Abstract
Despite substantial clinical advances over the past 65 years, cardiovascular disease remains the leading cause of death in America. The past 15 years has witnessed major basic and translational interest in the use of stem and/or precursor cells as a therapeutic agent for chronically injured organs. Among the cell types under investigation, adult mesenchymal stem cells (MSCs) are widely studied and in early stage clinical studies show promise for repair and regeneration of cardiac tissues. The ability of MSCs to differentiate into mesoderm and non-mesoderm derived tissues, their immunomodulatory effects, their availability and their key role in maintaining and replenishing endogenous stem cell niches have rendered them one of the most heavily investigated and clinically tested type of stem cell. Accumulating data from preclinical and early phase clinical trials document their safety when delivered as either autologous or allogeneic forms in a range of cardiovascular diseases, but also importantly define parameters of clinical efficacy that justify further investigation in larger clinical trials. Here, we review the biology of MSCs, their interaction with endogenous molecular and cellular pathways, and their modulation of immune responses. Additionally, we discuss factors that enhance their proliferative and regenerative ability and factors that may hinder their effectiveness in the clinical setting.
Keywords: stem cells, regeneration, cell differentiation, mesenchymal stem cell
Introduction
Cardiovascular disease is the leading cause of mortality and morbidity in the US and worldwide1. Attributed mainly to myocardial infarction (MI) and its fatal sequelae, heart failure and sudden cardiac death, cardiovascular diseases carry an enormous psychological and financial burden to patients, their families and society. Over the past half a century conventional medicine and surgery have offered many breakthroughs, resulting in a dramatic decline in CV mortality1. Despite the major advances, medical or surgical treatment of chronic heart disease yields only temporary delay in a progressive disease process2, with the only definite cure remaining heart transplantation.
The idea of using stem or precursor cells has emerged in the last decade as a leading approach for a regenerative strategy to address cardiac disease3. In this context, mesenchymal stem cells are lead candidates for cellular therapy not only for heart disease, but multiple diseases characterized by fibrosis4. Bone marrow (BM) and adipose derived MSCs are easily isolated, expanded and immunologically tolerated allowing for allogeneic, “off the shelf” transplantation5. The ability to use pre-prepared allogeneic cells for cell-based therapy allows for a level of quality control and scalability that far exceeds autologous strategies5. In this review we describe the biology, the mechanisms of action, the emerging preclinical and clinical trial results, and discuss potential strategies that will refine the development of MSCs as a therapeutic tool.
What constitutes a Mesenchymal Stem Cell?
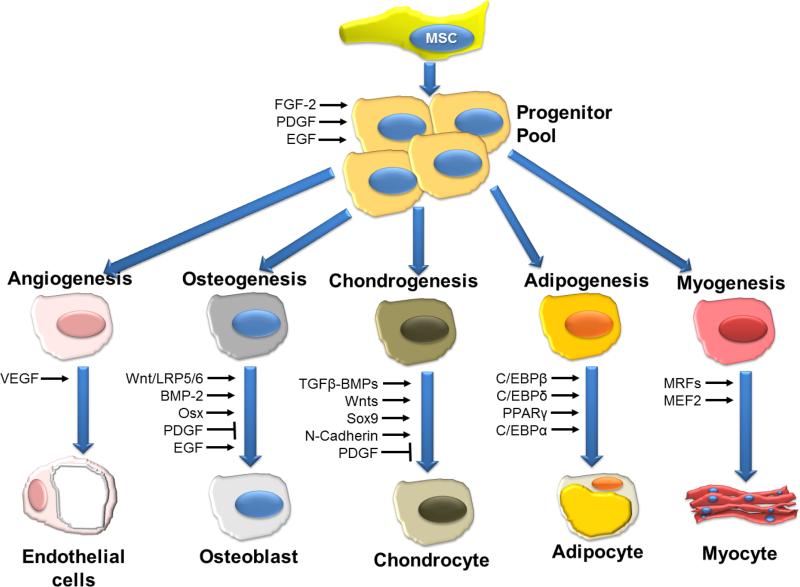
In 1970 Friedenstein6 discovered a rare (0.0001%-0.01% of nucleated cells in human BM) population of plastic adherent stromal cells residing in the BM. These cells, which readily expand in culture, are now commonly called mesenchymal stem or stromal cells, and are recognized to play an integral role in the hematopoietic niche7. In 2006, the International Society for Cellular Therapy established minimal requirements8 for MSC definition: 1) adherence to plastic in standard culture conditions; 2) expression of the surface molecules CD73, CD90, and CD105 in the absence of CD34, CD45, HLA-DR, CD14 or CD11b, CD79a, or CD19 surface molecules and 3) a capacity for differentiation into osteoblasts, adipocytes, and chondroblasts in vitro (Figure 1). The rationale behind the selection of these criteria was to facilitate the comparison of different studies, to foster a more uniform characterization of MSC and render the exchange of data among investigators easier. However, these markers represent a range of MSC differentiation potential. Furthermore, these criteria apply to human MSCs, but do not necessarily extend to other species9, and also following culture these markers may be lost or new markers may arise. Some reports fail to meet these criteria, thus making an across the board comparison difficult. The convention of referring to human BMMSCs as stem cells results from their proven self-renewal capability and capacity for multilineage differentiation10 (Figure 1).
Figure 1. Schematic overview of signaling molecules and transcription factors involved in the regulation of differentiation of MSCs.
VEGF, vascular endothelial growth factor; BMP-2, bone morphogenetic protein-2; EGF, epidermal growth factor; FGF-2, Fibroblast growth factor-2; LRP5/6, low-density lipoprotein receptor-related protein-5/6; Osx, Osterix; PDGF, platelet-derived growth factor; RUNX2, runt-related transcription factor-2; TGF-b, transforming growth factor-b; MRF, myogenic regulatory factors; MEF2, myocyte enhancer factor-2. Figure modified and reporduced with permission from Augello et al. Hum Gene Ther 201045
Sources and Types of MSCs
MSCs are broadly distributed throughout the body11 outside BM, and reside in adipose tissue, gut, lung, liver, placenta, amniotic fluid, dental pulp, periodontal ligament and recently in the heart12, 13. The cells most commonly used in clinical trials to date originate from BM (MSCs and MPCs), adipose tissue, and umbilical cord3 (Table 1). Umbilical cord blood-derived MSCs have been extensively studied in models of heart disease, but their isolation can be difficult14. Wharton's jelly of the umbilical cord is also a rich source of MSCs, but mainly studied in the context of heart valve tissue engineering15. Cells that share some of the characteristics of MSCs can be identified in peripheral blood16.
Table 1.
Comparison of characteristics of MPC, BMMSC, ATMSC, and UCMSC
| Feature | MPC | BMMSC | ATMSC | UCMSC |
|---|---|---|---|---|
| Yield | 1-3% of BM mononucleated cells | 0.001-0.01% of total marrow nucleated cells | 0.5-5% of adipose tissue | |
| Immunophenotype | ||||
| CD90 | - | + | + | + |
| CD105 | + | + | + | + |
| CD73 | - | + | + | + |
| CD31 | + | - | - | - |
| CD146 | - | ± | - | + |
| CD271 | + | + | + | + |
| CD34 | - | - | ± | - |
| CD49d | + | - | - | - |
| CD106 | + | + | - | ± |
| CD54 | + | - | + | + |
| MHC I | + | + | + | + |
| MHC II | - | - | - | - |
| CD40 | - | - | - | - |
| CD80 | - | - | - | - |
| CD86 | - | - | - | - |
| STRO-1/STRO-3 | + | - | - | - |
| Differentiation potential* | ||||
| Adipogenic | + | - | + | + |
| Osteogenic | + | + | - | + |
| Chondrogenic | ± | + | + | + |
| Smooth muscle cells | + | + | + | + |
| Endothelial | + | + | + | + |
| Transcriptome and proteome** | IFN-γ | WNT11, WNT7B, SOX6 | CCL3, FGF9, IL1R2, KDR, PAX3, SPI1, ZNF45 | Vimentin, nph3, gelsolin, prohibitin, |
MPC – Mesenchymal precursor cell, BMMSC – BM derived mesenchymal stem cell, ATMSC – Adipose tissue derived mesenchymal stem cell, UCMSC – Umbilical Cord derived mesenchymal stem cell.
There are conflicting data that imply that MSCs from different sources can respond differently to different stimuli.
uniquely expressed or in significantly higher levels in each population
Amniotic fluid derived MSCs possess some of the characteristics of embryonic stem cells. In't Anker et al17 described amniotic fluid derived MSCs (AFMSC), with full in vitro multipotential differentiation capacity18. In addition, AFMSCs have a ckit+ subpopulation19 that also expresses embryonic stem cell markers (Oct-4, Nanog, and SSEA-4). AFMSCs are phenotypically similar to BMMSCs20, sharing similar immunologic profiles. Like BMMSCs, AFMSC express proteins and genes of HLA-ABC (MHC class I) but not those of HLA-DR (MHC class II)21.
Another subpopulation of BM derived mononuclear cells, called Mesenchymal Precursor Cells (MPCs), can be isolated from human BM aspirates22. A widely used methodology to identify MPCs is by selecting cells bearing the Stro-1 or Stro-3 receptor from bone marrow and then culture expanding that cell (Table 1). In humans, several surface proteins, including Stro-1, CD271, and CD146 may be used as markers for MPCs23. Recently, heart derived MSC-like cells have been identified12, 13 and have been used both in animal models of heart disease and in the CADUCEUS study24. It has also been shown that cardiac stromal cells treated with a cocktail of epigenetic drugs differentiate into functional cardiovascular precursors25. Cardiac stromal cells exhibit many phenotypical similarities26 to their bone marrow counterpart, however they demonstrate an ability to acquire a cardiomyocyte phenotype more efficiently27. In 2002, Jiang et al28 isolated adult cells from rodent bone marrow and suggested that under some conditions may have broad differentiation potency. They named those cells multipotent adult progenitor cells (MAPCs). MAPCs have been shown under appropriate circumstances to be able to differentiated into hematopoietic cells, osteoblasts, hepatocytes, neurons and also possess immunomodulatory capabilities29.
Tissue sources of MSCs
Adipose and BMMSCs are the most heavily investigated and tested3. The abundance of MSCs derived from adipose tissue and the ease of acquiring them with a liposuction, makes this source attractive and feasible for clinical trials (Table 1, supplementary table V). However, there has been a degree of uncertainty on whether adipose derived MSCs are truly MSCs, hence they are often termed as “adipose tissue stem cells”30. Adipose derived and BMMSCs share many biological characteristics; however, there are some differences in their immunophenotype, differentiation potential, transcriptome, proteome, and immunomodulatory activity (Table 1). Some of these differences may represent specific features, while others are suggestive of the inherent heterogeneity of both populations31. To date there is no conclusive head to head in vivo comparison of those two sources and BMMSCs are more widely studied.
Isolation and Expansion of MSCs
It is possible to obtain (after expansion) 50-400 million or even more cells from a BM aspirate of 10ml10. The 3 critical steps that allow MSCs to be isolated from other BM cells are: 1) use of density gradient centrifugation (i.e Ficoll or Percoll) to separate non nucleated red blood cells from nucleated cells or cell mobilization and isolation; 2) the ability of MSCs to adhere to plastic; and 3) the ability of monocytes to be separated from MSCs by trypsinization32. To isolate MSC from a BM aspirate, cord blood or peripheral blood, the samples are fractionated by density gradient for mononuclear cell isolation, resuspended in appropriate culture medium containing selected batches of fetal bovine serum (FBS) and allowed to adhere to plastic dishes for 2 days; then, non-adherent cells are removed and the remaining cells allowed to grow for 2–3 weeks. Cells initially generate a heterogeneous adherent cell layer including fibroblast-like and small round-shaped cells, while they appear uniformly spindle shaped after several passages in culture. Confluent cells are trypsinized and allowed to expand for as many as 40 generations without loss of multipotentiality. A panel of monoclonal antibodies against epitopes expressed on their surface is used for phenotypic characterization10.
Biochemical cross-talk and other factors regulating MSC differentiation
Colony Forming Units (CFU), once isolated, can commit to particular cell lineages through treatment with bioactive factors in vitro or in vivo (Figure 1). When vascular endothelial growth factor (VEGF)33 is present, CFUs preferentially take up vascular endothelial fate. Similarly, 5-azacytidine treatment induces their cardiac differentiation in vitro34. However, other factors shown to induce CFU differentiation into cardiac cells recently, including dexamethasone and ascorbic acid35, bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-4 (FGF-4)36, represent alternative avenues of CFU pretreatment. Treating CFU with dexamethasone, 1 3 -glycerol phosphate, and ascorbic acid, prompts differentiation into osteogenic cells that forms a mineralized extracellular matrix37. Chondrogenic differentiation is achieved by using dexamethasone and TGF-β338, adipogenic through dexamethasone, insulin, indomethacin and 1-methyl-3-isobutylxanthine10. Exposed to basic fibroblast factor, dimethylsulfoxide, β-mercaptoethanol, and butylated hydroxyanisole, MSC differentiate into cells expressing neuronal phenotype39. MSCs also differentiate into other mesoderm-derived tissue, notably myocytes, as shown in some but not all studies40.
MSC themselves also secrete several cytokines. Many cytokines pertinent to hematopoietic cells proliferation and differentiation are among them (interleukin-6, Flt-3 ligand, G-CSF, GM-CSF). MSCs express several adhesion-related antigens (CD166, CD54, CD31, CD106) and integrins (CD49, CD29, CD11, b4-integrin)10. Mechanical loading, as shown by Pijnappels et al41, may explain the increased propensity for MSC differentiation to occur in vivo or in co-culture situations that add mechanical forces.
When mouse MSC and rat ventricular myocytes were co-cultured, MSCs became actin positive and formed gap junctions with the native myocytes42. On the contrary, when a semipermeable membrane divided the two cell types there were no such findings, suggesting that differentiation also requires direct cell-cell contact42. Although this is not always true40, in a similar experiment the MSCs started to contract, express SERCA2 and ryanodine receptor and were positive for troponin, sarcomeric actinin and desmin40. All these suggest that cell-cell contact, cardiac microenvironment and factors secreted by myocytes play intertwined roles in MSC transdifferentiation to cardiomyocytes.
Two main molecular pathways govern MSC differentiation: the Wnt and the TGF-β superfamily pathway43 (Figure 1). The Wnt pathway is critical in skeletogenesis by promoting osteoblast proliferation44 and by suppressing chondrocyte formation. Activation of Wnt receptors on MSCs leads to downstream signal transduction that regulates cell proliferation and differentiation44. The TGF-β pathway is involved in skeletal tissue growth and regulation of MSC differentiation into chondrocytes45. This is achieved by up-regulation of gene expression in MSCs via several intracellular cascades, including extracellular-signal regulated kinase (ERK1/2), SMAD proteins, mitogen-activated protein (MAP) kinases, p38, and JNK45.
Immunomodulatory properties of MSCs
MSCs are potent modulators of the immune system by suppressing white blood cells and triggering anti-inflammatory subsets (Figure 2). MSCs were used to treat therapy resistant severe graft-versus-host disease46 based on the fact that MSCs inhibit T-cell proliferation in vitro47. The success of MSCs has sparked vigorous investigation of their immunomodulatory capabilities. Moreover, the immunomodulatory properties in addition to the immunosuppressive properties further underlie the ability of MSCs to be used as an allograft5.
Figure 2. Immunomodulatory capabilities of MSCs.
Schematic overview of the interactions between MSC and the immune system. Via multiple pathways MSC suppress proliferation of both T helper (TH) and cytotoxic T cells (Tc). In addition, differentiation to TH2 and regulatory T-cells (Treg) is triggered, resulting in an anti-inflammatory environment. Maturation of dendritic cells (DC) is inhibited via IL-6, blocking upregulation of CD40, CD80, and CD86, which in turn reduce T-cell activation. Monocytes are induced by MSC to differentiate preferentially towards the M2 phenotype. IL-10 produced by the M2 macrophages can boost the formation of Treg while simultaneously reducing tissue migration of neutrophils. Neutrophils (polymorphonuclear granulocytes; PMN) are allowed longer life span but reactive oxygen species production is decreased. Natural Killer (NK) cell proliferation is suppressed as well as their cytotoxic activity. B-cell proliferation is inhibited and the production of antibodies is reduced. Modified from van den Akker et al175.
MSCs when co-cultured with T-cells up-regulate indoleamine-pyrrole-2-3-dioxygenase (IDO) leading to tryptophan depletion and accumulation of metabolites such as kynurenine, both of which reduce T-cell proliferation48. MSCs also express PD-L1 and PD-L2 ligands that activate PD-1 receptor on a T-cell resulting in decreased production of the pro-inflammatory cytokines IFN-γ, TNF-α and IL-2. In addition, MSCs have been shown to secrete TSG-6, a powerful anit-inflammatory factor49. Moreover MSCs also down-regulate the activating receptors of natural killer cells NKp30, NKp44 and NKG2D50. MSCs are able to arrest B-cell maturation in G0/G1 phase and simultaneously reduce the chemotactic activity of these cells and block maturation of dendritic cells resulting in reduced expression of antigens and costimulatory molecules necessary to activate T-cells51.
After an MI two major types of macrophages can be found in the heart: 1) M1 (inducible nitric oxidase synthase, MHC class II, CD80, CD86) that is clearing the debris and produces proinflammatory IL-1β, TNF-α and IFN-γ52 and after five days 2)M2 (arginase, macrophage mannose receptor CD206) which has an anti-inflammatory phenotype reducing the release of proinflammatory cytokines and in the same time stimulating scar formation and angiogenesis53. In the presence of MSC, differentiation into the M2 subtype was boosted54 while the debris-cleaning function remained intact55.
The effects described above were observed in controlled in vitro studies. In vivo, transplanted cells first have to survive in order to then suppress or modulate the immunologic milieu. Their survival is aided by the fact that MSCs express moderate levels of HLA class I, lack HLA class II, B7 and CD40 ligand56 expression. In some57 but not all58 in vivo preclinical studies allogeneic MSCs may lose immunoprivilege. The most important assessment of MSC immunology in humans is The Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON; NCT01087996) clinical trial where allogeneic MSCs did not induce an immunologic reaction5 twelve months after transplantation. That was also confirmed in the clinical trial by Ascheim et al59 in which MPCs were administered to LVAD patients.
In Vivo Mechanism of Action
There is substantial accumulating data from in vitro60, 61, pre-clinical62-65 and clinical66-68 settings supporting a multi-factorial mechanism of action for the cardioreparative effects of MSCs. Three main mechanisms of action underlie the favorable actions of MSCs in disease: 1) reduction of fibrosis69, 2) Stimulation of angiogenesis70 and 3) restoration of contractile function66, 67 through engraftment71, differentiation and stimulation of endogenous cardiac stem cells to proliferate and differentiate63 (Figure 3). These effects occur in concert and together lead to the replacement of scarred or dysfunctional myocardial tissue with contractile and perfused tissue65, 66.
Figure 3. Mechanisms of action of MSCs.
The proposed mechanism of action of MSCs form an intertwined cycle of paracrine, autocrine and direct effects that include vascular regeneration, myocardial protection, cardiomyocyte regeneration that ultimately lead to cardiac repair.
Most studies that examine engraftment of cell therapy reveal low retention rates72, 73. Toma and colleagues71, reported that after 4 days, 0.44% of transplanted MSCs resided in the myocardium. This low retention rate prompted the exploration of other mechanisms of action driving the recovery in cardiac structure and function.
Cardioprotection
Many early studies revealed that the border zone of an experimentally induced myocardial infarction was characterized by a reduction in apoptotic myocytes and an augmentation of vascularity62, 74. Scar tissue reduction and cardioprotection after MSC transplantation is well described in both preclinical models and clinical trials. Amado et al64, 65 using serial CT imaging showed in-vivo reappearance of myocardial tissue and restoration of contractility after MSC implantation in swine. Gnecchi et al60 showed that cell culture medium conditioned by hypoxic MSC reduces apoptosis and necrosis of isolated rat cardiomyocytes exposed to low oxygen tension. These investigators interpreted the findings as consistent with paracrine signaling, given the use of cell culture medium as opposed to cells themselves. This cardioprotective effect could be enhanced when MSCs were engineered to overexpress Akt-160. This was later confirmed in vivo75 by a study showing enhanced survival of Akt-MSCs post transplantation. Additionally, Akt-MSCs secrete a protein (Sfrp2) that exerts a prosurvival effect through modulation of Wnt signaling76. Rehman et al77 showed that adipose derived stem cells secrete angiogenic and antiapoptotic factors providing further evidence of cardioprotection. The responsible pathways may include activation of inflammation and stress response associated signaling pathways mediated by IGF-1 and inhibition of transcription factor NF-kB78. The cardioprotective effect was further enhanced by preconditioning the MSCs with TGF-β or by activating the TNF receptor-261. In vivo, MSC cardioprotection leads to reduced number of apoptotic cells around the MSC injections79. Thus, together these findings support the idea that in addition to other key cardioreparative effects, MSCs also create a milieu that protects or reduces stimuli driving ongoing loss of myocytes.
Neoangiogenesis
The formation of new vessels is the cornerstone of any meaningful cardiac repair. There are three mechanisms of postnatal neovascularization: 1) angiogenesis, 2) arteriogenesis, and 3) postnatal vasculogenesis80 where endothelial precursors originating from the BM assemble to create new blood vessels. It is still under debate whether the observed increase in capillary density and tissue perfusion is due to differentiation of MSCs to endothelial cells and vascular smooth muscle cells or due to secretion of paracrine mediators and generation of new pericytes81. There is evidence that MSCs act as pericytes, perivascular cells that are essential to vascularization by stimulating the endothelial cells to form tube-like structures and subsequently vascular networks82. Expression of MSC markers was also detected at the surface of native, non-cultured perivascular cells. Thus, blood vessel walls harbor a reserve of progenitor cells that may be integral to the origin of the MSCs and other related adult stem cells83.
In vitro, MSCs express α-smooth muscle actin and β-actin filaments84 while in in vivo studies it is shown that MSCs expresses an endothelial phenotype that enhanced microvascular density70. Despite this evidence, several groups suggest that only a small number of vessels contain donor cells. Therefore it is proposed that it is the release of proangiogenic and proarteriogenic factors from the MSCs that play the most important role in neovasculogenesis85. In their experiments, there was a significant increase in the levels of VEGF and bFGF in the MSC treated animals, and that was also documented in a gene expression profiling of MSCs under hypoxia. Further supporting this theory, Markel and colleagues86 showed that MSCs underexpressing VEGF have significantly less cardioreparative capabilities. Therefore, MSCs either directly through exosomes, or indirectly through soluble factors are strong proangiogenic factors.
Antifibrosis triggers neomyogenesis
The dominant lesion in the remodeling heart following infarction is the replacement of necrotic myocardium with scar tissue. It is the scar burden that leads to both infarct expansion and extension, and the former sets the stage for the overall remodeling and transformation of the shape of the ventricle from ellipsoid to spherical. In a fibrotic environment, type 1 collagen accumulates resulting in decreased expression of a wide array of genes, growth factors and cytokines that inhibits endogenous reparative potential of muscle87. In addition degradation of extracellular matrix components play a key role in regulating muscular tissue regeneration88. Thus, reducing the fibrotic tissue directly enhances endogenous myogenesis89. Willems et al90 showed that a 1,4-dihydropyridine inducer of type 2 TGF-β receptor (TGFBR2) degradation-1 (ITD-1) selectively enhanced the differentiation of uncommitted mesodermal cells to cardiomyocytes. Extensive studies in the field of bone and cartilage regeneration have shown us that mechanical forces and extracellular matrix components significantly influence MSCs91 but also MSCs modulate the matrix by secreting metalloproteinases and their tissue inhibitors by shifting the balance towards domination of matrix-degrading effects69. This may require the secretion of hepatocyte growth factor92 and heme oxygenase-193 by MSCs. However, MSCs do not only interact with the matrix directly. It is reported that MSCs suppress the proliferation of fibroblasts and promote their metalloproteinase secretion94.
Direct MSC stimulation of endogenous repair
MSC transplantation has now been shown by multiple groups to stimulate proliferation and differentiation of endogenous cardiac stem cells63, 95, 96 (Figure 4). This discovery provides a highly plausible explanation for the replacement of scarred tissue with new contractile myocardium. Neomyogenesis occurs by two related mechanisms: stimulation of endogenous cardiac stem cells (c-kit+ and other lineages) and enhancement of myocyte cell cycling63. In the first demonstration of this phenomenon, GFP+ allogeneic MSCs were injected in infarcted swine hearts and led to the formation of chimeric clusters containing immature MSCs and endogenous ckit+ cardiac stem cells. These clusters exhibited cell-cell interactions mediated by connexin-43-mediated gap junctions and N-cadherin mechanical connections. Importantly, there was a 20-fold increase in the endogenous ckit+ population in MSC treated animals relative to controls, and the c-kit+ cells had much greater capacity for myocyte lineage commitment63. Beltrami and colleagues co-cultured MSCs and resident cardiac stem cells and they reported maturation and increased viability of the latter97. Loffredo et al95 used a lineage tracing mouse to examine the capacity of cell therapy to stimulate endogenous myogenesis, and compared the ability of BM derived c-kit+ stem cells and BM-MSCs to induce proliferation of endogenous CSCs after MI. Mice were genetically modified to express GFP in cardiomyocytes. At 8 weeks after transplantation, BM c-kit+ stem cells led to a significant reduction in the GFP+ cardiomyocyte pool and parallel increases in β-galactosidase+ cardiomyocytes compared to control, suggesting increased progenitor activity induced by BM c-kit+ stem cells. However, BM-MSCs did not exhibit similar findings, suggesting that MSCs may not stimulate endogenous progenitors. This murine finding contrasts with several studies in pigs and may reflect species differences. Suzuki et al96 found that in pigs with hibernating myocardium induced by left anterior descending artery stenosis, treatment with intracoronary injections of autologous GFP+-MSCs led to an improvement in regional wall thickening at both 2 and 6 weeks after injection compared with the control. They also noted a 4-fold increase in c-kit+ and CD133+ populations that also co-expressed GATA-4 and NKx2.5 at 3 days through 2 weeks in animals receiving MSCs. Markers of proliferation (Ki67) were significantly increased in hibernating myocardium in MSC treated animals. More rarely, fusion of transplanted MSC with resident cells may take place as proposed by certain groups98. In a preclinical study by our group99, the combination of human MSCs and ckit+ CSCs led to enhanced cardioreparative than that seen in either cell type alone. Together these findings exhibit important biological interactions between c-kit+ CSCs and MSCs.
Figure 4. Direct MSC stimulation of endogenous repair.
(A) Graph depicting the contribution of cardiomyocyte precursors following exogenous administration of MSCs (green line) and endogenous CSCs (orange line), during cardiac repair after MI. MSC differentiation occurs rapidly after delivery. At 2-weeks, MSCs activate endogenous expansion c-kit+ CSCs (orange line). (B) Two weeks following TEI, the number of C-kit+ cells co-expressing GATA-4 is greater in MSCs vs. non-MSCs treated hearts. The cardiac precursors are preferentially located in the IZ and BZ of the MI, indicating an active process of endogenous regeneration (‡p=0.019 and †p<0.0001) (C,D) The 2-week old chimeric myocardium contains mature cardiomyocytes (open arrow), immature MSCs (arrowheads, inset) and cardiac precursors of MSCs origin (arrow), coupled to host myocardium by connexin-43 gap junctions; Interestingly, endogenous c-kit+ CSCs are found in close proximity to MSCs (D). (E) Cluster of c-kit+ CSCs in an MSCs-treated heart; numerous CSCs are committed to cardiac lineage documented by GATA-4 and MDR-1 co-expression (arrows). (F) Few, isolated c-kit+ cells were found in non-MSC treated animals. Figure reproduced with permission from Hatzistergos et al. Circulation Research 201063
Cellular Effect of MSCs
Although the paracrine theory seems to play a role, it is the direct cellular mechanisms (exosomes, mitochondrial transfer, connexin43, etc.) that convincingly explain the effects observed in preclinical and clinical studies (Figure 3).
Extracellular Vesicles and Exosomes
MSCs exert a host of direct cellular effects by transmitting exosomes and mitochondria into recipient cells (Figure 3). Cells100 continuously secrete a large number of extracellular vesicles (EV), microvesicles, macromolecular complexes, and small molecules called exosomes. Exosomes have been reported to contain significant amounts of miRNA, other non-coding RNAs, as well as mRNA101. Valadi et al102 reported that some full-length molecules are present, and translated extracted RNA to identifiable full-length. Several papers100, 103 indicate that the RNA content of exosomes differs from that of the parental cell. They facilitate immune responses and participate in antigen presentation104, play roles in programmed cell death, angiogenesis, inflammation, and coagulation105. Exosomes' most unique function might be specific interaction with a target recipient cell, enabling cell–cell communication, putatively between widely separated locations in the body. More recent studies have demonstrated that exosomes are not only specifically targeted to recipient cells to exchange proteins and lipids or to trigger downstream signaling events, but also deliver specific nucleic acid cargo106. Embryonic stem cells secrete EVs containing Wnt-3107, the protein Oct-4 but also mRNA. Those EVs derived from endothelial progenitor cells triggered angiogenesis in endothelial cells by horizontal transfer of mRNA108. In addition to mRNAs those vesicles can also transfer miRNAs, further supporting the pivotal role of EVs in signaling within the stem cell niche109. Both mRNAs and miRNAs contained in EVs have been suggested to mediate a bidirectional exchange of genetic information between the stem cells and the injured cells110. EVs of endothelial origin that were released by ischemic muscle, were able to induce the differentiation of BMMNCs into endothelial cells and thus promoting post natal vasculogenesis111.
MSC derived exosomes were first investigated in 2010 in a mouse model of ischemia/reperfusion injury112. MSCs produce increased quantities of exosomes relative to myoblasts and human embryonic kidney cell line113. MSC derived exosomes express both the common surface markers of exosomes and markers expressed on MSCs (CD29, CD44, and CD73). Pretreating MSCs with RNase completely abolishes their renal protective effect114. Treatment of neurons and astrocytes with MSC-derived exosomes leads to an increase of miR-133b in these cells, which promotes functional recovery in Parkinson's disease and spinal cord injury. This finding suggests that MSCs regulate neurite outgrowth at least partly by transferring miR-133b to neurons and astrocytes via the release of exosomes115. Purified exosomes administered to a mouse ischemia-reperfusion injury model revealed that MSCs mediate their cardioprotective paracrine effect by exosome secretion112. Thus, MSC derived exosomes play a key role in cell-cell interactions, regulating everything from immune responses to neoangiogenesis in their surrounding tissue.
Mitochondrial transfer
In addition to exosomes, MSCs may transfer mitochondria through tunneling nanotubes to injured cells. Spees et al116 co-cultured somatic cells depleted of their mitochondrial DNA with MSC, and demonstrated rescue of respiration via the transfer of mitochondria from the healthy cells to the respiratory deficient cells. Similar results can be reproduced using cardiomyocytes117. While full details of the molecular mechanism remain to be elucidated, it seems to be mediated by actin-based extensions named tunneling nanotubes (TNT) and by gap junctions containing connexin 43118. Ahmad et al119 showed that Miro1 (mitochondrial Rho-GTPase) plays a key role mediating the mitochondrial transfer between cells. Interestingly, an association between Miro1 levels and mitochondrial transfer was also reported when comparing different cell types with different mitochondrial transfer capacity, with MSC expressing higher levels of Miro1 compared to lung epithelial cells and fibroblasts. Thus, MSCs through TNTs and gap junctions are able to directly rescue impaired myocytes.
Improvement of cardiac function by reconstituting the cardiac stem-cell niche
Stem cell niches are described in the bone marrow120, hair follicles121, intestinal epithelium122, and in the heart123. In the heart, the niche is comprised of supporting cells and cell-cell interactions that have crucial regulatory roles. MSC transplantation triggers chemokine and cytokine cascades that initiate and boost an endogenous repair mechanism through the restoration of a cellular and molecular collective with the properties of a stem cell niche124. One key axis that modulates the niches is the SDF-1α/CXCR4 axis that also regulates the homing of hematopoietic stem cells to the injured myocardium. Shi and colleagues125 reported high levels of CXCR4 expression both on the surface and intracellularly in BMMSCs even after several passages. When the MSCs were exposed to Flt-3 ligand, stem cell factor, IL-6, hepatocyte growth factor, and IL-3 they up-regulated the expression of CXCR4. Cardiac myocytes can be induced to reenter the cell cycle after treatment with periostin, FGF-1/p38 MAP kinase inhibitor126, neuropeptidase and TGF-β. The fact that some of these factors are secreted by MSCs underlines their role in activating the niche's proliferative potential and in fact modulating the cardiovascular microenvironment towards a reparative mode.
Harnessing and enhancing the therapeutic potential of MSCs
Numerous efforts to further enhance the therapeutic potential of MSCs by genetically modifying or pretreating MSCs with various drugs and cytokines are under way. While combining MSCs and simvastatin127 did not impact the reduction in perfusion defect, scar size or EDV, it did potentiate increases in EF and systolic wall thickening as well as improved MSC retention and survival. Trimetazidine pre-conditioned MSCs decreased LDH levels and enhanced production of survival proteins including HIF-1α, survivin, phosphorylated Akt, and Bcl-2 protein levels and Bcl-2 gene expression128.
Guo and colleagues129 pretreated MSCs with IGF-1 that led to overexpression of CXCR4 resulting in improved wall thickness and left ventricular function as compared to naïve MSCs alone. Similarly, preconditioning MSCs with VEGF resulted in increased activation of the Akt pathway, lower expression of cell aging markers and cell cycle inhibitors (p16 and p21)130. In another study, heme oxygenase-1 was used, that improved MSC survival in hypoxic conditions and up-regulated the secretion of various cytoprotective cytokines by MSCs131. The combination of various factors was also quickly put to test by various research groups. One such combination132 was a cocktail of FGF-2, IGF-1, and BMP-2 to pretreat MSCs before transplantation that led to increased cardiac specific markers and also interestingly enhanced expression of phosphorylated Akt and phosphorylated cAMP response by native cardiomyocytes. Surprising Akt activation not only enhanced the survival of the transplanted MSCs but also decreased the apoptosis of the surrounding myocytes132.
Another intriguing and more difficult approach is that of genetic modification of MSCs. MSCs that were modified to overexpress Bcl-2, had reduced apoptosis and increased secretion of VEGF that led to longer survival even at 6 weeks post transplantation in vivo79. Transfected MSCs to overexpress SDF-1 receptor, a chemotactic factor for lymphocytes, resulted in both increased MSC retention and expression of SDF-1 by the ischemic myocardium that triggered increased intracellular activation of Akt133. Genetic modification of MSCs, although tremendously interesting, will have greater challenges in reaching the clinic due to ethical and safety concerns.
Preclinical Trials of MSC Therapy
In a pioneering study by Toma et al71, human MSCs were injected in murine hearts and were shown to adopt cardiac fate. The immunoprivileged capabilities of MSCs were shown by Amado et al64, where allogeneic MSCs were transplanted into 3 day old immunocompetent porcine infarcted hearts that resulted in long term engraftment and a large decrease of scar tissue without any inflammatory response.
Subsequent to those early groundbreaking studies, MSCs have been tested in numerous cardiovascular settings. First, in acute myocardial infarction where the inflammatory microenvironment and the necrotic/apoptotic signals are the dominant opposing forces to the therapeutic activities of MSCs. However, the presence of homing signals and the antifibrotic milieu may be of benefit. Another study134 where MSCs were intracoronarily infused into porcine heart 5 days after infarction, showed improvement in EF and scar reduction in MSC treated animals. In another study135, where different doses of MSCs were used three days after MI, there was no dose dependent effect on scar size reduction or EF improvement 12 weeks post transplantation. In contrast, in a study136 where four different doses of BM derived STRO-3+ MSCs were directly injected into sheep hearts 1 hour post MI, there were improvements in EDV only in the two lower doses, although EF increased universally. These seemingly contradicting reports suggest that there might be a therapeutic threshold in the total number of cells that can be injected, although other factors certainly play a role. A preclinical study137 where Stro-3+ MPCs were intracoronarily infused in sheep's hearts immediately after MI showed a 40% decrease of infarct size, attenuation of remodeling, and a 50% increase of blood vessel density in border and remote zones.
In the chronic setting the repair processes have been completed, the scar has stabilized and the newly formed network of blood vessels is disorganized and inadequate to halt any disease deterioration. Silva and colleagues74 injected MSCs into canine hearts that resulted in increase in EF, vascular density and decrease in scar tissue. Schuleri and colleagues62 in a similar setting reported that autologous MSCs produce reverse remodeling and functional recovery. Our group73, has transendocardially injected allogeneic MSCs in swine hearts 3 months post infarction. Although adverse remodeling is nearly complete in that setting, we have observed significant decrease of scar tissue and improved contractility in MSC treated animals, three months post transplantation. Left ventricular global function was also improved thus indicating that MSCs may be able to reverse chronic adverse remodeling.
The intermediate setting between chronic and acute, the subacute model also presents with its own challenges. Although, there are fewer preclinical studies64, 138 in this setting, the accumulating data appear consistent with a benefit of MSCs. Our group64 administered allogeneic MSCs to the border and infarct zones of infarcted porcine myocardium 3 days after MI via intramyocardial injections. Eight weeks after transplantation there was a 50% reduction in scar size that was coupled with improvements in EF, LV end diastolic pressure, relaxation time and systolic compliance in the treated animals. Another group138 directly injected autologous MSCs into 2 week old swine infarcts. Four weeks after transplantation, a trend towards improved wall thickness and systolic thickening was observed in the MSC treated animals. Supplementary table I summarizes the preclinical trials described here.
Non-ischemic heart disease models
Ohnishi and colleagues139 in a rat model of non-ischemic dilated cardiomyopathy reported that MSCs attenuate myocardial injury and ventricular dysfunction. In canines, Plotnikov and colleagues140 suggest that MSCs may provide a platform for sustained biological pacemaker function. That experiment was rather provocative because whether or not cell therapy exerts pro- or anti-arrhythmic effects is crucial to address. These early concerns though have been largely entertained especially after the encouraging results from clinical trials supported the anti-arrhythmic effects68.
Autologous MSCs were even used to recellularize tissue-engineered heart porcine valves that were then transplanted to lambs141. Four months later, the MSC valves exhibited better transvalvular and distal gradients as well as less inflammatory reaction and structural deterioration than the BMMNC counterparts.
Safety of MSC Therapy
Although the vast amount of preclinical and clinical data suggest that MSC possess a great potential in treating cardiac diseases and in spite of all the evidence for the safety of MSC transplantation142, it is important to point out that very long term safety requires additional study. There are two main theoretic concerns regarding MSC therapy, arrhythmogenicity and tumorogenicity. The former, as discussed earlier has been proven not to be an issue in all the clinical trials that have been completed. Miura and colleagues143 showed that there is accumulating chromosomal instability in murine BMMSCs that may lead to malignant transformation. Although seen in rodent models, this observation has yet to be described in numerous preclinical and clinical studies5, 62-64, 66, 67, 74, 136.
Clinical Trials of MSC Therapy for Cardiac Repair
Acute Myocardial Infarction
Several studies have examined both autologous and allogeneic MSCs for acute MI. In a phase I randomized study68, 53 patients were randomized to receive either allogeneic MSCs or placebo 7 to 10 days after MI and in different doses. The intravenous infusion of allogeneic MSCs in this study resulted in improvement in overall clinical status 6 months after infusion, fewer arrhythmic events, and improved EF. The success of this pilot study led to a phase II trial where as it was preliminary reported by Osiris, the trial sponsor, that intravenous infusion of allogeneic MSCs within 7 days of an acute MI resulted in significantly reduced cardiac hypertrophy, stress-induced ventricular arrhythmia, heart failure, and rehospitalizations for cardiac complications144. Chen and colleagues145, administered autologous MSCs intracoronarily in patients with subacute MI and observed decreased perfusion defect, improved LVEF and LV remodeling 3 months after therapy. In CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (CADUCEUS) trial24 where autologous cardiospheres (that included MSC-like cardiac cells) were injected 2-4 weeks after MI, there were scar reduction, increase in viable heart mass, and regional contractility. However, changes in end-diastolic volume, end-systolic volume, and LVEF did not differ between groups by 6 months.
In addition to BMMSCs, adipose derived MSCs have also been tested for acute MI in the APOLLO trial146, a trial of 14 patients, which exhibited the safety of intracoronary infusion of freshly isolated adipose derived MSCs in the acute setting of an STEMI. This study also demonstrated a trend toward improved cardiac function, accompanied by a significant improvement of the perfusion defect and a 50% reduction of myocardial scar formation (Figure 5). Thus, these studies suggest a potential role for MSCs in the setting of acute MI.
Figure 5. Adipose Derived MSCs (PRECISE and APOLLO trials) and autologous CSC (C-CURE) in clinical trials.
(A) In the PRECISE trial, Adipose Derived Regenerative Cells (ADRCs) failed to reduce scar size in patients suffering from HF, 6 months after implantation. (B) In the APOLLO trial, where similar cells were employed in the setting of an acute myocardial infarction, there was a significant scar size reduction. (C and D) In the C-CURE trial, where autologous cardiopoietic stem cells (CSCs) were employed, LVEF increased significantly compared to baseline and to control group 6-months following treatment. That was coupled with decreases in left ventricular end-diastolic (ED) and end-systolic (ES) volumes. Panel A is reproduced with permission from Perin et al. American Heart Journal, 2014147, panel B from Houtgraaf et al, Journal of American College of Cardiology, 2012146 and panels C and D from Bartunek et al, JACC, 2013149.
Chronic Ischemic Cardiomyopathy
Currently, chronic ischemic cardiomyopathy is the setting in which there is the highest level of consensus for MSC efficacy. There are 6 published or ongoing clinical trials that together show that MSCs have anti-fibrotic effects, induce neoangiogenesis, enhance contractility and improve the quality of life of the recipient patients. Thus, the mechanisms of actions identified preclinically appear to be operative in humans with chronic ischemic cardiomyopathy. Perhaps the most consistent finding is that of a 30-50% decrease in the size of the MI scar in BMMSC trials5, 66, 67 (Figure 6). Importantly, a decrease in MI size is not evident in the one published trial of adipose MSCs for ischemic cardiomyopathy (Figure 5). Currently, no clinical study has directly compared head-to-head BMMSCs with ADMSCs.
Figure 6. Bone marrow derived MSCs in clinical trials (POSEIDON, TAC-HFT, PROMETHEUS).
(A) In the POSEIDON trial, autologous and allogeneic MSCs both decreased the scar size (as measured by MDCT) and (B) the end-systolic volume 13 months after transplantation. (C) In the TAC-HFT trial, twelve months after injection, scar mass reduced as the percentage of left ventricular mass for patients treated with mesenchymal stem cells (MSCs) and those in the placebo group. (D) In the PROMETHEUS trial, at 18 months after injection of autologous MSCs in patients undergoing CABG, scar tissue, perfusion, wall thickness and thickening,andsystolic strain improved preferentially in the MSC injected segments. There was a progressive drop-off in regional phenotypic improvement as distance from injection site increased. Panels A and B reproduced with permission from Telukuntla et al, Journal of American Heart Association, 2013144. Panel C reproduced with permission from Heldman et al. Journal of American Medical Association, 201467. Panel D reproduced with permission from Karantalis et al, Circulation Research, 201466
Importantly scar reduction is shown to be accompanied by reperfusion and restoration of contractile performance. Perfusion was also preserved or increased in the PRECISE147 (A Randomized Clinical Trial of Adipose Derived Stem and Regenerative Cells In the Treatment of Patients With Non Revascularizable Ischemic Myocardium) and in the PROMETHEUS trials66. These effects also led to a restoration of contractile function as measured by echocardiography147 or Eulerian circumferential strain using MRI at the site of injection66, 67. The last is also an interesting point and highlights the importance of imaging studies in the assessment of stem cell efficacy. In two studies from our group, a substudy of POSEIDON148 and the PROMETHEUS trial66 it was shown that MSC therapy exerts its strongest impact at the site of injection and there is a drop off effect in the adjacent myocardial segments that drive the global functional improvement (Figure 6). Maybe one of the most interesting findings that the clinical trials have offered is the improvement in functional capacity and quality of life these patients experience after cell therapy5, 67, 149
There is also an ongoing clinical trial employing adipose derived MSCs, the MesenchYmal STROMAL CELLTherapy in Patients With Chronic Myocardial Ischemia (MyStromalCell)150, that is using culture-expanded adipose tissue-derived MSCs, and is designed to investigate the safety and efficacy of intramyocardial delivery of VEGF-A165 stimulated autologous adipose tissue-derived MSCs to improve myocardial perfusion and exercise capacity and reduce symptoms in patients with chronic ischemic cardiomyopathy.
In a recently published clinical trial59 where allogeneic MPCs were injected in the hearts of patients that were undergoing left ventricular assist device (LVAD) implantation, 50% of the patients were successfully temporarily weaned from LVAD at 90 days post transplantation. Although this trial was small and exploratory it demonstrated the safety of MPC implantation. The efficacy end points though were derived only from a comparison of 10 patients in the MPC group and only two in the control group. In addition, an ongoing clinical trial, the Allogeneic Mesenchymal precursor cell Infusion in myoCardial Infarction (AMICI) trial, aims to prove safety, feasibility, and efficacy of MPC therapy in the acute ST-elevation MI (NCT01781390). Supplementary tables II-V summarize important completed and ongoing clinical trials.
Phase III Clinical trials
There are currently two phase III clinical trials employing MSCs. the C-CURE trial149 where MSCs were treated ex-vivo with cytokines in order to enhance their commitment to cardiopoietic lineage (Figure 5). Bartunek and colleagues reported significant improvements in EF, end systolic volume and walked distance compared to controls. Another phase III study is currently underway aiming to enroll 1730 patients with CHF, in which MPCs will be injected intramyocardially (NCT02032004).
Non Ischemic Cardiomypoathy
The PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis in Dilated CardioMyopathy (The POSEIDON-DCM; NCT01392625) ongoing clinical trial151 is testing transendocardial injections of autologous and allogeneic MSCs in a non-ischemic setting of dilated cardiomyopathy.
Demographic and Biologic Factors affecting MSC therapy efficacy
When the results of preclinical studies are compared to those of clinical trials there is a relative decrease of the impact seen in the former. Simply the fact that the animals used in preclinical trials are younger, healthier and followed up in a closed and controlled environment compared to humans enrolled in clinical trials, could account for the best part of this discrepancy in translation. But there are many lessons to be drawn from this phenomenon that will lead to further optimizing the cell therapy itself but also detecting the patient population that will benefit the most from it.
The cell products produced in different laboratories as well as the treated population are very heterogeneous, heavily influencing the clinical outcome. Timing, delivery method, and optimal dose are still open to debate although already there are hints for the existence of time windows and maximal and minimal effective doses.
Sethe and colleagues152 reported that the proportion of MSCs in the BM decreases with age and the MSC yield is lower in direct association with the age of the donor. Although the inherent ability to differentiate in different cell lineages seems to be preserved in older MSCs there are quantitative differences between older and younger cells153. Zhang and colleagues154 in a rat model found that increasing donor age adversely impacts the beneficial effect of MSCs. Khan et al155 suggested that only MSCs derived from young and healthy donors can effectively regenerate senescent rat hearts. Understanding the effect of aging on MSCs is crucial for autologous therapy for older patients, who are typically afflicted by cardiovascular diseases. In a recently published sub-study156 from the TAC-HFT and POSEIDON trials, it was reported that older individuals did not have an impaired response to MSC therapy.
Gender may influence the biology of MSCs. Some studies suggest that females have a lower reservoir of MSCs but compared to male MSCs exhibit greater resistance to injury157. But different conditions in the patients themselves may play a role. Less injury and inflammation is found in female patients compared to males after acute cardiac injuries. Estradiol contributes by decreasing the expression of the Bcl-2 family of genes and JNK pathways158.
It is also important to understand whether and what differences exist between MSCs obtained from normal and diseased individuals. Zhao and colleagues159 described the characteristics of MSCs derived from various malignant hematopoietic diseases. The morphology and phenotype of MSCs remained unchanged even after 20 passages. Patients with rheumatoid arthritis on the other hand, although having normal reserves of MSCs they seem to display decreased proliferative and clonogenic potential compared to normal160. Some studies suggest that in diabetics, MSCs become exhausted and lose their differentiation potential. These changes appear to be due to induction of apoptosis and senescence by advanced glycation end-products161. Interestingly the changes in MSCs caused by the diabetic environment are probably permanent, because culturing MSCs in medium without glucose does not reverse the effect. Another risk factor for cardiovascular disease, cigarette smoking, seems that adversely affects MSCs. A study demonstrated that nicotine decreased the proliferation of MSCs in a dose dependent manner162.
The Future of MSC therapy
Indications, timing, delivery of MSCs
MSCs have been used to treat pediatric cardiomyopathy163, congenital heart diseases (hypoplastic left heart syndrome)164, refractory angina165, acute MI68, 145, 146, 166, and chronic ischemic cardiomyopathy5, 66, 67, 147, 150, are being investigated in non-ischemic dilated cardiomyopathy151, and have been proposed for doxorubicin induced cardiomyopathy167. In the setting of refractory angina and in acute MI, the chemokines, cytokines, and growth factors released by the injured myocardium provide migratory cues for endogenous resident stem cells as well as bone marrow derived stem cells168; hence, the rationale behind locally (intracoronary) and systemically (intravenous) administered stem cells, respectively, in these settings. However, both delivery methods have limitations. After intravenous infusion, there is wide distribution of MSCs throughout the body, with entrapment of these cells in the lungs, spleen, and liver169-171, while the intracoronary technique requires a transient ischemic period through the inflation of a balloon to give the cells the chance to be distributed and not washed out. In addition, there is the potential for microvascular obstruction by the infused cells, which can result in myocardial necrosis. The intracoronary delivery technique is also limited by the inaccessibility of some myocardial distributions in patients with advanced coronary artery disease. In acute MI, intramyocardial injections have not been a preferred mode of delivery due to concerns about perforation in the setting of acute ischemia and necrosis. However, Perin et al172 have shown that transendocardial delivery was more efficacious than intracoronary delivery in a canine model of acute MI.
On the other hand, in the chronic setting of heart failure due to chronic ischemic and nonischemic heart disease, the migratory cues for stem cells are thought to be absent or minimal168 and a more targeted approach, namely intramyocardial injection, has been investigated. In an analysis from the POSEIDON148 clinical trial, transendocardial injection of MSCs reduced scar size in both injected and non-injected myocardial segments but segmental contractility improved only in the injected scar segments and was greatest in those territories with severe baseline dysfunction. Moreover, analysis of the effects of direct surgical myocardial injection in the PROMETHEUS66 clinical trial found a concordant reduction in scar size and improvement in perfusion and contractility in MSC-injected myocardial segments when compared to “revascularization only” and non-MSC treated segments in patients who underwent coronary artery bypass graft (CABG) surgery. In light of the PROMETHEUS trial66 and POSEIDON substudy148 findings, special attention should be invested on designing the strategy of the precise placement of the intramyocardial injections in order to cover as much of the impaired myocardium as possible. When there is no ischemia, as in the setting of non-ischemic dilated cardiomyopathy, this strategy makes even more sense, and effort should be made to inject as much area of the left ventricle as possible.
There is evidence5 that autologous and allogeneic sources of MSCs produce similar beneficial effects in patients with ischemic cardiomyopathy and this is currently being investigated in patients with non-ischemic cardiomyopathy as well151. Given these findings, the allogeneic source provides an “off-the-shelf” therapeutic option that is advantageous over the autologous source, especially in the urgent setting of an acute MI. Although there are clinical trials (TIME173, LateTIME174) that were designed to detect the optimal window of delivery of bone marrow mononuclear stem cells after an acute MI, the effect of timing of MSC delivery on therapeutic efficacy has not been specifically investigated. However, MSCs have been employed in the chronic setting of ischemic cardiomyopathy in the PROMETHEUS, POSEIDON, and TAC-HFT studies67 and have been shown to produce favorable outcomes.
Future directions
There is now a large body of evidence from preclinical62, 63 and early phase clinical trials5, 67 that elucidates the mechanism of action and detects potential pitfalls of MSC therapy. It is also largely accepted that one of the main limitations to the long term efficacy of MSC therapy is the low retention and survival of the transplanted cells72. Genetic engineering and cell pretreatment may in the near future provide the appropriate lifespan to the transplanted MSCs and thus render them the new nearly permanent regenerative niche in the impaired myocardium79 (Figure 7).
Figure 7. The evolution of MSC therapy for cardiac disease.
MSCs will play the central role in combination therapies supporting and orchestrating different cell types. Cytokines may also be given with or pretreat the target organ before MSC transplantation. A more challenging but promising way is the genetic modification of MSCs.
One of the primary advantages of the MSC is their ability to home to sites of injury, respond dynamically to the extent of injury and secrete a broad range of factors, many of whom not yet discovered81. Many research groups around the world are now moving towards enhancing the effectiveness of MSC therapy via various pretreatment cocktails of factors, although this has yet to be translated clinically132. The employment of sophisticated imaging like CT148 and MRI66 and the advanced understanding those two modalities offer us on the regional effect of MSC therapy, will also play a big role on accurately detecting target areas for therapy. Additionally and in conjunction to the latter, there is significant potential for improvement in design of cell delivery methods.
It is also becoming increasingly evident, that although MSC remains one of the promising cell types for a successful cell therapy, complete myocardial regeneration cannot be meaningfully achieved by just one cell type99. The future of MSC therapy may lie in being the main supporting, trophic and orchestrating cell type in a cell therapy that will combine different cell types taking advantage of their unique characteristics and benefits.
We now have enough evidence for the safety and encouraging signs of efficacy of MSC therapy. Currently, there are ongoing phase 3 clinical trials testing whether various MSC-based strategies offer clinical benefits to patients. These trials based upon substantial basic, preclinical, and phase I/II data will take the next step in addressing the appropriate use of cell-based therapy in the armamentarium of approaches for chronic heart disease.
Supplementary Material
Acknowledgements
We would like to thank Dr. Ivonne H. Schulman for her valuable assistance in critical review.
Sources of Funding
Dr. Hare is supported by National Institutes of Health Grants R01HL110737, R01HL084275, R01HL094849, R01HL107110 and UM1HL113460 and the Starr Foundation. Dr. Karantalis is supported by an award from the American Heart Association.
Non-standard Abbreviations and Acronyms
- MSC
Mesenchymal Stem Cell
- MNC
Mononuclear Cell
- AFMSC
Amniotic Fluid Derived Mesenchymal Stem Cell
- MPC
Mesenchymal Precursor Cell
- CFU
Colony Forming Unit
- VEGF
Vascular Endothelial Growth Factor
- Miro-1
Mitochondrial Rho-GTPase
- TNT
Tunneling Nanotubes
- EV
Extracellular Vesicles
- miRNA
microRNA
Footnotes
Disclosures
Dr. Hare disclose a relationship with Vestion that includes equity, board membership, and consulting.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 3.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303:H256–270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: The mesenchymal stromal cells breakthrough. Stem Cells Int. 2014;2014:340257. doi: 10.1155/2014/340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (mscs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (msc): A comparison of adult and neonatal tissue-derived msc. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelekanos RA, Li J, Gongora M, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac msc-like populations supports strong congruence with bone marrow msc despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73. doi: 10.1016/j.scr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident msc-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada Y, Yokoyama S, Fukuda N, Kidoya H, Huang XY, Naitoh H, Satoh N, Takakura N. A novel approach for myocardial regeneration with educated cord blood cells cocultured with cells from brown adipose tissue. Biochem Biophys Res Commun. 2007;353:182–188. doi: 10.1016/j.bbrc.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2:87–92. doi: 10.1007/s12015-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 16.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 18.Perin L, Sedrakyan S, Da Sacco S, De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008;86:85–99. doi: 10.1016/S0091-679X(08)00005-8. [DOI] [PubMed] [Google Scholar]
- 19.De Coppi P, Bartsch G, Jr., Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 20.Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 21.Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 22.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, stro-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 23.Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P, Muller I, Schewe B, Skutella T, Fibbe WE, Kanz L, Buhring HJ. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against cd56, cd271, and mesenchymal stem cell antigen-1 Haematologica. 2009;94:173–184. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecellio M, Meraviglia V, Nanni S, Barbuti A, Scavone A, DiFrancesco D, Farsetti A, Pompilio G, Colombo GI, Capogrossi MC, Gaetano C, Rossini A. In vitro epigenetic reprogramming of human cardiac mesenchymal stromal cells into functionally competent cardiovascular precursors. PLoS One. 2012;7:e51694. doi: 10.1371/journal.pone.0051694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossini A, Frati C, Lagrasta C, et al. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res. 2011;89:650–660. doi: 10.1093/cvr/cvq290. [DOI] [PubMed] [Google Scholar]
- 27.Meraviglia V, Azzimato V, Piacentini L, Chiesa M, Kesharwani RK, Frati C, Capogrossi MC, Gaetano C, Pompilio G, Colombo GI, Rossini A. Syngeneic cardiac and bone marrow stromal cells display tissue-specific microrna signatures and microrna subsets restricted to diverse differentiation processes. PLoS One. 2014;9:e107269. doi: 10.1371/journal.pone.0107269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 29.Sohni A, Verfaillie CM. Multipotent adult progenitor cells. Best Pract Res Clin Haematol. 2011;24:3–11. doi: 10.1016/j.beha.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Choi YH, Kurtz A, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther. 2011;22:3–17. doi: 10.1089/hum.2010.211. [DOI] [PubMed] [Google Scholar]
- 31.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 32.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1604–1605. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular vegf regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Song J, Liu W, Wan Y, Chen X, Hu C. Growth and differentiation of rat bone marrow stromal cells: Does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res. 2003;58:460–468. doi: 10.1016/s0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 35.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 36.Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol. 2005;60:277–284. doi: 10.2143/AC.60.3.2005005. [DOI] [PubMed] [Google Scholar]
- 37.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 39.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AA, van der Laarse A, Ypey DL, Atsma DE. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 43.Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013;786:213–229. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 44.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 46.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 47.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress t-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 48.Mandi Y, Vecsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 49.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hmscs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein tsg-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin e2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 52.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barin JG, Rose NR, Cihakova D. Macrophage diversity in cardiac inflammation: A review. Immunobiology. 2012;217:468–475. doi: 10.1016/j.imbio.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 57.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 58.English K, Mahon BP. Allogeneic mesenchymal stem cells: Agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 59.Ascheim DD, Gelijns AC, Goldstein D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129:2287–2296. doi: 10.1161/CIRCULATIONAHA.113.007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 61.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 62.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 66.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (prometheus) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr., Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molina EJ, Palma J, Gupta D, Torres D, Gaughan JP, Houser S, Macha M. Reverse remodeling is associated with changes in extracellular matrix proteases and tissue inhibitors after mesenchymal stem cell (msc) treatment of pressure overload hypertrophy. J Tissue Eng Regen Med. 2009;3:85–91. doi: 10.1002/term.137. [DOI] [PubMed] [Google Scholar]
- 70.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: Mesenchymal stromal cells: Potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 71.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 72.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 73.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 75.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 76.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (sfrp2) is the key akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 78.Rogers TB, Pati S, Gaa S, Riley D, Khakoo AY, Patel S, Wardlow RD, 2nd, Frederick CA, Hall G, He LP, Lederer WJ. Mesenchymal stem cells stimulate protective genetic reprogramming of injured cardiac ventricular myocytes. J Mol Cell Cardiol. 2011;50:346–356. doi: 10.1016/j.yjmcc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered mscs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 80.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 81.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA, Zhang Z, Vacanti JP, Hauschka PV, Grottkau BE. Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev. 2009;5:437–445. doi: 10.1007/s12015-009-9097-6. [DOI] [PubMed] [Google Scholar]
- 83.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 85.Gomes SA, Rangel EB, Premer C, Dulce RA, Cao Y, Florea V, Balkan W, Rodrigues CO, Schally AV, Hare JM. S-nitrosoglutathione reductase (gsnor) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110:2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR. Vegf is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295:H2308–2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Lisio M, Jensen T, Sukiennik RA, Huntsman HD, Boppart M. Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res Ther. 2014;5:74. doi: 10.1186/scrt463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adh Migr. 2009;3:337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 90.Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P, Bushway PJ, Lanier M, Walsh C, Kirchhausen T, Izpisua Belmonte JC, Cashman J, Mercola M. Small molecule-mediated tgf-beta type ii receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell. 2012;11:242–252. doi: 10.1016/j.stem.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan G, Shim W, Gu Y, Qian L, Chung YY, Lim SY, Yong P, Sim E, Wong P. Differential effect of myocardial matrix and integrins on cardiac differentiation of human mesenchymal stem cells. Differentiation. 2010;79:260–271. doi: 10.1016/j.diff.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, Zhang L, Huang Y. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29:9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 93.Shu T, Zeng B, Ren X, Li Y. Ho-1 modified mesenchymal stem cells modulate mmps/timps system and adverse remodeling in infarcted myocardium. Tissue Cell. 2010;42:217–222. doi: 10.1016/j.tice.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 95.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suzuki G, Iyer V, Lee TC, Canty JM., Jr. Autologous mesenchymal stem cells mobilize ckit+ and cd133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 97.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 99.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microrna-loaded exosomes from t cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gusachenko ON, Zenkova MA, Vlassov VV. Nucleic acids in exosomes: Disease markers and intercellular communication molecules. Biochemistry (Mosc) 2013;78:1–7. doi: 10.1134/S000629791301001X. [DOI] [PubMed] [Google Scholar]
- 102.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 103.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small rna. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 105.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 106.Verweij FJ, van Eijndhoven MA, Middeldorp J, Pegtel DM. Analysis of viral microrna exchange via exosomes in vitro and in vivo. Methods Mol Biol. 2013;1024:53–68. doi: 10.1007/978-1-62703-453-1_5. [DOI] [PubMed] [Google Scholar]
- 107.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mrna. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 109.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of micrornas by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 111.Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 112.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by msc reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic potential of the msc exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ahmad T, Mukherjee S, Pattnaik B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 121.Pasolli HA. The hair follicle bulge: A niche for adult stem cells. Microsc Microanal. 2011;17:513–519. doi: 10.1017/S1431927611000419. [DOI] [PubMed] [Google Scholar]
- 122.Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. 2011;68:2513–2523. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: Potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 125.Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of cxcr4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in nod/scid mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 126.Engel FB, Hsieh PC, Lee RT, Keating MT. Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 128.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through bcl-2 expression. J Pharmacol Exp Ther. 2009;329:543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo J, Lin G, Bao C, Hu Z, Chu H, Hu M. Insulin-like growth factor 1 improves the efficacy of mesenchymal stem cells transplantation in a rat model of myocardial infarction. J Biomed Sci. 2008;15:89–97. doi: 10.1007/s11373-007-9207-x. [DOI] [PubMed] [Google Scholar]
- 130.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. Vegf improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Zeng B, Ren X, Lin G, Zhu C, Chen H, Yin J, Jiang H, Yang B, Ding D. Paracrine action of ho-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol Int. 2008;32:1256–1264. doi: 10.1016/j.cellbi.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 132.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 133.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. Sdf-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 134.Qi CM, Ma GS, Liu NF, Shen CX, Chen Z, Liu XJ, Hu YP, Zhang XL, Teng GJ, Ju SH, Ma M, Tang YL. Transplantation of magnetically labeled mesenchymal stem cells improves cardiac function in a swine myocardial infarction model. Chin Med J (Engl) 2008;121:544–550. [PubMed] [Google Scholar]
- 135.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 136.Hamamoto H, Gorman JH, 3rd, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: The effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 138.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 139.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Jr., Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 141.Vincentelli A, Wautot F, Juthier F, Fouquet O, Corseaux D, Marechaux S, Le Tourneau T, Fabre O, Susen S, Van Belle E, Mouquet F, Decoene C, Prat A, Jude B. In vivo autologous recellularization of a tissue-engineered heart valve: Are bone marrow mesenchymal stem cells the best candidates? J Thorac Cardiovasc Surg. 2007;134:424–432. doi: 10.1016/j.jtcvs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 142.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Canadian Critical Care Trials G. Safety of cell therapy with mesenchymal stromal cells (safecell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 144.Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: Insights and lessons from clinical trials. J Am Heart Assoc. 2013;2:e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 146.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 147.Perin EC, Sanz-Ruiz R, Sanchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The precise trial. Am Heart J. 2014;168:88–95 e82. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 148.Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the percutaneous stem cell injection delivery effects on neomyogenesis (poseidon) randomized trial. Circ Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 150.Qayyum AA, Haack-Sorensen M, Mathiasen AB, Jorgensen E, Ekblond A, Kastrup J. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (mystromalcell trial): Study design. Regen Med. 2012;7:421–428. doi: 10.2217/rme.12.17. [DOI] [PubMed] [Google Scholar]
- 151.Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM. Rationale and design of the percutaneous stem cell injection delivery effects on neomyogenesis in dilated cardiomyopathy (the poseidon-dcm study) : A phase i/ii, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. Allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Transl Res. 2014;7:769–780. doi: 10.1007/s12265-014-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Fan M, Chen W, Liu W, Du GQ, Jiang SL, Tian WC, Sun L, Li RK, Tian H. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–438. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 154.Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, Li RK. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289:H2089–2096. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- 155.Khan M, Mohsin S, Khan SN, Riazuddin S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15:1515–1527. doi: 10.1111/j.1582-4934.2009.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65:125–132. doi: 10.1016/j.jacc.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and vegf, tnf, il-6 expression: Role of the 55 kda tnf receptor (tnfr1). J Mol Cell Cardiol. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen HY, Zhang X, Chen SF, Zhang YX, Liu YH, Ma LL, Wang LX. The protective effect of 17beta-estradiol against hydrogen peroxide-induced apoptosis on mesenchymal stem cell. Biomed Pharmacother. 2012;66:57–63. doi: 10.1016/j.biopha.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 159.Zhao ZG, Liang Y, Li K, Li WM, Li QB, Chen ZC, Zou P. Phenotypic and functional comparison of mesenchymal stem cells derived from the bone marrow of normal adults and patients with hematologic malignant diseases. Stem Cells Dev. 2007;16:637–648. doi: 10.1089/scd.2007.0008. [DOI] [PubMed] [Google Scholar]
- 160.Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos GD, Jorgensen C, Charbord P, Haupl T, Boumpas DT, Papadaki HA. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67:741–749. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 161.Yang Q, Chen C, Wu S, Zhang Y, Mao X, Wang W. Advanced glycation end products downregulates peroxisome proliferator-activated receptor gamma expression in cultured rabbit chondrocyte through mapk pathway. Eur J Pharmacol. 2010;649:108–114. doi: 10.1016/j.ejphar.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 162.Kim BS, Kim SJ, Kim HJ, Lee SJ, Park YJ, Lee J, You HK. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90:109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 163.Selem SM, Kaushal S, Hare JM. Stem cell therapy for pediatric dilated cardiomyopathy. Curr Cardiol Rep. 2013;15:369. doi: 10.1007/s11886-013-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ishigami S, Ohtsuki S, Tarui S, et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome (ticap): A prospective phase 1 controlled trial. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.304671. [DOI] [PubMed] [Google Scholar]
- 165.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Quyyumi AA, Waller EK, Murrow J, et al. Cd34(+) cell infusion after st elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 167.Mohammadi Gorji S, Karimpor Malekshah AA, Hashemi-Soteh MB, Rafiei A, Parivar K, Aghdami N. Effect of mesenchymal stem cells on doxorubicin-induced fibrosis. Cell J. 2012;14:142–151. [PMC free article] [PubMed] [Google Scholar]
- 168.Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: Strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. doi: 10.1155/2012/342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 170.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–239. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 171.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112:I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 172.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 173.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Traverse JH, Henry TD, Vaughan DE, et al. Latetime: A phase-ii, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 175.van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. 2013;2013:181020. doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.