Abstract
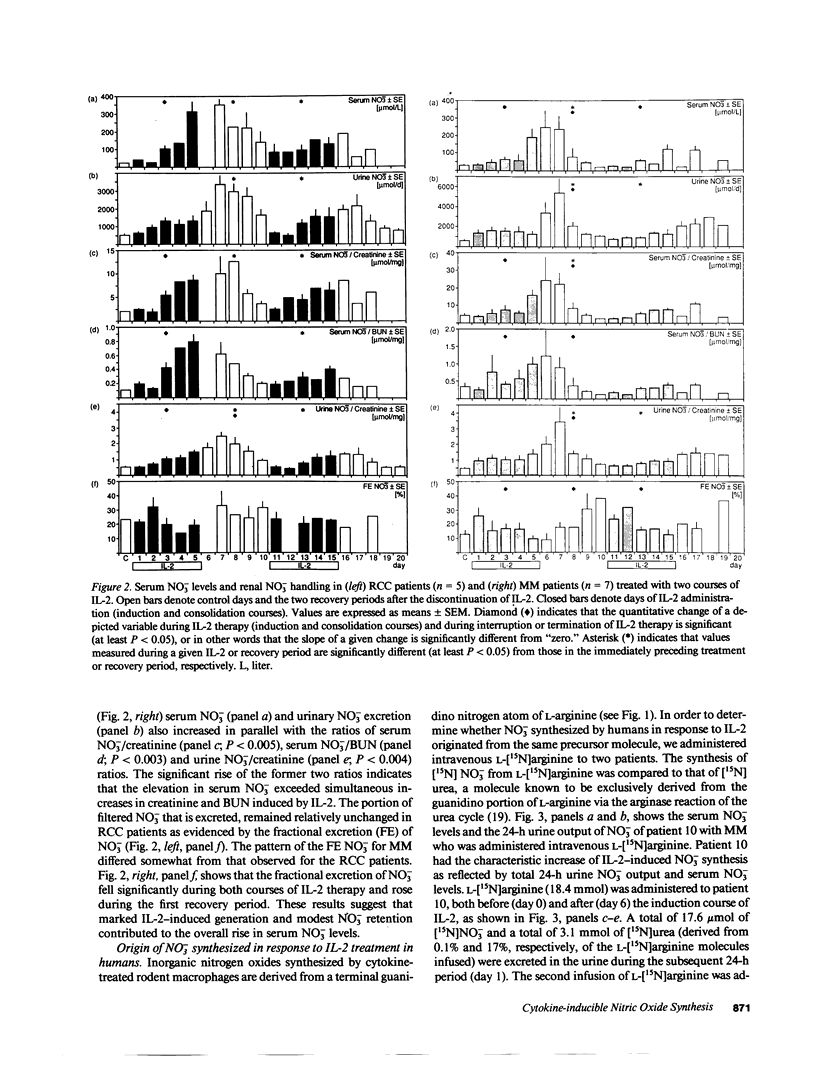
An interferon-gamma, tumor necrosis factor, and interleukin-1-inducible, high-output pathway synthesizing nitric oxide (NO) from L-arginine was recently identified in rodents. High-dose interleukin-2 (IL-2) therapy is known to induce the same cytokines in patients with advanced cancer. Therefore, we examined renal cell carcinoma (RCC; n = 5) and malignant melanoma (MM; n = 7) patients for evidence of cytokine-inducible NO synthesis. Activity of this pathway was evaluated by measuring serum and urine nitrate (the stable degradation product of NO) during IL-2 therapy. IL-2 administration caused a striking increase in NO generation as reflected by serum nitrate levels (10- and 8-fold increase [P less than 0.001, P less than 0.003] for RCC and MM patients, respectively) and 24-h urinary nitrate excretion (6.5- and 9-fold increase [both P less than 0.001] for RCC and MM patients, respectively). IL-2-induced renal dysfunction made only a minor contribution to increased serum nitrate levels. Metabolic tracer studies using L-[guanidino-15N2]arginine demonstrated that the increased nitrate production was derived from a terminal guanidino nitrogen atom of L-arginine. Our results showing increased endogenous nitrate synthesis in patients receiving IL-2 demonstrate for the first time that a cytokine-inducible, high-output L-arginine/NO pathway exists in humans.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Cytokines induce an L-arginine-dependent effector system in nonmacrophage cells. J Leukoc Biol. 1988 Jul;44(1):58–65. doi: 10.1002/jlb.44.1.58. [DOI] [PubMed] [Google Scholar]
- Bartholomew B. A rapid method for the assay of nitrate in urine using the nitrate reductase enzyme of Escherichia coli. Food Chem Toxicol. 1984 Jul;22(7):541–543. doi: 10.1016/0278-6915(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Baylis C., Harton P., Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol. 1990 Dec;1(6):875–881. doi: 10.1681/ASN.V16875. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belldegrun A., Webb D. E., Austin H. A., 3rd, Steinberg S. M., White D. E., Linehan W. M., Rosenberg S. A. Effects of interleukin-2 on renal function in patients receiving immunotherapy for advanced cancer. Ann Intern Med. 1987 Jun;106(6):817–822. doi: 10.7326/0003-4819-106-6-817. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Blay J. Y., Favrot M. C., Negrier S., Combaret V., Chouaib S., Mercatello A., Kaemmerlen P., Franks C. R., Philip T. Correlation between clinical response to interleukin 2 therapy and sustained production of tumor necrosis factor. Cancer Res. 1990 Apr 15;50(8):2371–2374. [PubMed] [Google Scholar]
- Boccoli G., Masciulli R., Ruggeri E. M., Carlini P., Giannella G., Montesoro E., Mastroberardino G., Isacchi G., Testa U., Calabresi F. Adoptive immunotherapy of human cancer: the cytokine cascade and monocyte activation following high-dose interleukin 2 bolus treatment. Cancer Res. 1990 Sep 15;50(18):5795–5800. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Cameron M. L., Granger D. L., Weinberg J. B., Kozumbo W. J., Koren H. S. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1313–1319. doi: 10.1164/ajrccm/142.6_Pt_1.1313. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Fraker D. L., Langstein H. N., Norton J. A. Passive immunization against tumor necrosis factor partially abrogates interleukin 2 toxicity. J Exp Med. 1989 Sep 1;170(3):1015–1020. doi: 10.1084/jem.170.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Tannenbaum S. R., Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981 Apr 3;212(4490):56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: regulation of enzyme activity by porphyrins and metalloporphyrins. Semin Hematol. 1989 Jan;26(1):63–76. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kasid A., Director E. P., Rosenberg S. A. Induction of endogenous cytokine-mRNA in circulating peripheral blood mononuclear cells by IL-2 administration to cancer patients. J Immunol. 1989 Jul 15;143(2):736–739. [PubMed] [Google Scholar]
- Kikeri D., Pennell J. P., Hwang K. H., Jacob A. I., Richman A. V., Bourgoignie J. J. Endotoxemic acute renal failure in awake rats. Am J Physiol. 1986 Jun;250(6 Pt 2):F1098–F1106. doi: 10.1152/ajprenal.1986.250.6.F1098. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Imaizumi K., Imai K., Tyuma I. Stoichiometry of the reaction of oxyhemoglobin with nitrite. Biochim Biophys Acta. 1979 Nov 23;581(1):184–188. doi: 10.1016/0005-2795(79)90235-6. [DOI] [PubMed] [Google Scholar]
- Kozeny G. A., Nicolas J. D., Creekmore S., Sticklin L., Hano J. E., Fisher R. I. Effects of interleukin-2 immunotherapy on renal function. J Clin Oncol. 1988 Jul;6(7):1170–1176. doi: 10.1200/JCO.1988.6.7.1170. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf C. D., Wishnok J. S., Tannenbaum S. R. L-arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1032–1037. doi: 10.1016/0006-291x(89)92325-5. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Chenais B., Yapo A., Lemaire G., Thelander L., Tenu J. P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990 Aug 25;265(24):14143–14149. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. A., Brock T. A., Ballermann B. J. Glomerular endothelial cells respond to calcium-mobilizing agonists with release of EDRF. Am J Physiol. 1990 May;258(5 Pt 2):F1295–F1303. doi: 10.1152/ajprenal.1990.258.5.F1295. [DOI] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991 Jan;49(1):73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Mertelsmann R., Welte K. Human interleukin 2: molecular biology, physiology and clinical possibilities. Immunobiology. 1986 Sep;172(3-5):400–419. doi: 10.1016/S0171-2985(86)80121-8. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Vachino G., van der Meer J. W., Numerof R. P., Adams S., Cannon J. G., Bernheim H. A., Atkins M. B., Parkinson D. R., Dinarello C. A. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988 Nov;8(6):426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Polissar L., Diehr P. Regression analysis in health services research: the use of dummy variables. Med Care. 1982 Sep;20(9):959–966. doi: 10.1097/00005650-198209000-00008. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Ratcliffe P. J., Richardson A. J., Kirby J. E., Moyses C., Shelton J. R., Morris P. J. Effect of intravenous infusion of atriopeptin 3 on immediate renal allograft function. Kidney Int. 1991 Jan;39(1):164–168. doi: 10.1038/ki.1991.21. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Mulé J. J. NIH conference. New approaches to the immunotherapy of cancer using interleukin-2. Ann Intern Med. 1988 Jun;108(6):853–864. doi: 10.7326/0003-4819-108-6-853. [DOI] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Seifert R., Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989 Feb 27;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Shaw S. G., Weidmann P., Hodler J., Zimmermann A., Paternostro A. Atrial natriuretic peptide protects against acute ischemic renal failure in the rat. J Clin Invest. 1987 Nov;80(5):1232–1237. doi: 10.1172/JCI113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P. J., Schorer A. E., Raij L. Effects of endothelium-derived relaxing factor and nitric oxide on rat mesangial cells. Am J Physiol. 1990 Jan;258(1 Pt 2):F162–F167. doi: 10.1152/ajprenal.1990.258.1.F162. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Kalhan S. C. Gas chromatography/mass spectrometric determination of [15N]urea in plasma and application to urea metabolism study. Anal Chem. 1982 Mar;54(3):489–491. doi: 10.1021/ac00240a031. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Shah S. V. Reactive oxygen metabolites in endotoxin-induced acute renal failure in rats. Kidney Int. 1990 Dec;38(6):1125–1132. doi: 10.1038/ki.1990.322. [DOI] [PubMed] [Google Scholar]
- Wardle N. Acute renal failure in the 1980s: the importance of septic shock and of endotoxaemia. Nephron. 1982;30(3):193–200. doi: 10.1159/000182461. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Tetrahydrobiopterin-dependent formation of nitrite and nitrate in murine fibroblasts. J Exp Med. 1990 Dec 1;172(6):1599–1607. doi: 10.1084/jem.172.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenfelder C., Arevalo G. J., Crawford P. W., Zerwer P., Baranowski R. L., Birch F. M., Earnest W. R., Hamburger R. K., Coleman R. D., Kurtzman N. A. Renal tubular function in glycerol-induced acute renal failure. Kidney Int. 1980 Oct;18(4):432–444. doi: 10.1038/ki.1980.156. [DOI] [PubMed] [Google Scholar]
- Westenfelder C., Birch F. M., Baranowski R. L., Riebman J. B., Olsen D. B., Burns G. L., Kablitz C. Volume homeostasis in calves with artificial atria and ventricles. Am J Physiol. 1990 Apr;258(4 Pt 2):F1005–F1017. doi: 10.1152/ajprenal.1990.258.4.F1005. [DOI] [PubMed] [Google Scholar]
- Westenfelder C., Hamburger R. K., Garcia M. E. Effect of vanadate on renal tubular function in rats. Am J Physiol. 1981 Jun;240(6):F522–F529. doi: 10.1152/ajprenal.1981.240.6.F522. [DOI] [PubMed] [Google Scholar]
- White J. W., Jr Relative significance of dietary sources of nitrate and nitrite. J Agric Food Chem. 1975 Sep-Oct;23(5):886–891. doi: 10.1021/jf60201a034. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Escherichia coli endotoxin injections potentiate experimental ischemic renal injury. Am J Physiol. 1986 Dec;251(6 Pt 2):F988–F994. doi: 10.1152/ajprenal.1986.251.6.F988. [DOI] [PubMed] [Google Scholar]





