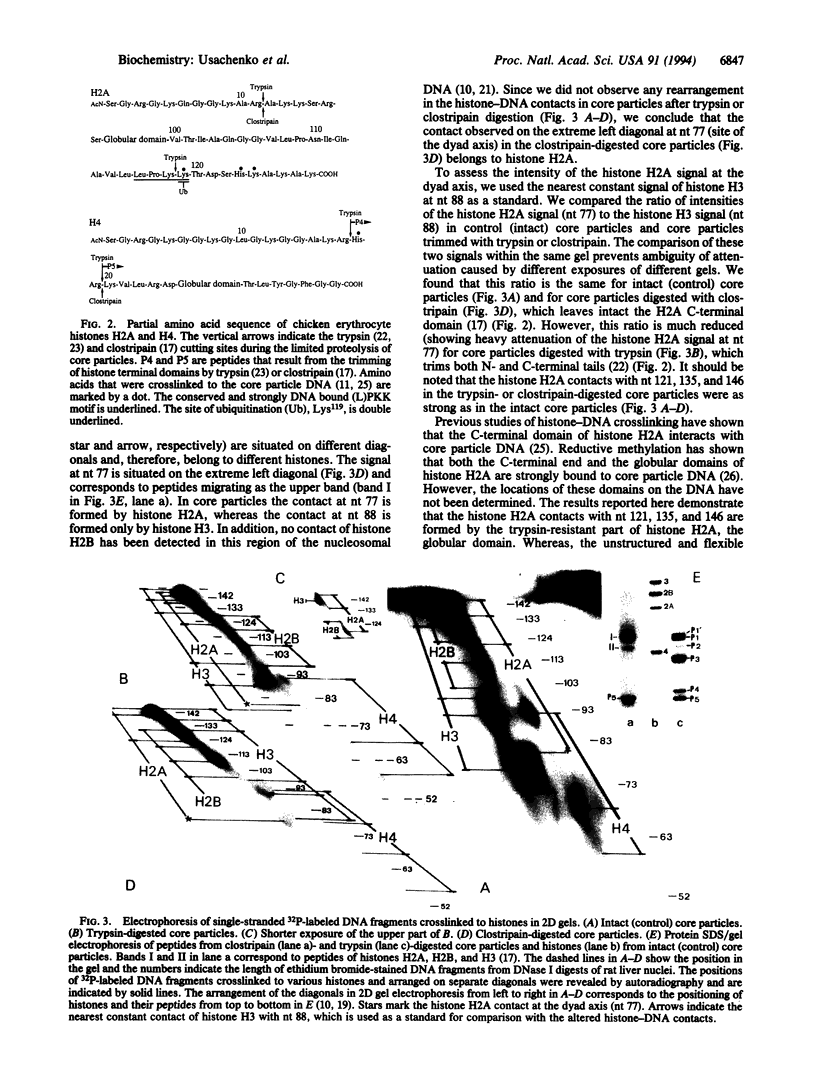
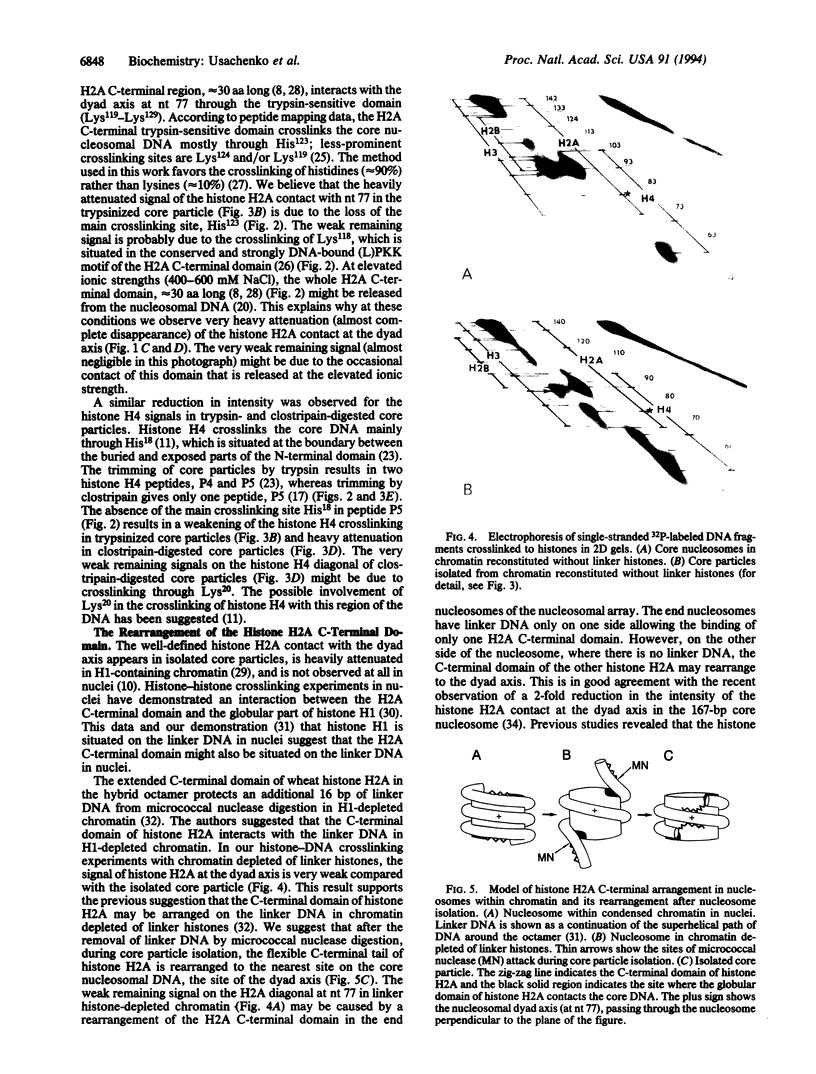
Abstract
Using zero-length covalent protein-DNA crosslinking, we have mapped the histone-DNA contacts in nucleosome core particles from which the C- and N-terminal domains of histone H2A were selectively trimmed by trypsin or clostripain. We found that the flexible trypsin-sensitive C-terminal domain of histone H2A contacts the dyad axis, whereas its globular domain contacts the end of DNA in the nucleosome core particle. The appearance of the histone H2A contact at the dyad axis occurs only in the absence of linker DNA and does not depend on the absence of linker histones. Our results show the ability of the histone H2A C-terminal domain to rearrange. This rearrangement might play a biological role in nucleosome disassembly and reassembly and the retention of the H2A-H2B dimer (or the whole octamer) during the passing of polymerases through the nucleosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Harborne N., Rau D. C., Gould H. Participation of core histone "tails" in the stabilization of the chromatin solenoid. J Cell Biol. 1982 May;93(2):285–297. doi: 10.1083/jcb.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan J., Staynov D. Z., Gould H. Reversible dissociation of linker histone from chromatin with preservation of internucleosomal repeat. Proc Natl Acad Sci U S A. 1980 Feb;77(2):885–889. doi: 10.1073/pnas.77.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arents G., Burlingame R. W., Wang B. C., Love W. E., Moudrianakis E. N. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausio J., Dong F., van Holde K. E. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone "tails" in the stabilization of the nucleosome. J Mol Biol. 1989 Apr 5;206(3):451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- Bavykin S. G., Usachenko S. I., Lishanskaya A. I., Shick V. V., Belyavsky A. V., Undritsov I. M., Strokov A. A., Zalenskaya I. A., Mirzabekov A. D. Primary organization of nucleosomal core particles is invariable in repressed and active nuclei from animal, plant and yeast cells. Nucleic Acids Res. 1985 May 24;13(10):3439–3459. doi: 10.1093/nar/13.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavykin S. G., Usachenko S. I., Zalensky A. O., Mirzabekov A. D. Structure of nucleosomes and organization of internucleosomal DNA in chromatin. J Mol Biol. 1990 Apr 5;212(3):495–511. doi: 10.1016/0022-2836(90)90328-J. [DOI] [PubMed] [Google Scholar]
- Belyavsky A. V., Bavykin S. G., Goguadze E. G., Mirzabekov A. D. Primary organization of nucleosomes containing all five histones and DNA 175 and 165 base-pairs long. J Mol Biol. 1980 May 25;139(3):519–536. doi: 10.1016/0022-2836(80)90144-8. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992 Jan;14(1):9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- Böhm L., Briand G., Sautière P., Crane-Robinson C. Proteolytic digestion studies of chromatin core-histone structure. Identification of limit peptides from histone H2B. Eur J Biochem. 1982 Apr 1;123(2):299–303. doi: 10.1111/j.1432-1033.1982.tb19767.x. [DOI] [PubMed] [Google Scholar]
- Böhm L., Briand G., Sautière P., Crane-Robinson C. Proteolytic digestion studies of chromatin core-histone structure. Identification of the limit peptides of histones H3 and H4. Eur J Biochem. 1981 Sep;119(1):67–74. doi: 10.1111/j.1432-1033.1981.tb05577.x. [DOI] [PubMed] [Google Scholar]
- Böhm L., Crane-Robinson C., Sautière P. Proteolytic digestion studies of chromatin core-histone structure. Identification of a limit peptide of histone H2A. Eur J Biochem. 1980 May;106(2):525–530. doi: 10.1111/j.1432-1033.1980.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Moss T., Bradbury E. M. High-resolution proton-magnetic-resonance studies of chromatin core particles. Eur J Biochem. 1978 Sep 1;89(2):475–482. doi: 10.1111/j.1432-1033.1978.tb12551.x. [DOI] [PubMed] [Google Scholar]
- Clark D. J., Felsenfeld G. A nucleosome core is transferred out of the path of a transcribing polymerase. Cell. 1992 Oct 2;71(1):11–22. doi: 10.1016/0092-8674(92)90262-b. [DOI] [PubMed] [Google Scholar]
- Davies N., Lindsey G. G. Histone-DNA contacts in the 167 bp 2-turn core particle. Biochim Biophys Acta. 1991 Dec 2;1129(1):57–63. doi: 10.1016/0167-4781(91)90212-5. [DOI] [PubMed] [Google Scholar]
- Dong F., Hansen J. C., van Holde K. E. DNA and protein determinants of nucleosome positioning on sea urchin 5S rRNA gene sequences in vitro. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5724–5728. doi: 10.1073/pnas.87.15.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis-Kervabon A., Encontre I., Etienne G., Jauregui-Adell J., Méry J., Mesnier D., Parello J. A chromatin core particle obtained by selective cleavage of histones by clostripain. EMBO J. 1986 Jul;5(7):1735–1742. doi: 10.1002/j.1460-2075.1986.tb04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebralidse K. K., Grachev S. A., Mirzabekov A. D. A highly basic histone H4 domain bound to the sharply bent region of nucleosomal DNA. Nature. 1988 Jan 28;331(6154):365–367. doi: 10.1038/331365a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramirez M., Dong F., Ausio J. Role of the histone "tails" in the folding of oligonucleosomes depleted of histone H1. J Biol Chem. 1992 Sep 25;267(27):19587–19595. [PubMed] [Google Scholar]
- Greyling H. J., Schwager S., Sewell B. T., von Holt C. The identity of conformational states of reconstituted and native histone octamers. Eur J Biochem. 1983 Dec 1;137(1-2):221–226. doi: 10.1111/j.1432-1033.1983.tb07818.x. [DOI] [PubMed] [Google Scholar]
- Hayes J. J., Clark D. J., Wolffe A. P. Histone contributions to the structure of DNA in the nucleosome. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6829–6833. doi: 10.1073/pnas.88.15.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov V. L., Bavykin S. G., Preobrazhenskaya O. V., Belyavsky A. V., Mirzabekov A. D. Alignment of nucleosomes along DNA and organization of spacer DNA in Drosophila chromatin. Nucleic Acids Res. 1982 Jul 24;10(14):4321–4337. doi: 10.1093/nar/10.14.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Rhodes D., Smith J., Finch J. T., Thomas J. O. A low resolution structure for the histone core of the nucleosome. Nature. 1980 Oct 9;287(5782):509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert S. F., Thomas J. O. Lysine-containing DNA-binding regions on the surface of the histone octamer in the nucleosome core particle. Eur J Biochem. 1986 Oct 1;160(1):191–201. doi: 10.1111/j.1432-1033.1986.tb09957.x. [DOI] [PubMed] [Google Scholar]
- Lindsey G. G., Orgeig S., Thompson P., Davies N., Maeder D. L. Extended C-terminal tail of wheat histone H2A interacts with DNA of the "linker" region. J Mol Biol. 1991 Apr 20;218(4):805–813. doi: 10.1016/0022-2836(91)90268-b. [DOI] [PubMed] [Google Scholar]
- Meersseman G., Pennings S., Bradbury E. M. Chromatosome positioning on assembled long chromatin. Linker histones affect nucleosome placement on 5 S rDNA. J Mol Biol. 1991 Jul 5;220(1):89–100. doi: 10.1016/0022-2836(91)90383-h. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Bavykin S. G., Belyavsky A. V., Karpov V. L., Preobrazhenskaya O. V., Shick V. V., Ebralidse K. K. Mapping DNA-protein interactions by cross-linking. Methods Enzymol. 1989;170:386–408. doi: 10.1016/0076-6879(89)70058-6. [DOI] [PubMed] [Google Scholar]
- Moss T., Cary P. D., Abercrombie B. D., Crane-Robinson C., Bradbury E. M. A pH-dependent interaction between histones H2A and H2B involving secondary and tertiary folding. Eur J Biochem. 1976 Dec 11;71(2):337–350. doi: 10.1111/j.1432-1033.1976.tb11120.x. [DOI] [PubMed] [Google Scholar]
- Nacheva G. A., Guschin D. Y., Preobrazhenskaya O. V., Karpov V. L., Ebralidse K. K., Mirzabekov A. D. Change in the pattern of histone binding to DNA upon transcriptional activation. Cell. 1989 Jul 14;58(1):27–36. doi: 10.1016/0092-8674(89)90399-1. [DOI] [PubMed] [Google Scholar]
- O'Neill T. E., Smith J. G., Bradbury E. M. Histone octamer dissociation is not required for transcript elongation through arrays of nucleosome cores by phage T7 RNA polymerase in vitro. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6203–6207. doi: 10.1073/pnas.90.13.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Shick V. V., Belyavsky A. V., Bavykin S. G., Mirzabekov A. D. Primary organization of the nucleosome core particles. Sequential arrangement of histones along DNA. J Mol Biol. 1980 May 25;139(3):491–517. doi: 10.1016/0022-2836(80)90143-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Streeck R. E., Zachau H. G. Closely spaced nucleosome cores in reconstituted histone.DNA complexes and histone-H1-depleted chromatin. Eur J Biochem. 1978 Feb;83(2):615–628. doi: 10.1111/j.1432-1033.1978.tb12131.x. [DOI] [PubMed] [Google Scholar]
- Struck M. M., Klug A., Richmond T. J. Comparison of X-ray structures of the nucleosome core particle in two different hydration states. J Mol Biol. 1992 Mar 5;224(1):253–264. doi: 10.1016/0022-2836(92)90588-b. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holde K. E., Lohr D. E., Robert C. What happens to nucleosomes during transcription? J Biol Chem. 1992 Feb 15;267(5):2837–2840. [PubMed] [Google Scholar]





