Abstract
Importance
Higher intake of whole grains has been associated with a lower risk of major chronic diseases, such as type 2 diabetes and cardiovascular disease (CVD), although limited prospective evidence exists regarding whole grains’ association with mortality.
Objective
To examine the association between dietary whole grain consumption and risk of mortality.
Design, Setting and Participants
Two large prospective cohort studies. The study population comprised 74,341 women in the Nurses’ Health Study (1984–2010) and 43,744 men in the Health Professionals Follow-Up Study (1986–2010), who were free of CVD and cancer at baseline.
Main Outcomes and Measures
Hazard ratio for total mortality and mortality due to CVD and cancer according to quintiles of whole grain consumption, which was updated every two or four years by using validated food frequency questionnaires.
Results
We documented 26,920 deaths during 2,727,006 person-years of follow-up. After multivariate adjustment for potential confounders, including age, smoking, body mass index, physical activity, and a modified alternative healthy eating index, higher whole grain intake was associated with a lower total and CVD mortality but not cancer mortality: the pooled hazard ratios (HRs) and 95% confidence intervals (CIs) across quintiles of whole grain intake were 1.0, 0.99 (0.95–1.02), 0.98 (0.95–1.02), 0.97 (0.93–1.01), and 0.91 (0.88–0.95) for total mortality (Ptrend<0.001); 1.0, 0.94 (0.88–1.01), 0.94 (0.87–1.01), 0.87 (0.80–0.94) and 0.85 (0.78–0.92) for CVD mortality (Ptrend<0.001); or 1.0, 1.02 (0.96–1.08), 1.05 (0.99–1.12), 1.04 (0.98–1.11), and 0.97 (0.91–1.04) for cancer mortality (Ptrend=0.43). The pooled multivariable-adjusted HRs (95%CIs) for each serving/day increment of whole grain consumption were 0.95 (0.93–0.98), 0.91 (0.87–0.96), and 0.98 (0.94–1.02) for total, CVD and cancer mortality, respectively. Similar inverse associations were observed between bran intake and CVD mortality, with a pooled HR of 0.80 (95%CI 0.73–0.87, Ptrend<0.0001), whereas germ intake was not associated with CVD mortality after adjustment for bran intake.
Conclusions and Relevance
These data indicate that higher whole grain consumption is associated with lower total and CVD mortality in U.S. men and women, independent of other dietary and lifestyle factors. These results are in line with recommendations that promote increased whole grain consumption to facilitate disease prevention.
Keywords: whole grains, mortality, prospective cohort study
INTRODUCTION
Whole grains have been widely recommended in numerous dietary guidelines as a healthful food.1,2 In comparison to refined carbohydrates, whole grains contain many beneficial nutrients and phytochemicals that primarily reside in the outer layers of grains that are removed during milling processes to produce refined grain products. In laboratory research and human feeding trials, whole grains, as well as constituents of whole grains, such as insoluble fiber, magnesium, and phytochemicals, consistently have beneficial effects on glucose metabolism,3–5 blood lipids,6 endothelial function,7 antioxidant activity,8 and inflammation.9,10 In addition, epidemiological studies have consistently found inverse associations between whole grain intake and lower risk of type 2 diabetes (T2D)11–13 and cardiovascular diseases (CVD).12–14
While these lines of evidence suggest beneficial effects of whole grain intake on the overall health, data regarding whole grain intake and mortality was not entirely consistent. For instance, whole grain intake was significantly associated with lower total mortality risk in the Iowa Women’s Health Study,15,16 the Atherosclerosis Risk in Communities (ARIC) study,17 and the Norwegian County Study.18 In contrast, null associations were found among a healthy elderly population19 or diabetes patients.20 Regarding cause-specific mortality, an inverse association between whole grain intake and CVD mortality was consistently observed in previous studies,15,16,18–21 although only three studies evaluated cancer mortality and reported a non-significant reduced cancer mortality risk among whole grain eaters.15,16,18 The inconsistency of results may be explained in part by the heterogeneity in dietary assessments, baseline exclusion criteria, and population demographic and lifestyle characteristics. In particular, most previous studies only assessed the eating frequency of whole grain foods that may contain various proportions of actual whole grain contents. For example, some studies defined whole grain foods using the proportion of whole grain or bran ≥ 25% as the criterion and examined total servings per day of whole grain foods in relation to disease risk.17,19 This approach might bring in residual measurement error because the absolute amount of whole grain varies among the foods. Moreover, only one study, which was conducted among type 2 diabetic women, explicitly examined the intake of added bran and germ in relation to total and cause-specific mortality.20
Therefore, in the current study we investigated the association between whole grain intake and total and cause-specific mortality in two large cohort studies with repeated assessments of diet and extended length of follow-up: the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). We also evaluated the association between bran and germ consumption and total and cause-specific mortality.
METHODS
Study Populations
The NHS is a prospective cohort study of 121,700 female registered nurses from 11 U.S. states aged 30–55 years initiated in 1976. The HPFS cohort was established in 1986 with an enrollment of 51,529 U.S. male health professionals from all 50 states aged 32–87 years. Through 2010, a response rate exceeding 90% has been achieved in both cohorts.
For the current investigation, we excluded participants with cancer, stroke, or coronary heart disease (CHD) at baseline (1984 for NHS, 1986 for HPFS); those who had incomplete information for dietary data, or who reported implausible total energy intake (<500 or >3500 kcal/day for NHS, and <800 or >4200 kcal/day for HPFS). After exclusions, a total of 74,341 women and 43,744 men remained in the analysis.
The study protocol has been approved by the Human Research Committee of Brigham and Women’s Hospital in Boston and the Harvard School of Public Health. The completion of the self-administered questionnaire was considered to imply informed consent.
Ascertainment of Diet
Intakes of whole grains and other foods were assessed using validated food frequency questionnaires (FFQs) every 2 to 4 years.22,23 The FFQs inquired about average consumption of foods (with a pre-specified portion size) during the previous year using nine categories of intake frequency ranging from “<1/month” to “6+/day”. Open-ended questions were included for breakfast cereal brand names and foods that were not listed on the FFQ.
Intakes of whole grain (g/d) were estimated from all grain-containing foods (rice, bread, pasta, and breakfast cereals) according to the dry weight of the whole grain ingredients in each food.24–26 Whole grain consumption from breakfast cereal was derived from more than 250 brand name cereals based on information provided by product labels and breakfast cereal manufacturers. In our study, whole grains included both intact and pulverized forms containing the expected proportion of bran, germ, and endosperm for the specific grain types. By definition, the following foods/ingredients were considered as whole grains: whole wheat and whole wheat flour, whole oats and whole oat flour, whole cornmeal and whole corn flour, whole rye and whole rye flour, whole barley, bulgur, buckwheat, brown rice and brown rice flour, popcorn, amaranth, and psyllium. In the FFQ, we further asked the frequency of consuming added bran (oat bran and other bran) and added wheat germ. Intakes of bran and germ were derived directly from whole grain foods and those added to foods. Total bran and total germ are the sum of intakes from both sources.
Ascertainment of Mortality
Deaths were reported by the next of kin or the postal authorities or by searching the National Death Index.27 More than 97% of deaths can be identified in these cohorts.27 For all deaths, we sought death certificates and, when appropriate, requested permission from the next of kin to review medical records. The underlying cause of death was assigned according to the International Classification of Diseases, 8th Revision (ICD-8). In this analysis we also specifically considered deaths due to CVD (ICD-8 390.0–458.9 or 795.0–795.9) or cancer (ICD-8 140.0–207.9).
Assessment of Covariates
In both cohorts, information on body weight, medical history, lifestyle characteristics (e.g., cigarette smoking and physical activity), disease diagnoses (diabetes, hypertension, and hypercholesterolemia), and other characteristics were collected at baseline and in biennial validated questionnaires. Alcohol and other dietary information was assessed and updated by validated FFQs. Detailed descriptions on the validity and reproducibility of self-reported body weight, physical activity, and alcohol consumption have been published elsewhere.28–30 A modified alternative healthy eating index (aHEI) was calculated based on intakes of ten foods and nutrients predictive of chronic disease risk, including fruits, vegetables, nuts and legumes, red/processed meat, sugar sweetened beverages, alcohol, sodium, trans fat, long-chain n-3 fats and other polyunsaturated fats.31
Statistical Analysis
We calculated person-years of follow-up from the return date of the first FFQ to the date of death or January 31, 2010 for HPFS or June 30, 2010 for NHS, whichever came first. We calculated and used the energy-adjusted residuals of whole grain intakes to control for total energy intake. To better represent long-term or habitual intake, and to minimize within-person variation, we created and used the cumulative average of energy-adjusted whole grain intake from all available dietary questionnaires from baseline through the end of follow-up.32 We replaced missing values in each FFQ with cumulative averages based on prior assessments. To minimize the possibility of reverse causation bias, we stopped updating diet after participants reported a diagnosis of diabetes, stroke, or CHD, because diagnosis of these conditions led to changes in whole grain intake (Table S1). We then carried forward the cumulative averages of dietary variables prior to the development of these diseases to represent diet for later follow-up.33 Due to differences in sex, follow-up time, and the questionnaires in the two cohorts, we conducted analyses separately for each cohort to facilitate better control of confounding. Cox proportional hazard models stratified on age (months) and calendar time (2-year intervals) were used to investigate the association of whole grain, bran and germ consumption with total and cause-specific mortality. We used hazard ratios (HRs) to measure relative risks in higher intake quintiles in comparison with participants in the lowest quintiles, and 95% confidence intervals (CIs) were calculated for HRs. In multivariate analysis, we controlled for age and ethnicity, as well as time-varying covariates, including BMI, smoking status, alcohol intake, physical activity, multivitamin use, aspirin use, a family history of heart disease, cancer, or diabetes, and history of hypertension, high cholesterol, or diabetes at baseline, total energy (kcal; in quintiles), and modified aHEI (whole grain excluded). In NHS, we also adjusted for menopausal status and postmenopausal hormone use. Proportional hazards assumption was tested by evaluating the significance of the interaction term between quintiles of whole grain consumption and time period of follow-up, and no violation of the proportional-hazards assumption was found (P>0.05 for all tests). Tests for trend were conducted by assigning the median value to each category and modeled this value as a continuous variable. In addition, we used restricted cubic spline regressions with 4 knots to examine dose-response relationships between whole grain intake and risk of mortality. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. We estimated the association of substituting whole grains for refined grains, red meat, and potato. The calculation of substitution effects was based on the differences of β coefficients between whole grains and a specific food, and their variances and covariance matrix were used to derive the 95% CI for the point estimate.34 The estimates of association across the two studies were pooled using a fixed-effects model.
All analyses were performed using SAS, version 9.3 (SAS Institute Inc, Cary, NC). All p values presented are two-tailed, and p values below 0.05 were considered statistically significant.
RESULTS
Table 1 presents baseline characteristics by quintiles of whole grain intake. Men and women with higher intake of whole grains were more likely to be physically active and have a history of high cholesterol, were less likely to be current smokers, and had lower alcohol intake. In addition, a higher whole grain intake was associated with a better diet quality as reflected by the higher aHEI score.
Table 1.
Baseline Characteristics of Study Participants by Intake Levels of Whole Grains, the Nurses’ Health Study (1984) and Health Professionals Follow-up Study (1986).*
| Variable | Quintiles of whole grain intake in NHS
|
Quintiles of whole grain intake in HPFS
|
||||
|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
|
|
|
|||||
| N | 14819 | 14630 | 14868 | 8724 | 8777 | 8776 |
| Whole grains, gram/day | 4.3 | 14.7 | 35.6 | 5.8 | 21.8 | 52.6 |
| Bran, gram/day | 0.4 | 2.2 | 8.7 | 0.5 | 3.8 | 11.7 |
| Germ, gram/day | 0.1 | 0.6 | 1.8 | 0.2 | 0.9 | 2.7 |
| Age, years | 50.2 | 50.2 | 50.3 | 53.3 | 53.2 | 53.2 |
| BMI, kg/m2 | 25.3 | 25.2 | 24.4 | 25.2 | 25.1 | 24.3 |
| Alcohol, g/d | 9.4 | 6.8 | 4.7 | 14.5 | 11.6 | 8.1 |
| Physical activity, METs-hr/week | 11.4 | 13.6 | 15.7 | 17.8 | 20.4 | 25.8 |
| Total energy, kcal/day | 1711 | 1786 | 1653 | 1984 | 2060 | 1879 |
| Alternative healthy eating index† | 42.8 | 45.6 | 49.5 | 46.0 | 49.9 | 54.6 |
| Smoking status, % | ||||||
| Never smoker | 36.3 | 44.6 | 50.2 | 41.9 | 50.6 | 56.1 |
| Past smoker | 27.1 | 32.1 | 35.2 | 40.7 | 41.0 | 39.5 |
| Current smoker | 36.6 | 23.3 | 14.6 | 17. 5 | 8.4 | 4.3 |
| Family history of diabetes, % | 29.9 | 29.5 | 29.0 | 23.7 | 24.1 | 24.0 |
| Family history of cancer, % | 14.3 | 15.2 | 16.6 | 34.9 | 34.7 | 36.7 |
| Family history of MI, % | 19.7 | 19.6 | 18.9 | 31.3 | 30.8 | 33.0 |
| Diabetes, % | 2.7 | 2.7 | 3.5 | 2.2 | 2.4 | 3.3 |
| Hypertension, % | 22.0 | 20.4 | 21.2 | 22.9 | 19.1 | 19.9 |
| High cholesterol, % | 6.6 | 7.6 | 10.1 | 9.2 | 10.1 | 13.2 |
| Aspirin use, % | 66.3 | 67.4 | 64.8 | 25.3 | 27.8 | 26.5 |
| Multivitamin use, % | 30.2 | 37.0 | 44.9 | 34.7 | 41.0 | 50.3 |
Abbreviations: BMI, body mass index; MI, myocardial infarction; MET, metabolic equivalent task, NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study.
Values are mean for continuous variables or % for categorical variables. All variables were age-standardized, except age per se.
Whole grains were excluded when calculating the alternative healthy eating index.
In NHS, during 26 years of follow-up (1,798,063 person-years), we documented 15,106 deaths, of which 2,989 were CVD deaths and 5,964 were cancer deaths (of which 499 due to colorectal cancer, 1417 lung cancer, and 895 breast cancer). In HPFS, with up to 24 years of follow-up (928,943 person-years), we documented 11,814 deaths, of which 3,621were CVD deaths and 3,921 were cancer deaths (of which 423 due to colorectal cancer, 739 lung cancer, and 564 prostate cancer). Table 2 shows associations between whole grain consumption and total and cause-specific mortality. In age-adjusted analyses, higher intake of whole grain was significantly associated with lower total and cause-specific mortality. Further adjustment for other potential confounders, especially physical activity, smoking, and BMI, attenuated these associations, although the statistical significance remained for associations with total and CVD mortality. The pooled HRs (CIs) comparing extreme whole grain intake levels were 0.91 (0.88–0.95; Ptrend<0.001) for total mortality, and 0.85 (0.78–0.92; Ptrend<0.001) for CVD mortality. In contrast, the same multivariate adjustment abolished the associations for cancer mortality; the pooled HR (95%CI) was 0.97 (0.91–1.04; Ptrend=0.43) comparing extreme intake levels. Further analyses showed that whole grain intake was not significantly associated with mortality due to major types of cancer, including colorectal cancer, lung cancer, breast cancer or prostate cancer (Table S2). The association for non-CVD and non-cancer mortality was showed in Table S3.
Table 2.
Hazard Ratio (95%CI) of Total Mortality and Cause-specific Mortality by Quintiles of Whole Grain Intake.
| Quintiles of Whole Grain Intake (g/d)
|
Ptrend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Total mortality | ||||||
| NHS | ||||||
| Median | 4.2 | 9.7 | 14.7 | 21.1 | 33.0 | |
| No. of cases | 4122 | 2990 | 2744 | 2550 | 2700 | |
| Person-years | 356,895 | 359,688 | 359,945 | 361,086 | 360,449 | |
| Model 1* | 1.0 | 0.72 (0.69–0.76) | 0.63 (0.60–0.66) | 0.54 (0.51–0.57) | 0.49 (0.47–0.51) | < 0.001 |
| Model 2† | 1.0 | 0.97 (0.93–1.02) | 0.98 (0.93–1.03) | 0.92 (0.87–0.97) | 0.86 (0.81–0.90) | < 0.001 |
| Model 3‡ | 1.0 | 0.98 (0.93–1.03) | 1.00 (0.95–1.05) | 0.94 (0.89–0.99) | 0.88 (0.84–0.93) | < 0.001 |
| HPFS | ||||||
| Median | 5.9 | 14.4 | 22.1 | 31.3 | 47.8 | |
| No. of cases | 3056 | 2392 | 2117 | 2112 | 2137 | |
| Person-years | 183,911 | 185,779 | 186,319 | 186,660 | 186,274 | |
| Model 1* | 1.0 | 0.80 (0.76–0.84) | 0.68 (0.65–0.72) | 0.66 (0.62–0.69) | 0.62 (0.59–0.66) | < 0.001 |
| Model 2† | 1.0 | 0.99 (0.94–1.05) | 0.95 (0.90–1.01) | 0.99 (0.93–1.05) | 0.92 (0.86–0.97) | 0.006 |
| Model 3‡ | 1.0 | 1.00 (0.94–1.05) | 0.97 (0.91–1.02) | 1.01 (0.95–1.07) | 0.95 (0.89–1.00) | 0.13 |
| Pooled§ | ||||||
| Model 1* | 1.0 | 0.75 (0.73–0.78) | 0.65 (0.63–0.68) | 0.59 (0.57–0.61) | 0.54 (0.52–0.56) | < 0.001 |
| Model 2† | 1.0 | 0.98 (0.95–1.02) | 0.97 (0.93–1.01) | 0.95 (0.91–0.99) | 0.88 (0.85–0.92) | < 0.001 |
| Model 3‡ | 1.0 | 0.99 (0.95–1.02) | 0.98 (0.95–1.02) | 0.97 (0.93–1.01) | 0.91 (0.88–0.95) | < 0.001 |
| CVD mortality | ||||||
| NHS | ||||||
| No. of cases | 860 | 597 | 535 | 450 | 547 | |
| Person-years | 359,859 | 361,853 | 361,937 | 362,951 | 362,354 | |
| Model 1* | 1.0 | 0.69 (0.62–0.77) | 0.59 (0.53–0.65) | 0.45 (0.40–0.50) | 0.46 (0.41–0.51) | < 0.001 |
| Model 2† | 1.0 | 0.96 (0.87–1.07) | 0.95 (0.85–1.06) | 0.81 (0.72–0.91) | 0.84 (0.75–0.93) | < 0.001 |
| Model 3‡ | 1.0 | 0.97 (0.87–1.08) | 0.96 (0.86–1.08) | 0.82 (0.73–0.92) | 0.86 (0.76–0.96) | 0.001 |
| HPFS | ||||||
| No. of cases | 973 | 721 | 664 | 630 | 633 | |
| Person-years | 185,791 | 187,312 | 187,630 | 187,998 | 187,645 | |
| Model 1* | 1.0 | 0.76 (0.69–0.84) | 0.68 (0.61–0.74) | 0.62 (0.56–0.68) | 0.58 (0.52–0.64) | < 0.001 |
| Model 2† | 1.0 | 0.92 (0.83–1.02) | 0.92 (0.83–1.02) | 0.90 (0.81–1.00) | 0.82 (0.74–0.91) | < 0.001 |
| Model 3‡ | 1.0 | 0.92 (0.84–1.02) | 0.92 (0.83–1.02) | 0.91 (0.82–1.01) | 0.84 (0.75–0.93) | 0.002 |
| Pooled§ | ||||||
| Model 1* | 1.0 | 0.73 (0.68–0.78) | 0.63 (0.59–0.68) | 0.54 (0.50–0.58) | 0.52 (0.48–0.56) | < 0.001 |
| Model 2† | 1.0 | 0.94 (0.87–1.01) | 0.93 (0.87–1.01) | 0.86 (0.80–0.93) | 0.83 (0.77–0.89) | < 0.001 |
| Model 3‡ | 1.0 | 0.94 (0.88–1.01) | 0.94 (0.87–1.01) | 0.87 (0.80–0.94) | 0.85 (0.78–0.92) | < 0.001 |
| Cancer mortality | ||||||
| NHS | ||||||
| No. of cases | 1449 | 1170 | 1162 | 1094 | 1089 | |
| Person-years | 359,291 | 361,309 | 361,345 | 362,318 | 361,852 | |
| Model 1* | 1.0 | 0.80 (0.74–0.86) | 0.77 (0.71–0.83) | 0.68 (0.63–0.73) | 0.60 (0.56–0.65) | < 0.001 |
| Model 2† | 1.0 | 1.02 (0.94–1.10) | 1.10 (1.02–1.19) | 1.06 (0.98–1.15) | 0.98 (0.90–1.07) | 0.73 |
| Model 3‡ | 1.0 | 1.02 (0.94–1.10) | 1.10 (1.02–1.19) | 1.06 (0.98–1.15) | 0.99 (0.91–1.07) | 0.82 |
| HPFS | ||||||
| No. of cases | 976 | 790 | 708 | 725 | 722 | |
| Person-years | 185,746 | 187,228 | 187,599 | 187,911 | 187,560 | |
| Model 1* | 1.0 | 0.84 (0.76–0.92) | 0.72 (0.66–0.80) | 0.71 (0.64–0.78) | 0.67 (0.61–0.74) | < 0.001 |
| Model 2† | 1.0 | 1.01 (0.92–1.11) | 0.97 (0.88–1.07) | 1.01 (0.91–1.11) | 0.94 (0.85–1.04) | 0.29 |
| Model 3‡ | 1.0 | 1.01 (0.92–1.11) | 0.98 (0.88–1.08) | 1.01 (0.91–1.12) | 0.95 (0.86–1.05) | 0.40 |
| Pooled§ | ||||||
| Model 1* | 1.0 | 0.81 (0.77–0.86) | 0.75 (0.70–0.80) | 0.69 (0.65–0.73) | 0.63 (0.59–0.67) | < 0.001 |
| Model 2† | 1.0 | 1.02 (0.96–1.08) | 1.05 (0.99–1.12) | 1.04 (0.97–1.10) | 0.96 (0.90–1.03) | 0.30 |
| Model 3‡ | 1.0 | 1.02 (0.96–1.08) | 1.05 (0.99–1.12) | 1.04 (0.98–1.11) | 0.97 (0.91–1.04) | 0.43 |
Abbreviations: CVD, cardiovascular diseases; CI, confidence interval; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study.
Age-adjusted.
Further adjusted for ethnicity (Caucasian, Asian, African American and Hispanic/others), BMI (<18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0 kg/m2), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d), pack years smoked (0, 1–9,10–24, 25–44, ≥45 pack-years), years since quitting for past smoker (not past smoker, years since smoking quitting <2, 3–5, 6–9 and ≥10years) alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥30.0 g/d for men; 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥15.0 g/d for women), physical activity (quintiles), family history of diabetes, cancer and heart disease (yes, no), multivitamin use (yes, no), aspirin use at least once per week (yes, no), history of hypertension, high cholesterol, or diabetes at baseline, total energy (kcal/day, in quintiles). For women, postmenopausal status and postmenopausal hormone use were further adjusted for.
Further adjusted for modified alternative healthy eating index (in quintiles), which did not include whole grains.
Pooled hazard ratios were calculated using a fixed-effects model.
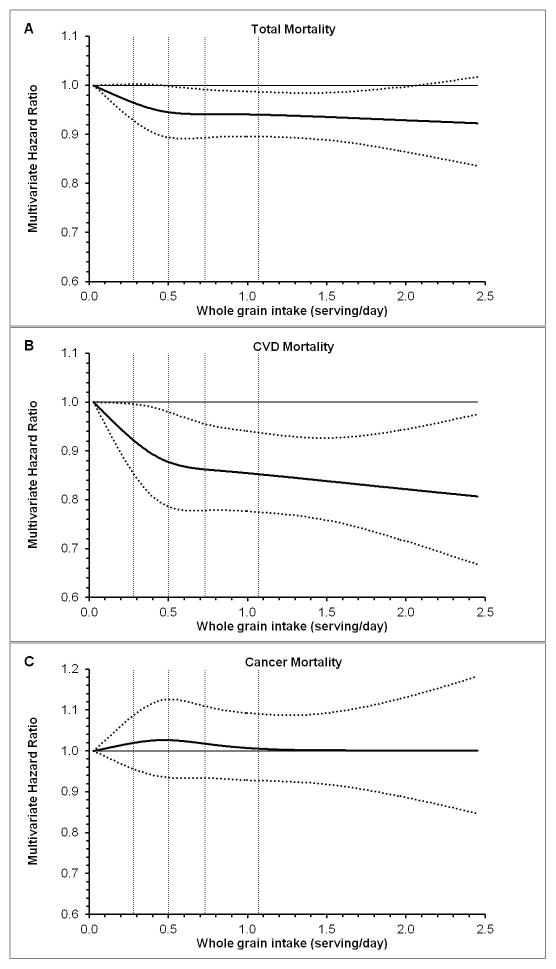
We further estimated that every serving (28 g)/day of whole grain consumption was associated with 5% (95%CI 2%–7%) lower total morality or 9% (95%CI 4%, 13%) lower CVD mortality. Consistently, we observed a monotonic relationships between whole grain consumption and total mortality (P for linearity=0.047), and CVD mortality (P for linearity=0.003), and no evidence of non-linearity was observed (Figure 1).
Figure 1. Dose-response Relationships between Whole Grain Consumption and Total and Cause-specific Mortality.
Multivariate hazard ratios are calculated in combined dataset of the Nurses’ Health Study and the Health Professionals Follow-up Study, using restricted cubic spline regression with adjustment for gender, age (continuous), ethnicity (Caucasian, Asian, African American and Hispanic/others), BMI (<18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥ 35.0 kg/m2), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥ 25 cigarettes/d), pack years smoked (0, 1–9,10–24, 25–44, >=45 pack-years), years since quitting for past smoker (not past smoker, years since smoking quitting <2, 3–5, 6–9 and ≥ 10years), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥ 30.0 g/d for men; 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥ 15.0 g/d for women), physical activity (quintiles), family history of diabetes, cancer and heart disease (yes, no), multivitamin use (yes, no), aspirin use at least once per week (yes, no), history of hypertension, high cholesterol, or diabetes at baseline, total energy (kcal/day, in quintiles), and modified alternative healthy eating index (in quintiles), which did not include whole grain. Solid lines represent point estimates and dashed lines are 95% confidence intervals. Vertical dashed lines represent cut-off points for quintiles of whole grain intake. A, total mortality; B, CVD mortality; C. cancer mortality.
By using the same model adjustment, we further observed that each serving (28 g)/day consumption of refined grains was associated with a small reduction in total mortality (pooled HR=0.98, 95%CI 0.97, 0.99), by not with CVD mortality (pooled HR=0.99, 95%CI 0.97, 1.01) or cancer mortality (pooled HR=0.98, 95%CI 0.97, 1.00). In the substitution analyses, replacing 1 serving of refined grains or total red meat with 1 serving of whole grains daily was associated with lower CVD mortality: 8% (pooled HR, 0.92; 95% CI, 0.88, 0.97) for replacing refined grains, and 20% (pooled HR, 0.80; 95% CI, 0.75, 0.86) for replacing red meat (Figure 2). The corresponding substitution estimates were 4% and 10% for total mortality, respectively. Replacement of potato was not significantly associated with total or CVD mortality. Meanwhile, no significant associations were found for cancer mortality in substitution analyses (Figure S1). The associations between whole grain intake and CVD mortality largely persisted among participants with various risk profiles defined by age, BMI, physical activity, smoking status, and aHEI score (Table 3, all Pinteraction ≥ 0.21). In addition, whole grain without added bran or germ was also associated with lower total and CVD mortality to a similar extent (Table S4).
Table 3.
Pooled Hazard Ratio (95%CI) of CVD Mortality according to Quintiles of Whole Grain Intake by Various Characteristics of Participants.*
| Quintiles of Whole Grain Intake
|
Ptrend | Pinteraction† | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Stratified by age | 0.37 | ||||||
| Age< 65 years | |||||||
| No. of cases | 320 | 216 | 201 | 146 | 150 | ||
| Person-years | 343,367 | 348,369 | 340,124 | 325,628 | 299,984 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.94 (0.78–1.12) | 1.01 (0.84–1.21) | 0.83 (0.68–1.02) | 0.88 (0.71–1.09) | 0.13 | |
| Age≥65 years | |||||||
| No. of cases | 1513 | 1102 | 998 | 934 | 1030 | ||
| Person-years | 202,282 | 200,795 | 209,443 | 225,321 | 250,015 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.96 (0.88–1.04) | 0.93 (0.86–1.01) | 0.88 (0.81–0.96) | 0.84 (0.78–0.92) | < 0.001 | |
| Stratified by BMI | 0.48 | ||||||
| BMI<25 kg/m2 | |||||||
| No. of cases | 1121 | 804 | 704 | 686 | 764 | ||
| Person-years | 263,214 | 256,847 | 261,339 | 278,390 | 316,187 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.97 (0.88–1.06) | 0.97 (0.87–1.08) | 0.92 (0.83–1.02) | 0.87 (0.79–0.96) | < 0.001 | |
| 25 ≤ BMI<30 kg/m2 | |||||||
| No. of cases | 408 | 317 | 323 | 250 | 288 | ||
| Person-years | 180,616 | 192,918 | 197,649 | 192,915 | 171,614 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.93 (0.80–1.08) | 0.96 (0.82–1.12) | 0.82 (0.72–0.95) | 0.81 (0.69–0.96) | 0.06 | |
| BMI ≥ 30 kg/m2 | |||||||
| No. of cases | 304 | 197 | 172 | 144 | 128 | ||
| Person-years | 101,820 | 99,400 | 90,578 | 79,643 | 62,198 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.85 (0.70–1.04) | 0.79 (0.67–0.92) | 0.99 (0.82–1.19) | 0.85 (0.68–1.06) | 0.11 | |
| Stratified by aHEI | 0.73 | ||||||
| aHEI ≥ median level | |||||||
| No. of cases | 624 | 536 | 562 | 553 | 742 | ||
| Person-years | 209,786 | 265,049 | 302,545 | 336,987 | 390,075 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.97 (0.88–1.06) | 0.95 (0.86–1.05) | 0.91 (0.81–1.01) | 0.86 (0.77–0.97) | 0.007 | |
| aHEI<median level | |||||||
| No. of cases | 1209 | 781 | 638 | 528 | 437 | ||
| Person-years | 335,560 | 283,811 | 246,682 | 213,584 | 159,723 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.94 (0.84–1.06) | 0.94 (0.83–1.06) | 0.85 (0.75–0.95) | 0.85 (0.76–0.95) | 0.002 | |
| Stratified by physical activity | 0.21 | ||||||
| Physical activity ≥ median level | |||||||
| No. of cases | 652 | 542 | 543 | 482 | 559 | ||
| Person-years | 290,024 | 328,705 | 351,504 | 368,725 | 383,318 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.93 (0.85–1.02) | 0.92 (0.84–1.02) | 0.90 (0.82–1.00) | 0.85 (0.77–0.95) | 0.006 | |
| Physical activity<median level | |||||||
| No. of cases | 1181 | 775 | 657 | 599 | 620 | ||
| Person-years | 255,324 | 220,155 | 197,721 | 181,847 | 166,480 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.96 (0.85–1.08) | 0.95 (0.84–1.06) | 0.83 (0.73–0.93) | 0.84 (0.74–0.95) | 0.002 | |
| Stratified by smoking status | 0.35 | ||||||
| Never | |||||||
| No. of cases | 587 | 492 | 494 | 449 | 553 | ||
| Person-years | 217,996 | 242,354 | 260,296 | 276,409 | 290,455 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 1.03 (0.91–1.16) | 1.03 (0.91–1.17) | 0.90 (0.79–1.02) | 0.90 (0.80–1.02) | 0.03 | |
| Ever | |||||||
| No. of cases | 1246 | 825 | 706 | 632 | 626 | ||
| Person-years | 327,351 | 306,506 | 288,930 | 274,163 | 259,344 | ||
| Multivariate-adjusted hazard ratio (95% CI) | 1.0 | 0.90 (0.82–0.98) | 0.89 (0.81–0.98) | 0.86 (0.78–0.96) | 0.81 (0.74–0.90) | < 0.001 | |
Abbreviations: CI, confidence interval; BMI, body mass index; aHEI, alternative health eating index
Models were adjusted for age (years), ethnicity (Caucasian, Asian, African American and Hispanic/others), BMI (<18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0 kg/m2), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥25 cigarettes/d), pack years smoked (0, 1–10,10–24, 25–44, ≥45 pack-years), years since quitting for past smoker (not past smoker, years since smoking quitting <2, 3–5, 6–9 and ≥10years), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥30.0 g/d for men; 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥15.0 g/d for women), physical activity (quintiles), family history of diabetes, cancer and heart disease(yes, no), multivitamin use (yes, no), aspirin use at least once per week (yes, no), history of hypertension, high cholesterol, or diabetes at baseline, total energy (kcal/day, in quintiles) and the modified aHEI which did not include whole grain in (quintiles). BMI (kg/m2), physical activity (METs-hr/week), past smoking history/number of cigarettes currently smoked per day, and aHEI score were further adjusted for in analyses that were stratified by these variables. For women, postmenopausal status and postmenopausal hormone use were further adjusted for.
Pinteraction was calculated using likelihood-ratio test.
In several sensitivity analyses we updated participants’ diet throughout follow-up regardless of disease occurrence and applied a 4-year lagged between exposure and the occurrence of deaths; further stopped updating diet after intermediate (hypertension and hypercholesterolemia) diagnosis; or adjusted for incidence of intermediate outcomes; or used baseline whole grain intakes which did not account for different trends of whole grain intakes over time; or used a multiple imputation procedure with 5 rounds of imputation to impute missing data of exposures and covariates and repeated the analyses. The association between whole grain intake and mortality did not change materially in these analyses (Table S5).
The age-adjusted correlation coefficient for association between intake of whole grain and total bran was 0.87 for women and 0.85 for men; and these figures were both 0.79 for association of whole grain and total germ in men and women. Total bran consumption was significantly associated with lower total and CVD mortality. The pooled HRs (95% CIs) comparing extreme quintiles of total bran intake were 0.80 (0.73–0.87, Ptrend<0.001) for CVD mortality (Table 4), and 0.94 (0.90–0.99, Ptrend=0.02) for total mortality (Table S6) in multivariate adjustment models. Added bran had similar benefits as naturally-occurring bran (Table S7). We did not find a significant association between total germ intake and risk of mortality after further adjustment for total bran intake.
Table 4.
Hazard Ratio (95%CI) of CVD Mortality by Quintiles of Total Bran and Germ Intakes.
| Quintiles of Total Bran or Germ Intake (g/d)
|
Ptrend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Total bran | ||||||
| NHS | ||||||
| Median | 0.7 | 2.0 | 3.5 | 5.7 | 10.4 | |
| No. of cases | 895 | 557 | 535 | 466 | 536 | |
| Person-years | 360,229 | 360,479 | 362,651 | 362,983 | 362,612 | |
| Model 1* | 1.0 | 0.65 (0.58–0.73) | 0.61 (0.54–0.68) | 0.46 (0.41–0.53) | 0.44 (0.39–0.50) | < 0.001 |
| Model 2† | 1.0 | 0.88 (0.79–0.99) | 0.94 (0.83–1.06) | 0.80 (0.70–0.91) | 0.79 (0.69–0.90) | < 0.001 |
| Model 3‡ | 1.0 | 0.89 (0.79–0.99) | 0.94 (0.83–1.07) | 0.80 (0.70–0.92) | 0.80 (0.70–0.91) | 0.001 |
| HPFS | ||||||
| Median | 0.7 | 2.6 | 5.0 | 8.2 | 15.0 | |
| No. of cases | 930 | 691 | 653 | 660 | 687 | |
| Person-years | 185,617 | 186,559 | 188,474 | 187,981 | 187,744 | |
| Model 1* | 1.0 | 0.80 (0.71–0.88) | 0.73 (0.65–0.82) | 0.64 (0.57–0.72) | 0.56 (0.50–0.63) | < 0.001 |
| Model 2† | 1.0 | 0.93 (0.84–1.04) | 0.93 (0.83–1.05) | 0.86 (0.77–0.97) | 0.79 (0.70–0.89) | < 0.001 |
| Model 3‡ | 1.0 | 0.93 (0.84–1.04) | 0.93 (0.83–1.05) | 0.86 (0.77–0.97) | 0.80 (0.71–0.90) | < 0.001 |
| Pooled§ | ||||||
| Model 1* | 1.0 | 0.72 (0.67–0.78) | 0.67 (0.62–0.73) | 0.56 (0.51–0.61) | 0.50 (0.46–0.55) | < 0.001 |
| Model 2† | 1.0 | 0.91 (0.84–0.98) | 0.93 (0.86–1.02) | 0.83 (0.76–0.91) | 0.79 (0.72–0.87) | < 0.001 |
| Model 3‡ | 1.0 | 0.91 (0.84–0.98) | 0.94 (0.86–1.02) | 0.84 (0.77–0.91) | 0.80 (0.73–0.87) | < 0.001 |
| Total germ | ||||||
| NHS | ||||||
| Median | 0.2 | 0.4 | 0.6 | 0.9 | 1.6 | |
| No. of cases | 1419 | 1176 | 1150 | 1090 | 1129 | |
| Person-years | 359,732 | 359,899 | 362,047 | 362,371 | 362,066 | |
| Model 1* | 1.0 | 0.94 (0.84–1.05) | 0.86 (0.76–0.98) | 0.86 (0.76–0.99) | 0.96 (0.84–1.09) | 0.02 |
| Model 2† | 1.0 | 1.06 (0.95–1.19) | 1.00 (0.88–1.14) | 1.03 (0.91–1.18) | 1.09 (0.95–1.24) | 0.49 |
| Model 3‡ | 1.0 | 1.07 (0.95–1.20) | 1.01 (0.89–1.15) | 1.04 (0.91–1.19) | 1.11 (0.97–1.27) | 0.30 |
| HPFS | ||||||
| Median | 0.2 | 0.6 | 0.9 | 1.3 | 2.3 | |
| No. of cases | 908 | 763 | 662 | 795 | 793 | |
| Person-years | 185,631 | 186,437 | 188,459 | 187,880 | 187,637 | |
| Model 1* | 1.0 | 0.91 (0.82–1.01) | 0.81 (0.72–0.90) | 0.89 (0.79–1.00) | 0.93 (0.82–1.04) | 0.08 |
| Model 2† | 1.0 | 1.03 (0.93–1.14) | 0.93 (0.83–1.05) | 1.02 (0.91–1.15) | 1.02 (0.90–1.14) | 0.93 |
| Model 3‡ | 1.0 | 1.03 (0.93–1.14) | 0.94 (0.84–1.05) | 1.03 (0.92–1.16) | 1.03 (0.92–1.16) | 0.71 |
| Pooled§ | ||||||
| Model 1* | 1.0 | 0.93 (0.86–1.00) | 0.83 (0.76–0.90) | 0.88 (0.80–0.96) | 0.94 (0.86–1.02) | 0.008 |
| Model 2† | 1.0 | 1.04 (0.97–1.13) | 0.96 (0.89–1.05) | 1.03 (0.94–1.12) | 1.05 (0.96–1.14) | 0.66 |
| Model 3‡ | 1.0 | 1.05 (0.97–1.13) | 0.97 (0.89–1.06) | 1.04 (0.95–1.13) | 1.07 (0.98–1.17) | 0.39 |
Abbreviations: CI, confidence interval; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study.
Total bran and total germ were mutually adjusted in all models.
Age-adjusted.
Further adjusted for ethnicity (Caucasian, Asian, African American and Hispanic/others), BMI (<18.5, 18.5–22.9, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥ 35.0 kg/m2), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes/d, 15–24 cigarettes/d, or ≥ 25 cigarettes/d), pack years smoked (0, 1–9,10–24, 25–44, ≥ 45 pack-years), years since quitting for past smoker (not past smoker, years since smoking quitting <2, 3–5, 6–9 and ≥ 10years), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥ 30.0 g/d for men; 0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥ 15.0 g/d for women), physical activity (quintiles), family history of diabetes, cancer and heart disease (yes, no), multivitamin use (yes, no), aspirin use at least once per week (yes, no), history of hypertension, high cholesterol, or diabetes at baseline, total energy (kcal/day, in quintiles). For women, postmenopausal status and postmenopausal hormone use were further adjusted for.
Further adjusted for modified alternative healthy eating index (in quintiles), which did not include whole grains.
Pooled hazard ratios were calculated using a fixed-effects model.
DISCUSSION
In these two cohorts of U.S. men and women, we found that a higher whole grain intake, with or without added bran or germ, was associated with a reduced mortality, especially deaths due to CVD. These associations were independent of demographic and lifestyle predictors of mortality, as well as the overall dietary quality, and largely persisted among participants with various risk profiles.
The associations between whole grain foods and total and cause-specific mortality have been examined in several prior investigations. In the first investigation among the Iowa Women’s Health Study, Jacobs et al. found that baseline whole grain foods (dark bread, whole grain breakfast cereal, popcorn, and other foods that contain various whole grain contents) were significantly associated with a lower CHD mortality, which was primarily ascribed to dark bread.16 In updated analyses in the same cohort with extended follow-up, total servings of whole grain foods were significantly associated with a lower all-cause and CVD mortality, but not cancer mortality.15 In the ARIC study, total whole grain food consumption was significantly associated with a reduced all-cause mortality, but associations with cause-specific mortality were not examined.17 In a Norwegian cohort, dark bread was associated with lower total and various cause-specific mortality with similar strength.18 In contrast, in studies conducted among a healthy elderly population19 or diabetes patients, 20 whole grain intakes were significantly associated with a lower CVD mortality only. Overall, results regarding lower CVD mortality are concordant with numerous previous studies showing inverse associations between whole grain intake and risk of diabetes,11–13 hypertension,17,35,36 and CVD.12–14 Several clinic trials also demonstrated beneficial effects of whole grain intake on CVD risk factors, such as lipid profiles,37,38 blood pressure,39,40 insulin sensitivity and glucose metabolism.5,21,39
In contrast, associations between whole grain intakes and cancer mortality remain inconclusive. Consistent with our results, several previous investigations have reported an inverse association between whole grain intake and cancer mortality that was much attenuated after further adjustment for other healthful lifestyle and dietary factors correlated with whole grain intake.15,16,18 Associations between whole grain intake and cancer incidence may depend on the population characteristics and vary by specific types of cancer. For example, a recent meta-analysis of 25 prospective studies demonstrated that whole grain intake was associated with a reduced risk of colorectal cancer,41 whereas other studies did not find significant associations with endometrial cancer,42,43 ovarian cancer,44 breast cancer,45,46 or prostate cancer.47 Consistent with the evidence of incident cancer, we observed that whole grain consumption was associated with lower mortality due to colorectal cancer in men, but no significant associations with mortality due to lung cancer, prostate cancer, or breast cancer were found. Future studies with larger sample size of various cancer caused death are needed to replicate our observation.
An interesting finding of our study is that intakes of bran but not germ were significantly associated with a reduced CVD mortality. Consistently, results from previous analyses in the NHS and HPFS also suggested that the bran component, but not germ, was significantly associated with reduced risk of diabetes,11 hypertension,36 CHD,25 or CVD mortality among those with diabetes,20 after mutual adjustments of bran and germ. These lines of evidence suggest that the association for germ may not be independent of that of bran. Another possible explanation for null associations for germ may be that absolute intake level for germ in our study is rather low. The observed significant associations for bran are in line with proposed mechanisms that attribute the benefits of whole grains primarily to nutrients and phytochemicals existing in the bran portion.25 Bran is a rich source of fiber, B-group vitamins, vitamin E, magnesium and phytochemicals, which may potentially explain whole grains’ favorable effects.48 For instance, fiber, primarily found in the bran, may reduce the risk of certain chronic diseases, in particular CVD, metabolic syndrome, diabetes, and certain cancers.49,50 Antioxidant phytochemicals found in wheat bran fractions, such as phenolic acids and alkylresorcinols may modulate cellular oxidative status and prevent biologically important molecules such as DNA, proteins, and membrane lipids from oxidative damage.51 In addition, magnesium has potentially favorable effects on insulin sensitivity and diabetes risk,3 blood pressure,52 and cardiovascular health.53
The strengths of our study include a large sample size, a high follow-up rate, long duration of follow-up, repeated assessments of diet, multivariate adjustment, and assessments of whole grain contents from various food sources. In addition, all participants were health professionals, which may help minimize potential confounding by educational attainment or socioeconomic status. There are also several limitations of our study. First, although we carefully adjusted for multiple dietary and lifestyle factors, residual or unmeasured confounding might still exist and may thus hinder causal inference based on these observations. Second, measurement errors in whole grain intake and other dietary factors are inevitable, although the FFQs used in our cohorts have been validated against diet records with reasonable reproducibility and validity.22,23 Because of the prospective study design, misclassification of whole grain intake was unlikely to be correlated with study outcome ascertainment and therefore more likely to attenuate true associations toward the null. Moreover, we calculated cumulative averages for dietary intakes to reduce random measurement errors and represent long-term dietary habits.32 On the other hand, despite that we calculated whole grain intake from all relevant foods in comparison to previous studies that focused on whole grain foods only, the inverse association with CVD mortality was consistently observed in the current and previous investigations, suggesting that the associations are largely robust to various degrees of measurement errors. Finally, the participants included in our study were predominantly middle-aged and older health professions with European ancestry, and it is unknown whether our findings could be generalized to other demographic or ethnic groups.
In summary, our data from two large prospective cohort studies consistently showed significant inverse associations of whole grain intake and mortality, especially CVD mortality. In addition, bran portion of the whole grain foods, as well as bran added to foods, was significantly associated with a lower CVD mortality. These findings further support current dietary guidelines that recommend increasing whole grain consumption to facilitate primary and secondary prevention of chronic diseases, and also provide promising evidence suggesting diet enriched with whole grains may confer benefits toward extended life expectancy.
Supplementary Material
Acknowledgments
This work was supported by the research grant, DK58845, P01 CA87969, R01 HL034594, UM1 CA167552, R01 HL35464, HL60712, U54CA155626, and CA055075 from National Institutes of Health, a career development award R00HL098459 from the National Heart, Lung, and Blood Institute (to Q.S.). We would like to thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-Up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
The authors’ responsibilities were as follows — HW: analyzed the data and drafted the manuscript. AJF: conduct technical review for the manuscript. FBH and QS: designed the study and supervised data analysis. All authors: contributed to the interpretation of results and the critical revision of the manuscript.
DISCLOSURE: None
References
- 1.European Food Information Council (EUFIC) [accessed November 2013];Whole grain fact sheet. http://www.eufic.org/article/en/page/BARCHIVE/expid/Whole-grain-Fact-Sheet.
- 2.The Department of Agriculture (USDA) and the Department of Health and Human Services (HHS) [accessed November 2013];Dietary Guidelines for Americans. http://www.choosemyplate.gov/dietary-guidelines.html.
- 3.McCarty MF. Magnesium may mediate the favorable impact of whole grains on insulin sensitivity by acting as a mild calcium antagonist. Med Hypotheses. 2005;64(3):619–627. doi: 10.1016/j.mehy.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Juntunen KS, Niskanen LK, Liukkonen KH, et al. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr. 2002;75(2):254–262. doi: 10.1093/ajcn/75.2.254. [DOI] [PubMed] [Google Scholar]
- 5.Pereira MA, Jacobs DR, Pins JJ, et al. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75(5):848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJA, Kendall CWC, Axelsen M, Augustin LSA, Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol. 2000;11(1):49–56. doi: 10.1097/00041433-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Katz DL, Nawaz H, Boukhalil J, et al. Effects of Oat and Wheat Cereals on Endothelial Responses. Preventive Medicine. 2001;33(5):476–484. doi: 10.1006/pmed.2001.0918. [DOI] [PubMed] [Google Scholar]
- 8.Adom KK, Liu RH. Antioxidant Activity of Grains. J Agric Food Chem. 2002;50(21):6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 9.Adlercreutz H. Lignans and Human Health. Crit Rev Clin Lab Sci. 2007;44(5–6):483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol. 2007;18(1):3–8. doi: 10.1097/MOL.0b013e328011c6e0. [DOI] [PubMed] [Google Scholar]
- 11.de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole Grain, Bran, and Germ Intake and Risk of Type 2 Diabetes: A Prospective Cohort Study and Systematic Review. PLoS Med. 2007;4(8):e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes, Cardiovascular Disease, and Weight Gain. J Nutr. 2012;142(7):1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SS, Qi L, Fahey GC, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98(2):594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 14.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18(4):283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs DR, Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr. 2007 Jun;85(6):1606–1614. doi: 10.1093/ajcn/85.6.1606. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs DR, Jr, Meyer KA, Kushi LH, Folsom AR. Is whole grain intake associated with reduced total and cause-specific death rates in older women? The Iowa Women’s Health Study. Am J Public Health. 1999 Mar;89(3):322–329. doi: 10.2105/ajph.89.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steffen LM, Jacobs DR, Stevens J, et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78(3):383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs DR, Jr, Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the Norwegian County Study. Eur J Clin Nutr. 2001 Feb;55(2):137–143. doi: 10.1038/sj.ejcn.1601133. [DOI] [PubMed] [Google Scholar]
- 19.Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr. 2006;83(1):124–131. doi: 10.1093/ajcn/83.1.124. [DOI] [PubMed] [Google Scholar]
- 20.He M, van Dam RM, Rimm E, Hu FB, Qi L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation. 2010;121(20):2162–2168. doi: 10.1161/CIRCULATIONAHA.109.907360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs DR, Jr, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr. 1998;68(2):248–257. doi: 10.1093/ajcn/68.2.248. [DOI] [PubMed] [Google Scholar]
- 22.Salvini S, Hunter DJ, Sampson L, et al. Food-Based Validation of a Dietary Questionnaire: The Effects of Week-to-Week Variation in Food Consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 23.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 24.Koh-Banerjee P, Franz M, Sampson L, et al. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr. 2004;80(5):1237–1245. doi: 10.1093/ajcn/80.5.1237. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MK, Koh-Banerjee P, Hu FB, et al. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am J Clin Nutr. 2004;80(6):1492–1499. doi: 10.1093/ajcn/80.6.1492. [DOI] [PubMed] [Google Scholar]
- 26.Franz M, Sampson L. Challenges in developing a whole grain database: Definitions, methods and quantification. Journal of Food Composition and Analysis. 2006;19(Supplement 0):S38–S44. [Google Scholar]
- 27.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 30.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Chiuve SE, Fung TT, Rimm EB, et al. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu FB, Stampfer MJ, Rimm E, et al. Dietary Fat and Coronary Heart Disease: A Comparison of Approaches for Adjusting for Total Energy Intake and Modeling Repeated Dietary Measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Spiegelman D, van Dam RM, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170(11):961–969. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83(2):284–290. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Gaziano JM, Liu S, et al. Whole- and refined-grain intakes and the risk of hypertension in women. Am J Clin Nutr. 2007;86(2):472–479. doi: 10.1093/ajcn/86.2.472. [DOI] [PubMed] [Google Scholar]
- 36.Flint AJ, Hu FB, Glynn RJ, et al. Whole grains and incident hypertension in men. Am J Clin Nutr. 2009;90(3):493–498. doi: 10.3945/ajcn.2009.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leinonen KS, Poutanen KS, Mykkänen HM. Rye Bread Decreases Serum Total and LDL Cholesterol in Men with Moderately Elevated Serum Cholesterol. J Nutr. 2000;130(2):164–170. doi: 10.1093/jn/130.2.164. [DOI] [PubMed] [Google Scholar]
- 38.Maki KC, Beiseigel JM, Jonnalagadda SS, et al. Whole-Grain Ready-to-Eat Oat Cereal, as Part of a Dietary Program for Weight Loss, Reduces Low-Density Lipoprotein Cholesterol in Adults with Overweight and Obesity More than a Dietary Program Including Low-Fiber Control Foods. Journal of the American Dietetic Association. 2010;110(2):205–214. doi: 10.1016/j.jada.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 39.Tighe P, Duthie G, Vaughan N, et al. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr. 2010;92(4):733–740. doi: 10.3945/ajcn.2010.29417. [DOI] [PubMed] [Google Scholar]
- 40.Saltzman E, Das SK, Lichtenstein AH, et al. An Oat-Containing Hypocaloric Diet Reduces Systolic Blood Pressure and Improves Lipid Profile beyond Effects of Weight Loss in Men and Women. J Nutr. 2001;131(5):1465–1470. doi: 10.1093/jn/131.5.1465. [DOI] [PubMed] [Google Scholar]
- 41.Aune D, Chan DSM, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343 doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aarestrup J, Kyrø C, Christensen J, et al. Whole Grain, Dietary Fiber, and Incidence of Endometrial Cancer in a Danish Cohort Study. Nutrition and Cancer. 2012;64(8):1160–1168. doi: 10.1080/01635581.2012.723786. [DOI] [PubMed] [Google Scholar]
- 43.Kasum CM, Nicodemus K, Harnack LJ, Jacobs DR, Folsom AR. Whole Grain Intake and Incident Endometrial Cancer: The Iowa Women’s Health Study. Nutrition and Cancer. 2001;39(2):180–186. doi: 10.1207/S15327914nc392_4. [DOI] [PubMed] [Google Scholar]
- 44.Hedelin M, Löf M, Andersson TM-L, Adlercreutz H, Weiderpass E. Dietary Phytoestrogens and the Risk of Ovarian Cancer in the Women’s Lifestyle and Health Cohort Study. Cancer Epidemiology Biomarkers & Prevention. 2011;20(2):308–317. doi: 10.1158/1055-9965.EPI-10-0752. [DOI] [PubMed] [Google Scholar]
- 45.Nicodemus K, Jacobs D, Jr, Folsom A. Whole and refined grain intake and risk of incident postmenopausal breast cancer (United States) Cancer Causes & Control. 2001;12(10):917–925. doi: 10.1023/a:1013746719385. [DOI] [PubMed] [Google Scholar]
- 46.Egeberg R, Olsen A, Loft S, et al. Intake of whole grain products and risk of breast cancer by hormone receptor status and histology among postmenopausal women. International Journal of Cancer. 2009;124(3):745–750. doi: 10.1002/ijc.23992. [DOI] [PubMed] [Google Scholar]
- 47.Egeberg R, Olsen A, Christensen J, et al. Intake of whole-grain products and risk of prostate cancer among men in the Danish Diet, Cancer and Health cohort study. Cancer Causes & Control. 2011;22(8):1133–1139. doi: 10.1007/s10552-011-9789-5. [DOI] [PubMed] [Google Scholar]
- 48.Slavin J. Why whole grains are protective: biological mechanisms. Proceedings of the Nutrition Society. 2003;62(01):129–134. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 49.Alan P-A, Ofelia R-Sn, Patricia T, Rosario Maribel R-Sn. Cereal bran and wholegrain as a source of dietary fibre: technological and health aspects. Int J Food Sci Nutr. 2013;63(7):882–892. doi: 10.3109/09637486.2012.676030. [DOI] [PubMed] [Google Scholar]
- 50.Lattimer JM, Haub MD. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients. 2010;2(12):1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou K, Su L, Yu L. Phytochemicals and Antioxidant Properties in Wheat Bran. J Agric Food Chem. 2004;52(20):6108–6114. doi: 10.1021/jf049214g. [DOI] [PubMed] [Google Scholar]
- 52.Kawano Y, Matsuoka H, Takishita S, Omae T. Effects of Magnesium Supplementation in Hypertensive Patients: Assessment by Office, Home, and Ambulatory Blood Pressures. Hypertension. 1998;32(2):260–265. doi: 10.1161/01.hyp.32.2.260. [DOI] [PubMed] [Google Scholar]
- 53.Shechter M, Merz CNB, Paul-Labrador M, et al. Oral magnesium supplementation inhibits platelet-dependent thrombosis in patients with coronary artery disease. Am J Cardiol. 1999;84(2):152–156. doi: 10.1016/s0002-9149(99)00225-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.