Abstract
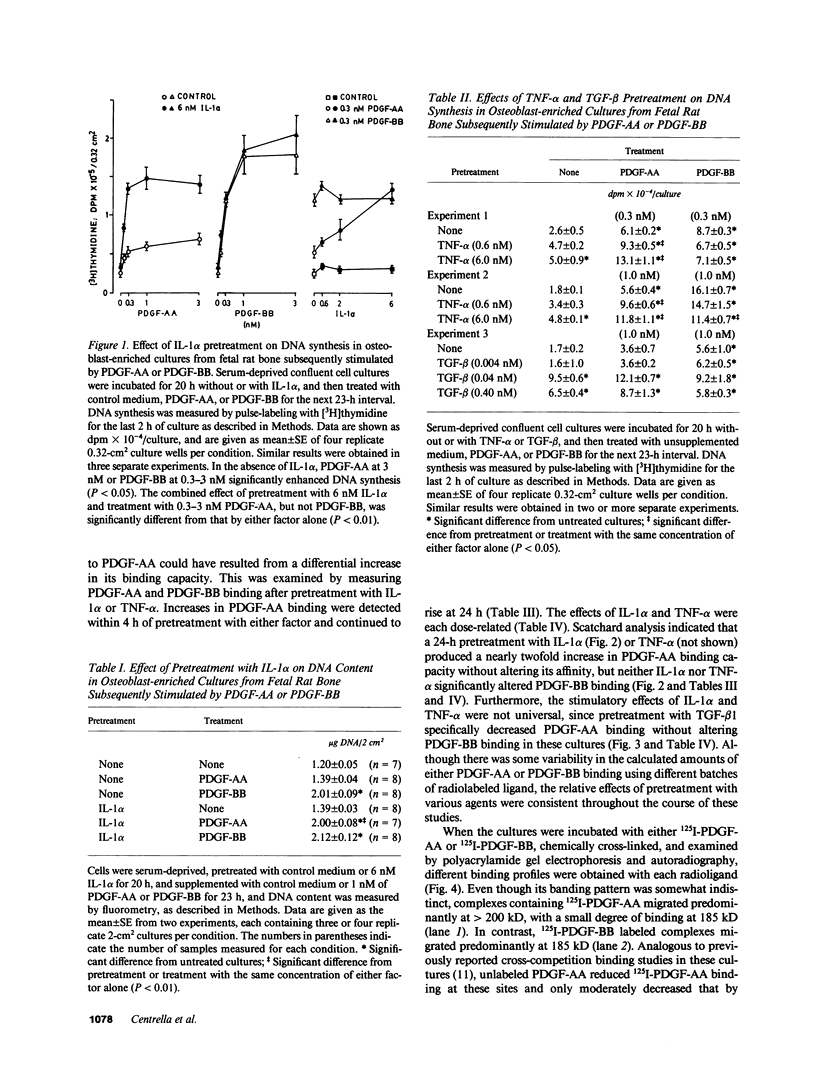
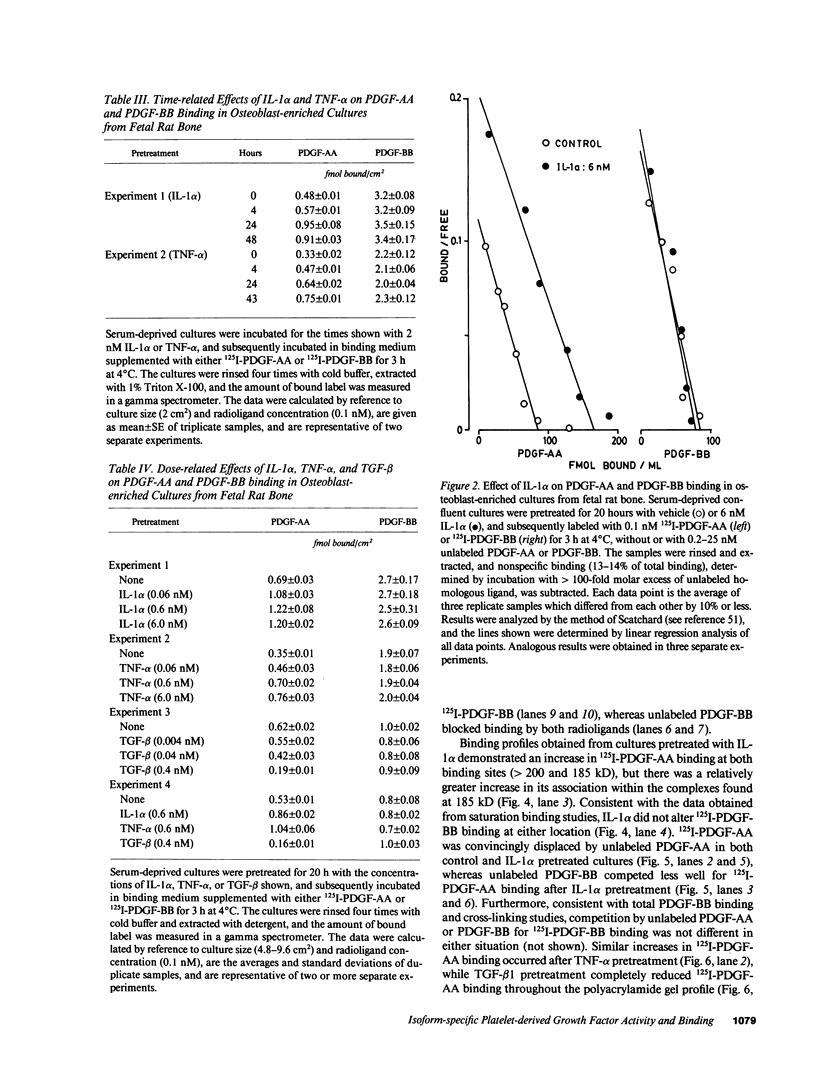
In osteoblast-enriched cultures from fetal rat bone, the A-chain homodimer of platelet-derived growth factor (PDGF-AA) is less potent than the PDGF isoforms containing B chain subunits (PDGF-AB and PDGF-BB), but normal osteoblasts appear to synthesize only PDGF-A subunit mRNA and polypeptide. However, other agents may regulate PDGF-AA activity in skeletal tissue. Pretreatment of osteoblast-enriched cultures with interleukin 1 alpha (IL-1 alpha) or tumor necrosis factor-alpha (TNF-alpha) synergistically enhanced the mitogenic effect of PDGF-AA coincident with increased binding site occupancy, but neither factor augmented PDGF-BB activity or binding. Polyacrylamide gel analysis showed 125I-PDGF-AA binding complexes predominantly at greater than 200 kD and faint labeling at 185 kD. After IL-1 alpha or TNF-alpha pretreatment, PDGF-AA binding increased at both sites, but this effect was more striking at 185 kD, which co-migrated with 125I-PDGF-BB-labeled complexes. PDGF-AA binding sites were rapidly lost by comparison to those for PDGF-BB in cycloheximide-treated cultures, but they remained relatively enhanced by IL-1 alpha and TNF-alpha pretreatment. These studies indicate that IL-alpha and TNF-alpha increase PDGF-AA binding and activity for osteoblasts by mechanisms that are at least in part independent of new receptor synthesis, and suggest regulatory events that could control how PDGF binding sites specifically recognize different ligands.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Canalis E. Effects of tumor necrosis factor on bone formation in vitro. Endocrinology. 1987 Nov;121(5):1596–1604. doi: 10.1210/endo-121-5-1596. [DOI] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol Cell Biol. 1991 Sep;11(9):4490–4496. doi: 10.1128/mcb.11.9.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Mitogenesis in fetal rat bone cells simultaneously exposed to type beta transforming growth factor and other growth regulators. FASEB J. 1987 Oct;1(4):312–317. doi: 10.1096/fasebj.1.4.3498658. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Platelet-derived growth factor enhances deoxyribonucleic acid and collagen synthesis in osteoblast-enriched cultures from fetal rat parietal bone. Endocrinology. 1989 Jul;125(1):13–19. doi: 10.1210/endo-125-1-13. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987 Feb 25;262(6):2869–2874. [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Canalis E. Tumor necrosis factor-alpha inhibits collagen synthesis and alkaline phosphatase activity independently of its effect on deoxyribonucleic acid synthesis in osteoblast-enriched bone cell cultures. Endocrinology. 1988 Sep;123(3):1442–1448. doi: 10.1210/endo-123-3-1442. [DOI] [PubMed] [Google Scholar]
- Centrella M., McCarthy T. L., Kusmik W. F., Canalis E. Relative binding and biochemical effects of heterodimeric and homodimeric isoforms of platelet-derived growth factor in osteoblast-enriched cultures from fetal rat bone. J Cell Physiol. 1991 Jun;147(3):420–426. doi: 10.1002/jcp.1041470306. [DOI] [PubMed] [Google Scholar]
- Chiou W. J., Bonin P. D., Harris P. K., Carter D. B., Singh J. P. Platelet-derived growth factor induces interleukin-1 receptor gene expression in Balb/c 3T3 fibroblasts. J Biol Chem. 1989 Dec 25;264(36):21442–21445. [PubMed] [Google Scholar]
- Circolo A., Pierce G. F., Katz Y., Strunk R. C. Antiinflammatory effects of polypeptide growth factors. Platelet-derived growth factor, epidermal growth factor, and fibroblast growth factor inhibit the cytokine-induced expression of the alternative complement pathway activator factor B in human fibroblasts. J Biol Chem. 1990 Mar 25;265(9):5066–5071. [PubMed] [Google Scholar]
- Claesson-Welsh L., Hammacher A., Westermark B., Heldin C. H., Nistér M. Identification and structural analysis of the A type receptor for platelet-derived growth factor. Similarities with the B type receptor. J Biol Chem. 1989 Jan 25;264(3):1742–1747. [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Hart C. E., Murray M. J., McPherson J. M. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989 Sep;84(3):1036–1040. doi: 10.1172/JCI114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Savage N. Interleukin-1 and its receptor. Crit Rev Immunol. 1989;9(1):1–20. [PubMed] [Google Scholar]
- Escobedo J. A., Navankasatussas S., Cousens L. S., Coughlin S. R., Bell G. I., Williams L. T. A common PDGF receptor is activated by homodimeric A and B forms of PDGF. Science. 1988 Jun 10;240(4858):1532–1534. doi: 10.1126/science.2836953. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Valentin-Opran A., Delgado R., Valente A. J., Mundy G., Piche J. The potential role of platelet-derived growth factor as an autocrine or paracrine factor for human bone cells. Connect Tissue Res. 1989;23(2-3):209–218. doi: 10.3109/03008208909002419. [DOI] [PubMed] [Google Scholar]
- Gronwald R. G., Seifert R. A., Bowen-Pope D. F. Differential regulation of expression of two platelet-derived growth factor receptor subunits by transforming growth factor-beta. J Biol Chem. 1989 May 15;264(14):8120–8125. [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factors: a family of isoforms that bind to two distinct receptors. Br Med Bull. 1989 Apr;45(2):453–464. doi: 10.1093/oxfordjournals.bmb.a072334. [DOI] [PubMed] [Google Scholar]
- Hock J. M., Canalis E., Centrella M. Transforming growth factor-beta stimulates bone matrix apposition and bone cell replication in cultured fetal rat calvariae. Endocrinology. 1990 Jan;126(1):421–426. doi: 10.1210/endo-126-1-421. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li W., Schlessinger J. Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Mol Cell Biol. 1991 Jul;11(7):3756–3761. doi: 10.1128/mcb.11.7.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomri A., Marie P. J. Bone cell responsiveness to transforming growth factor beta, parathyroid hormone, and prostaglandin E2 in normal and postmenopausal osteoporotic women. J Bone Miner Res. 1990 Nov;5(11):1149–1155. doi: 10.1002/jbmr.5650051110. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Centrella M. Interleukin-1 in combination with transforming growth factor-alpha produces enhanced bone resorption in vitro. Endocrinology. 1988 Nov;123(5):2194–2200. doi: 10.1210/endo-123-5-2194. [DOI] [PubMed] [Google Scholar]
- Marcelli C., Yates A. J., Mundy G. R. In vivo effects of human recombinant transforming growth factor beta on bone turnover in normal mice. J Bone Miner Res. 1990 Oct;5(10):1087–1096. doi: 10.1002/jbmr.5650051013. [DOI] [PubMed] [Google Scholar]
- Marusić A., Kalinowski J. F., Harrison J. R., Centrella M., Raisz L. G., Lorenzo J. A. Effects of transforming growth factor-beta and IL-1 alpha on prostaglandin synthesis in serum-deprived osteoblastic cells. J Immunol. 1991 Apr 15;146(8):2633–2638. [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Further biochemical and molecular characterization of primary rat parietal bone cell cultures. J Bone Miner Res. 1988 Aug;3(4):401–408. doi: 10.1002/jbmr.5650030406. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M. D., Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988 Jun 9;333(6173):560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Camilliere J. J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989 Jun;124(6):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Van Wyk J. J., Pledger W. J. Inhibition of the mitogenic effects of plasma by a monoclonal antibody to somatomedin C. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2389–2392. doi: 10.1073/pnas.81.8.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Smith D. D., Gowen M., Mundy G. R. Effects of interferon-gamma and other cytokines on collagen synthesis in fetal rat bone cultures. Endocrinology. 1987 Jun;120(6):2494–2499. doi: 10.1210/endo-120-6-2494. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Matsui T., Nakata H., Ito M., Natazuka T., Fukase M., Fujita T. Interleukin-1 enhances the response of osteoblasts to platelet-derived growth factor through the alpha receptor-specific up-regulation. J Biol Chem. 1991 Jun 5;266(16):10143–10147. [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. L., Cohn D. V. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]