;263(19):9360–9365. [PubMed] [Google Scholar]
- Levin E. G., Santell L. Thrombin- and histamine-induced signal transduction in human endothelial cells. Stimulation and agonist-dependent desensitization of protein phosphorylation. J Biol Chem. 1991 Jan 5;266(1):174–181. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R. A., Scheraga H. A. Steady-state kinetic study of the bovine thrombin-fibrinogen interaction. Biochemistry. 1980 May 27;19(11):2343–2350. doi: 10.1021/bi00552a010. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Loftus J. C., Du X. P., Glass A. A., Ruggeri Z. M., Shattil S. J., Plow E. F., Ginsberg M. H. Affinity modulation of the alpha IIb beta 3 integrin (platelet GPIIb-IIIa) is an intrinsic property of the receptor. Cell Regul. 1990 Nov;1(12):883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya B. V., Budzynski A. Z. Anticoagulant proteases from western diamondback rattlesnake (Crotalus atrox) venom. Biochemistry. 1984 Jan 31;23(3):460–470. doi: 10.1021/bi00298a010. [DOI] [PubMed] [Google Scholar]
- Pandya B. V., Cierniewski C. S., Budzynski A. Z. Conservation of human fibrinogen conformation after cleavage of the B beta chain NH2 terminus. J Biol Chem. 1985 Mar 10;260(5):2994–3000. [PubMed] [Google Scholar]
- Pandya B. V., Gabriel J. L., O'Brien J., Budzynski A. Z. Polymerization site in the beta chain of fibrin: mapping of the B beta 1-55 sequence. Biochemistry. 1991 Jan 8;30(1):162–168. doi: 10.1021/bi00215a024. [DOI] [PubMed] [Google Scholar]
- Ribes J. A., Francis C. W., Wagner D. D. Fibrin induces release of von Willebrand factor from endothelial cells. J Clin Invest. 1987 Jan;79(1):117–123. doi: 10.1172/JCI112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes J. A., Ni F., Wagner D. D., Francis C. W. Mediation of fibrin-induced release of von Willebrand factor from cultured endothelial cells by the fibrin beta chain. J Clin Invest. 1989 Aug;84(2):435–442. doi: 10.1172/JCI114184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland F. N., Donovan M. J., Picciano P. T., Wilner G. D., Kreutzer D. L. Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol. 1984 Dec;117(3):418–428. [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The effect of fibrin-stabilizing factor on the subunit structure of human fibrin. J Clin Invest. 1971 Jul;50(7):1506–1513. doi: 10.1172/JCI106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Skogen W. F., Griffin G. L., Wilner G. D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986 Mar;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. Integrin (alpha v beta 3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990 Feb 5;265(4):2168–2172. [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vali Z., Scheraga H. A. Localization of the binding site on fibrin for the secondary binding site of thrombin. Biochemistry. 1988 Mar 22;27(6):1956–1963. doi: 10.1021/bi00406a023. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weimar B., Delvos U. The mechanism of fibrin-induced disorganization of cultured human endothelial cell monolayers. Arteriosclerosis. 1986 Mar-Apr;6(2):139–145. doi: 10.1161/01.atv.6.2.139. [DOI] [PubMed] [Google Scholar]
- Weisel J. W. Fibrin assembly. Lateral aggregation and the role of the two pairs of fibrinopeptides. Biophys J. 1986 Dec;50(6):1079–1093. doi: 10.1016/S0006-3495(86)83552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J. I., Hudoba M., Massel D., Maraganore J., Hirsh J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990 Aug;86(2):385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol. 1988 Nov;107(5):1777–1783. doi: 10.1083/jcb.107.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron C., Ginsberg M. H., Plow E. F. Monoclonal antibodies specific for a conformationally altered state of fibrinogen. Thromb Haemost. 1990 Aug 13;64(1):41–46. [PubMed] [Google Scholar]