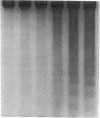
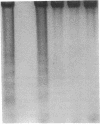
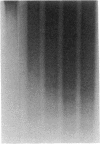
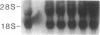Abstract
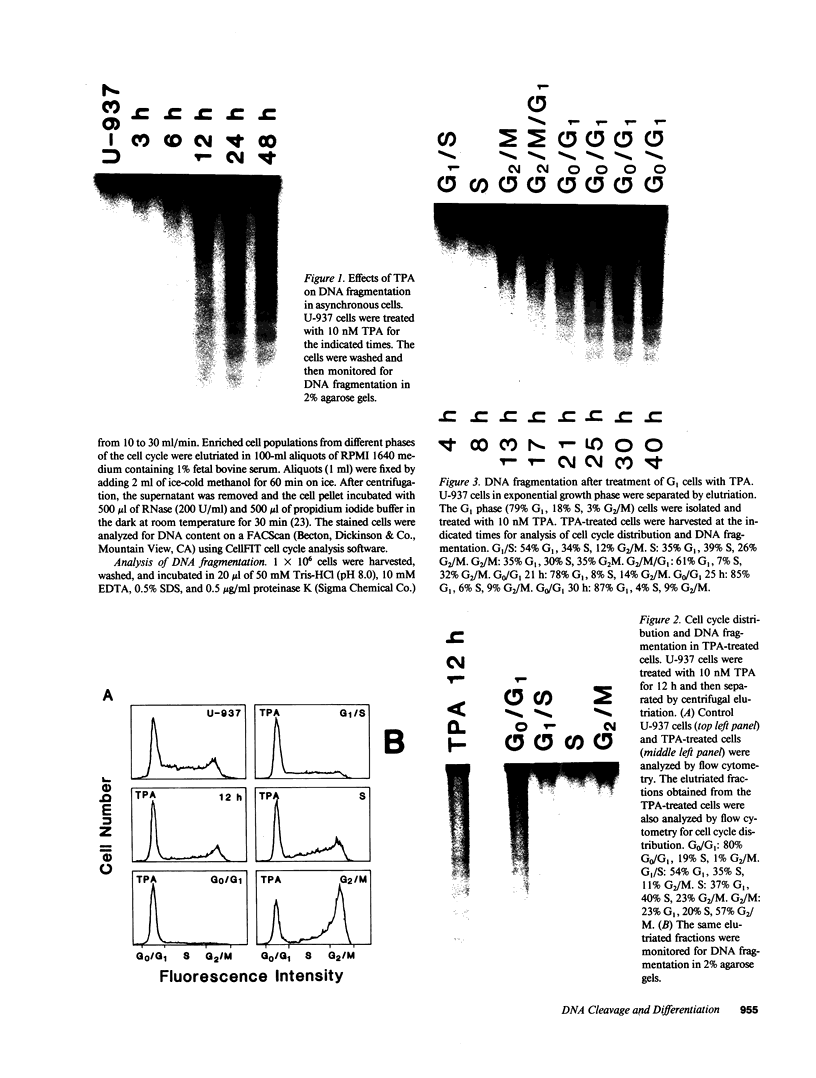
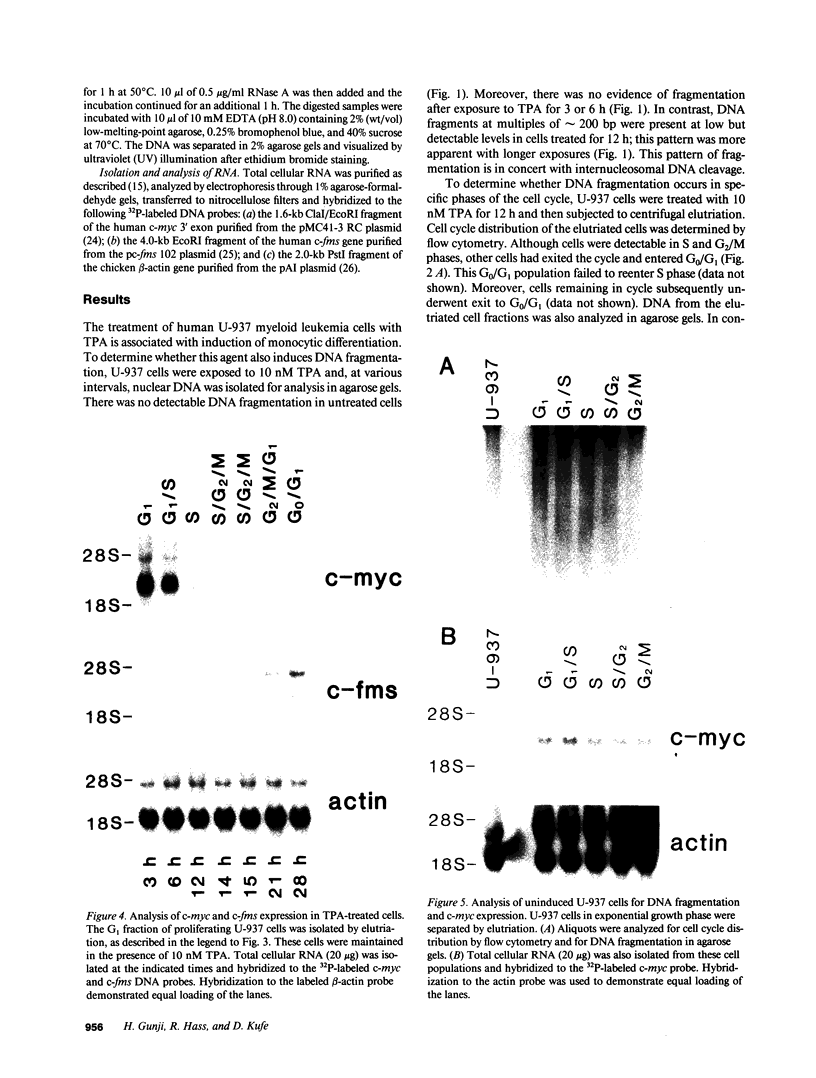
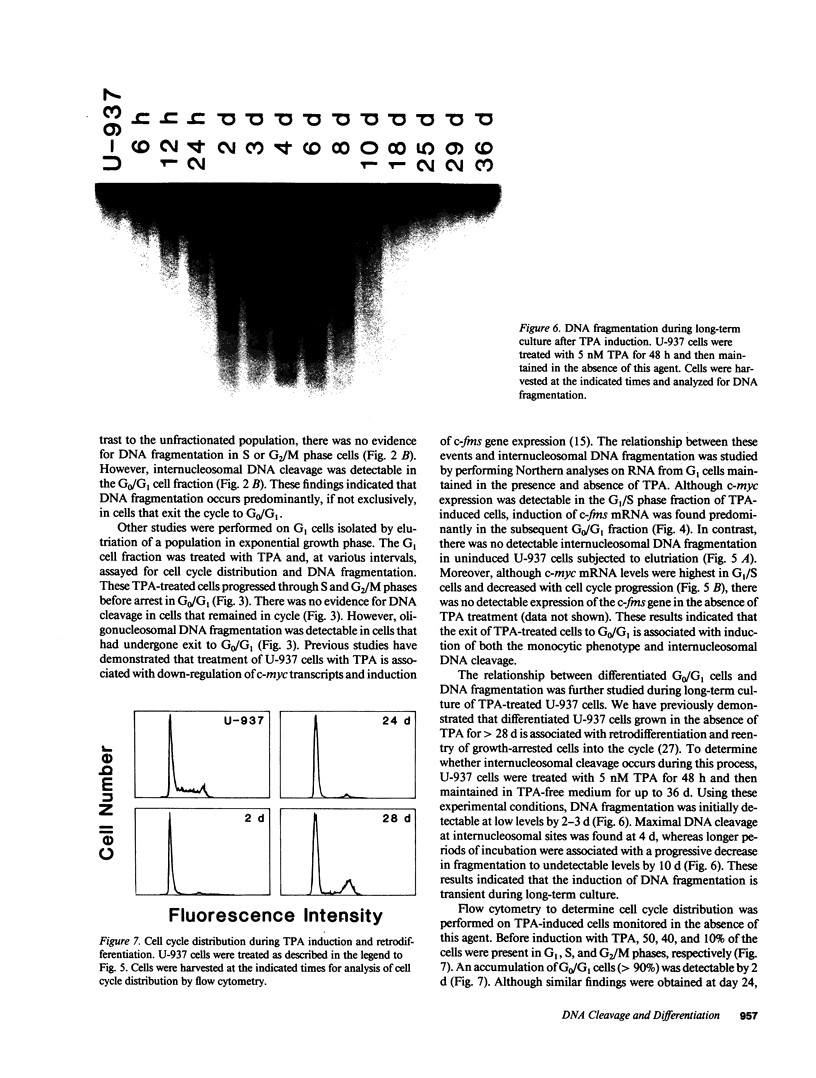
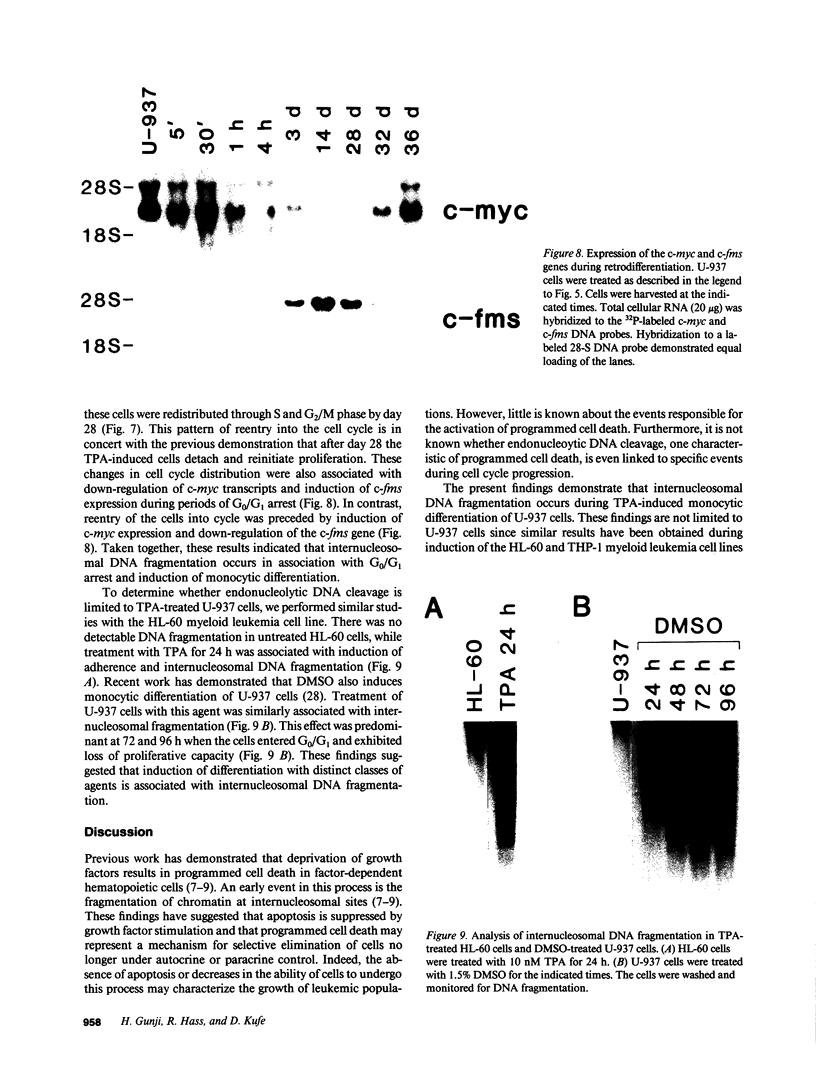
The treatment of human myeloid leukemia cell lines with phorbol esters, such as 12-O-tetradecanoylphorbol-13-acetate (TPA), is associated with loss of proliferative capacity and induction of monocytic differentiation. The present results demonstrate that treatment of asynchronous human U-937 leukemia cells with 10 nM TPA is also associated with oligonucleosomal DNA cleavage. This pattern of DNA fragmentation, which is observed in programmed cell death, was detectable in populations of TPA-treated cells that had entered a nonproliferative G0/G1 phase. Similar findings were obtained after TPA treatment of a synchronous population of G1 cells. These cells progressed through S and G2/M phases before undergoing internucleosomal DNA cleavage during G0/G1 arrest. These G0/G1 cells displayed characteristics of monocytic differentiation, including down-regulation of c-myc expression and induction of c-fms transcripts. DNA fragmentation was also studied in cells treated with 5 nM TPA for 48 h and then monitored in drug-free long-term culture. Endonucleolytic cleavage was similarly observed in the differentiated G0/G1 population. However, longer periods of culture were associated with a decrease in DNA fragmentation to undetectable levels. This effect was followed by retrodifferentiation and reentry of cells into cycle. Taken together, these findings demonstrate that internucleosomal DNA fragmentation occurs during induction of monocytic differentiation, and that both of these events are detectable in G0/G1 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Hass R., Giese G., Meyer G., Hartmann A., Dörk T., Köhler L., Resch K., Traub P., Goppelt-Strübe M. Differentiation and retrodifferentiation of U937 cells: reversible induction and suppression of intermediate filament protein synthesis. Eur J Cell Biol. 1990 Apr;51(2):265–271. [PubMed] [Google Scholar]
- Hass R., Lonnemann G., Männel D., Topley N., Hartmann A., Köhler L., Resch K., Goppelt-Strübe M. Regulation of TNF-alpha, IL-1 and IL-6 synthesis in differentiating human monoblastoid leukemic U937 cells. Leuk Res. 1991;15(5):327–339. doi: 10.1016/0145-2126(91)90008-h. [DOI] [PubMed] [Google Scholar]
- Hass R., Pfannkuche H. J., Kharbanda S., Gunji H., Meyer G., Hartmann A., Hidaka H., Resch K., Kufe D., Goppelt-Strübe M. Protein kinase C activation and protooncogene expression in differentiation/retrodifferentiation of human U-937 leukemia cells. Cell Growth Differ. 1991 Nov;2(11):541–548. [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983 Oct;62(4):709–721. [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Alsina J. L., Levy S. B. Induction of a Ca2+, Mg2+-dependent endonuclease activity during the early stages of murine erythroleukemic cell differentiation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7461–7465. doi: 10.1073/pnas.81.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Kharbanda S., Spriggs D., Kufe D. Effects of dexamethasone on induction of monocytic differentiation in human U-937 cells by dimethylsulfoxide. J Cell Physiol. 1990 Feb;142(2):261–267. doi: 10.1002/jcp.1041420207. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Ferrero D., Pagliardi G. L., Vartikar J., Pessano S., Bottero L., Abraham S., Lebman D. Induction of differentiation of human myeloid leukemias by phorbol diesters: phenotypic changes and mode of action. Ann N Y Acad Sci. 1982 Dec 10;397:211–220. doi: 10.1111/j.1749-6632.1982.tb43428.x. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Scher W., Friend C. Breakage of DNA and alterations in folded genomes by inducers of differentiation in Friend erythroleukemic cells. Cancer Res. 1978 Mar;38(3):841–849. [PubMed] [Google Scholar]
- Sherman M. L., Stone R. M., Datta R., Bernstein S. H., Kufe D. W. Transcriptional and post-transcriptional regulation of c-jun expression during monocytic differentiation of human myeloid leukemic cells. J Biol Chem. 1990 Feb 25;265(6):3320–3323. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Terada M., Nudel U., Fibach E., Rifkind R. A., Marks P. A. Changes in DNA associated with induction of erythroid differentiation by dimethyl sulfoxide in murine erythroleukemia cells. Cancer Res. 1978 Mar;38(3):835–840. [PubMed] [Google Scholar]
- Wakamiya N., Horiguchi J., Kufe D. Detection of c-fms and CSF-1 RNA by in situ hybridization. Leukemia. 1987 Jun;1(6):518–520. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]