Abstract
Narrative reviews conclude that behavioral therapies (BTs) produce better outcomes than control conditions for cannabis use disorders (CUDs). However, the strength and consistency of this effect has not been directly empirically examined. The present meta-analysis combined multiple well-controlled studies to help clarify the overall impact of behavioral interventions in the treatment of CUDs. A comprehensive literature search produced 10 randomized controlled trials (RCTs; n = 2,027) that were included in the final analyses. Analyses indicated an effect of BTs (including contingency management, relapse prevention, and motivational interviewing, and combinations of these strategies with cognitive behavioral therapy) over control conditions (including waitlist [WL], psychological placebo, and treatment as usual) across pooled outcomes and time points (Hedges’ g = 0.44). These results suggest that the average patient receiving a behavioral intervention fared better than 66% of those in the control conditions. BT also outperformed control conditions when examining primary outcomes alone (frequency and severity of use) and secondary outcomes alone (psychosocial functioning). Effect sizes were not moderated by inclusion of a diagnosis (RCTs including treatment-seeking cannabis users who were not assessed for abuse or dependence vs. RCTs including individuals diagnosed as dependent), dose (number of treatment sessions), treatment format (either group vs. individual treatment or in-person vs. non-in-person treatment), sample size, or publication year. Effect sizes were significantly larger for studies that included a WL control comparison versus those including active control comparisons, such that BT significantly outperformed WL controls but not active control comparisons.
Keywords: CBT, cognitive behavioral therapy, meta-analysis, cannabis, marijuana, review, substance use disorder, behavioral therapies
The need for public health awareness and evidence-based clinical care for cannabis use and its disorders remains a major health care priority in the United States and beyond. Cannabis represents the most widely used illicit substance for 30 consecutive years (Johnston, O’Malley, & Bachman, 2003; Substance Abuse and Mental Health Services Administration [SAMHSA], 2009). Correspondingly, 8.6% of the population report having used cannabis in the past year (Johnston, O’Malley, Bachman, & Schulenberg, 2005) and approximately 6.1% having used in the past month (SAMHSA, 2009). Furthermore, an estimated 10% of persons who have ever used cannabis are projected to become future daily users (Johnston, O’Malley, & Bachman, 1995), which is problematic considering the numerous health and psychosocial consequences associated with frequent (e.g., daily or weekly) cannabis use (Degenhardt, Hall, & Lynskey, 2001a, 2001b; Hall & Degenhardt, 2009). Moreover, escalating rates of cannabis use disorders (CUDs) in recent years are a significant public health concern (Anthony & Helzer, 1991; Anthony, Warner, & Kessler, 1994), with 35% of cannabis users in the United States currently meeting criteria for cannabis abuse or dependence, compared with 30% meeting criteria in the last decade (Compton, Grant, Colliver, Glantz, & Stinson, 2004).
Accumulating empirical evidence suggests that the clinical profile of CUDs is similar to that seen in the context of other substance use disorders (SUDs). For example, individuals attempting to quit cannabis experience marked difficulty in remaining abstinent whether they make a quit attempt on their own or seek professional treatment (Budney, Roffman, Stephens, & Walker, 2007; McRae, Budney, & Brady, 2003; Nordstrom & Levin, 2007). Moreover, several studies document high failure rates among self-quitters, as indexed by numerous unsuccessful quit attempts (Copersino et al., 2006; Weiner, Sussman, McCuller, & Lichtman, 1999). Individuals who find it particularly difficult to quit substances, including cannabis, are thought to possess elevated cognitive–affective vulnerability and reactivity to distressing states, such as withdrawal symptoms and stressors in daily life. Such coping-oriented cannabis use is predictive of greater dependence severity (Bonn-Miller, Zvolensky, & Bernstein, 2007; Johnson, Mullin, Marshall, Bonn-Miller, & Zvolensky, 2010).
For those who seek treatment, some narrative reviews conclude that behaviorally based psychotherapeutic interventions (e.g., contingency management [CM], motivational enhancement interviewing [ME], or a combination of behavioral interventions with cognitive components, i.e., cognitive behavioral therapy [CBT]) produce better outcomes than control conditions (McRae et al., 2003; Nordstrom & Levin, 2007; Zimmermann Mühlig, Sonntag, Bühringer, & Wittchen, 2004). Still, individual trials show inconsistent findings with behavioral therapies (BTs) outperforming controls on only some outcomes and not others (e.g., full abstinence vs. frequency reduction and harm reduction), limiting the conclusiveness of these findings. For example, one recent meta-analysis examining interventions for adolescent cannabis use found psychosocial substance use interventions to effectively reduce cannabis use frequency (Bender, Tripodi, Sarteschi, & Vaughn, 2011). Unfortunately, these findings are narrow in their conclusive scope, as they may be of limited relevance for adults, and for individuals seeking interventions for cannabis use abstinence or harm reduction.
We previously examined the effectiveness of psychosocial interventions in a meta-analysis for adult SUDs (Dutra et al., 2008). The aggregated treatment effect suggested that across outcomes, the average individual undergoing any active behavioral treatment for any SUD should fare significantly better than 67% of individuals in control conditions. Promisingly, collapsing across type of treatment, the greatest effects were found for CUDs compared to disorders of other illicit drugs. Specifically, the overall average pre- to posttreatment effect size calculation for the five CUD randomized controlled trials (RCTs) yielded a Cohen’s d of 0.81 (Dutra et al., 2008).
Despite the impressive magnitude and direction of the discussed findings, several limitations of the previous literature remain relevant to cannabis use. First, the aggregated effect reported in the Dutra’s study is not indicative of the aggregate effect of treatment for CUDs alone. Second, there are now 10 RCTs available compared to only 5 in the previous analysis. Additionally, by not including RCTs conducted among adolescents, these results may not be truly representative of individuals with CUD or problematic, high frequency use. Indeed, cannabis use among adolescence is particularly problematic, given research indicating potential links between cannabis use in adolescence and increased risk for mental health problems later in life (Large, Sharma, Compton, Slade, & Nielssen, 2011). Finally, over and above these results suggesting that CUDs respond to the same types of behavioral interventions as other SUDs (Dutra et al., 2008; McRae et al., 2003), whether treatment dose moderates the effectiveness of BT within CUDs remains in question. Thus, an updated examination of the strength and consistency of the effect of BT/CBTs for cannabis use exclusively (within both adult and adolescent populations) is warranted, and examining treatment dose as a moderator of response will provide us with further knowledge on the effects of BTs for CUDs.
Accordingly, the aim of the current study is to offer an updated empirical benchmark, clarifying the effectiveness of behaviorally based CUD psychotherapies in adolescents and adults through a comprehensive meta-analysis of 10 RCTs. We derived several hypotheses from the extant literature. First, overall, we expected that BT would outperform control conditions pooled across types of interventions, outcomes, and time variables combined (Hypothesis 1). We expected that BT (pooled across types of intervention and time variables) would also outperform control conditions on pooled primary outcomes (Frequency and Severity/Hypothesis 2) and pooled secondary outcomes (Psychosocial/Hypothesis 3). Finally, we expected that effect sizes would be moderated by dose (number of treatment sessions), with larger doses associated with greater response (Hypothesis 4). In addition, we explored the potential moderating effects of sample size, inclusion of a diagnosis (RCTs that did not assess for abuse or dependence vs. RCTs that only enrolled those diagnosed as dependent), and publication year as has been done in previous studies (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010; Powers, Sigmarsson, & Emmelkamp, 2008; Powers, Vedel, & Emmelkamp, 2008; Powers, Zum Vörde Sive Vörding, & Emmelkamp, 2009; Wolitzky-Taylor, Horowitz, Powers, & Telch, 2008). We also examined the potential moderating effects of in-person interventions versus computerized or telephone interventions.
Method
Study Selection
We conducted a literature search using Scopus, Medline, PsychINFO, PubMed, the Cochrane Register of Controlled Trials, and Dissertation Abstracts International. We searched for treatment outcome studies from the first available date until March 15, 2013. We used the search terms random* and cannabis or marijuana. These words were searched as key words, title, abstract, and MeSH subject heading terms. Also, we examined citation maps and used the “cited by” search tools. These findings were cross-referenced with references from reviews. Studies meeting the following inclusion criteria were selected for the meta-analysis: (a) at least one condition involving a behavioral component (e.g., CM, Relapse Prevention [RP], ME, or a combination of these behavioral interventions with CBT), (b) random assignment, (c) either an active (treatment as usual [TAU], psychological placebo [PsychPL], or other author-developed control comparisons without behavioral components) or inactive (waitlist [WL]) control group, and (d) a treatment-seeking sample. We chose to only include studies with a control condition (i.e., any conditions that did not appear to include the active components of BT) in order to compare controlled effect sizes. We could have included far more studies if we only compared active treatments. However, these uncontrolled effect sizes (pre- post-) are not standardized against a control condition. Thus, fluctuations from study to study (e.g., severity, treatment resistance, etc.) may explain effect size differences rather than treatment effects, which are isolated in a controlled effect, size comparison. It should be noted that throughout the article, active control treatments that were not PsychPL conditions, but author-developed control comparisons without behavioral components (but which were still thought to be potentially effective treatments), were referred to in a broad category labeled “TAU” for ease of labeling. This is consistent with methodology used in previous meta-analyses examining substance use (Dutra et al., 2008; Powers et al., 2008). We acknowledge that these treatments labeled as TAU in our article were not always specifically described as such; for example, the Stephens, Roffman, and Simpson (1994) study called their comparison condition “social support,” but described this treatment as using “a group process model of therapeutic change and was based on the content of local substance abuse programs.” Additionally, the Carroll et al. (2006) study comparison group, though labeled as “individual drug counseling,” was described as “a standardized version of the counseling that is typically offered in community-based clinics.”
We elected to include only studies that clearly denoted that the sample consisted of treatment-seeking individuals in order to obtain the effects of behavioral treatment among individuals who find their cannabis use problematic and would like to quit, as the findings of this meta-analysis may be more relevant to individuals who want to quit. Authors of selected studies were contacted directly when there were insufficient data provided in their articles to include in the meta-analysis. The initial search strategies identified 763 potential articles. A Quality of Reporting of Meta-Analyses (QUOROM) diagram is available upon request.
Software
Analyses were completed with Comprehensive Meta-Analysis (Borenstein & Rothstein, 1999). Comprehensive Meta-Analysis is a program funded by the National Institutes of Health Small Business Innovation Research program.
Procedure
Data on the following variables were collected: sample (Users/Dependent), treatment dose (number of sessions), sample size, treatment format (in-person, computerized, or telephone intervention), and year of publication. Dependent variables were classified into reliable outcome domains (Peters, Nich, & Carroll, 2011) including frequency and severity of cannabis use and psychosocial functioning. RCTs utilized a variety of measures to assess for frequency and severity of use, including number of days used in the past 30 days or number of joints assessed by a Timeline Follow-Back measurement (Sobell et al., 1980). RCTs also assessed frequency of cannabis use based on the number of clean/dirty urine samples, or percentage of sample abstinence. Severity was assessed using a number of measures, including the Marijuana Problems Scale (Stephens, Wertz & Roffman, 1993) and the number of abuse and dependence symptoms based on criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Psychosocial functioning was most frequently assessed using the Addiction Severity Index (McLellan et al., 1992). BT conditions were categorized as follows: RP; ME; CM; or combinations of CBT and these categories (i.e., CBT/RP, CBT/ME, CBT/ME/RP, CBT/ME/CM). Studies with multiple measures of one outcome domain were pooled into a single effect size per domain. This was completed by the analysis program after the raw data were converted to effect sizes to avoid the influence of differences in scales.
Effect Size Calculation and Analyses
Between-group effect sizes for each study were computed using Hedges’ g (Rosenthal, 1991). Using weights, the adjusted Hedges’ g corrects for small sample sizes (Deeks, Altman, & Bradburn, 2001; Hedges & Olkin, 1985; Rosenthal, 1994). These adjusted controlled effect sizes may then be conservatively interpreted with Cohen’s (1988) convention of small (0.2), medium (0.5), and large (0.8) effects. Studies with multiple outcomes were categorized as previously (Note: a table detailing the study outcomes is available upon request). Effect sizes were calculated with random effects analyses, which estimate the overall effect size assuming the studies included are only a sample of the entire population of studies. In addition, the random effects analysis is more appropriate if there is significant between-trial heterogeneity. Given our goal is to generalize these findings, we report the random effects analyses. Percent improvement is also reported to aid understanding and clinical relevance of the findings. This was calculated by subtracting the control group percentage improvement by the experimental group percentage improvement. Group improvements were calculated by dividing the posttest group mean minus the pretest group mean by the pretest group mean and multiplying by 100.
Heterogeneity was tested with the Q-statistic to determine whether individual study effect sizes varied significantly around the mean overall summary effect size of all studies (Hedges & Olkin, 1985; Raudenbush & Bryk, 2002). The magnitude of the variability or proportion of variance accounted for by true differences between studies was then estimated with the I2 index and interpreted with the conventions of small (25%), medium (50%), and large (75%; Higgins & Thompson, 2002; Huedo-Medina, Sanchez-Meca, Marin-Martinez, & Botella, 2006). Significant heterogeneity suggests that a random effects analysis is most appropriate and that between effect size differences may be explained by moderators.
We examined potential moderators using unrestricted maximum likelihood meta-regressions (Thompson & Higgins, 2002). Meta-regression allows the effect of continuous, as well as categorical, characteristics of studies to be investigated as potential effect size moderators.
Results
See Figure 1 for studies included in the meta-analysis. Across studies, the most common control condition was WL accounting for seven studies (Copeland, Swift, Roffman, & Stephens, 2001; Gates, Norberg, Copeland, & Digiusto, 2012; Martin & Copeland, 2008; Rooke, Copeland, Norberg, Hine, & McCambridge, 2013; Stephens, Roffman, & Curtin, 2000; The Marijuana Treatment Project Research Group [MTPRG], 2004). Two studies included TAU control conditions (Carroll et al., 2006; Stephens, Roffman, & Simpson, 1994) and one study included a PsychPL condition (Kadden, Litt, Kabela-Cormier, & Petry, 2007). The most common treatment condition component was ME, used in nine studies (Carroll et al., 2006; Copeland et al., 2001; Gates et al., 2012; Hoch et al., 2012; Kadden et al., 2007; Martin & Copeland, 2008; Rooke et al., 2013; Stephens et al., 2000; The MTPRG, 2004). Three studies included a condition with RP (Copeland et al., 2001; Stephens et al., 2000; Stephens et al., 1994) and two studies included conditions with CM (Carroll et al., 2006; Kadden et al., 2007).
Figure 1.
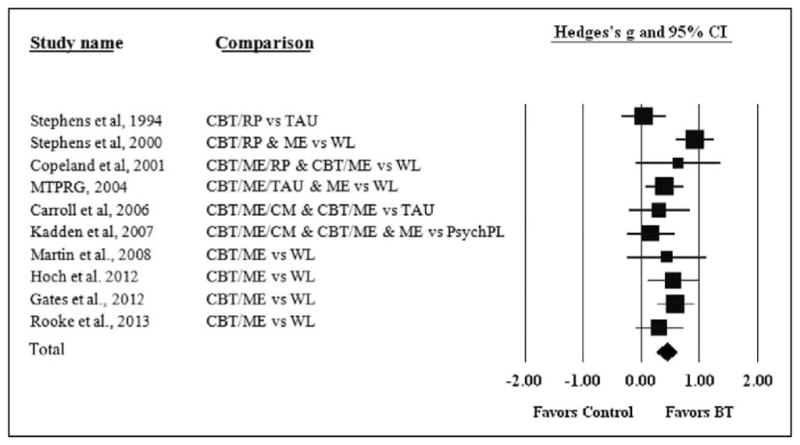
Forest plot of BT versus control with all outcomes and time points combined.
Studies Included in the Meta-Analysis
Stephens et al. (1994) randomized 212 treatment seeking cannabis-using adults to 10 sessions of (a) cognitive behavioral relapse prevention (RP) or (b) TAU (Roffman, Stephens, Simpson, & Whitaker, 1988; Stephens et al., 1994). There were no significant differences between the two conditions. However, men in the RP condition reported less use and problems at the 3-month follow-up. In a later study, Stephens, Roffman, and Curtin (2000) randomized 291 treatment-seeking cannabis-using adults to (a) 14 sessions of RP, (b) 2-sessions of ME, or (c) WL control (Stephens et al., 2000). The RP and ME conditions showed greater improvement at the 4-month follow-up compared to the WL condition. However, there were no significant differences in the two BTs (RP or ME) at any assessment. Copeland, Swift, Roffman, and Stephens (2001) randomized 229 treatment-seeking cannabis-using adults to (a) six sessions of CBT + ME + RP, (b) one session of CBT + ME, or (c) WL control (Copeland et al., 2001). Both active BTs outperformed the WL condition on use and use problems compared to the WL control condition. In addition, the CBT + ME + RP condition outperformed the WL condition in reduced levels of cannabis use. The MTPRG (2004) randomized 450 cannabis dependent adults to (a) nine sessions of CBT + ME + TAU, (b) two sessions of ME, or (c) WL control. The CBT + ME + TAU condition reduced cannabis use more than the ME condition which in turn outperformed the WL control. Improvements were largely maintained over the follow-up period. Carroll et al. (2006) randomized 136 cannabis dependent adults (aged 18–25) to 8 weeks of (a) CBT + ME + CM, (b) CBT + ME, or (c) TAU (Carroll et al., 2006). There was a main effect for more negative urine specimens. In addition, the CBT + ME + CM was more effective than the CBT + ME and TAU conditions on negative urine specimens and attendance. Kadden, Litt, Kabela-Cormier, and Petry (2007) randomized 240 cannabis dependent adults to 9 weekly sessions of (a) CBT + ME + CM, (b) CBT + ME, (c) CM, or (d) a supportive PsychPL condition (Kadden et al., 2007). This PsychPL condition included supportive contact with a therapist who did not provide suggestions to the participant regarding how to change cannabis use but instead addressed daily living concerns (e.g., family problems and depressed mood). The two CM conditions had the highest rates of abstinence. The CM-only condition had the highest rate of abstinence at posttreatment. The CBT + ME + CM condition had the highest rate of abstinence at later follow-up. Martin & Copeland (2008) randomized 40 adolescent (n = 40) cannabis users (past month) to (a) two sessions of CBT + ME or (b) WL control. The CBT + ME condition reduced cannabis use by the 3-month follow-up significantly more than the WL condition. Hoch et al. (2012) randomized 122 adolescents and adults (aged 16–44) with cannabis dependence to (a) 10 sessions of CBT + ME + TAU or (b) WL control (Hoch et al., 2012). The CBT condition significantly outperformed the WL condition in abstinence measures at posttreatment. Comparisons at follow-up were not possible, as the WL condition received treatment using the tested intervention following the posttreatment assessment. Gates, Norberg, Copeland, and Digiusto (2012) randomized 160 cannabis users to receive either (a) a telephone-based intervention consisting of four sessions of CBT + ME or (b) WL control. The CBT condition significantly outperformed the WL condition in dependence symptoms at both the 1-month and 3-month follow-up and in percentage of abstinence at the 3-month follow-up. Rooke et al. (2013) randomized 225 cannabis users to receive either (a) an Internet-based CBT/ME intervention or (b) WL control. At posttreatment, the CBT/ME group outperformed the WL condition on frequency and severity outcomes. These findings held at 3-month follow-up, though the past month quantity of cannabis consumed no longer differed between groups.
Hypothesis 1: BT versus control—All outcomes combined.
Consistent with prediction, BT outperformed control conditions when all outcome variables were combined across all time points. Using a random effects analysis, we obtained a mean overall effect size of Hedges’ g = 0.44 (standard error [SE] = 0.09, 95% confidence interval [CI]: [0.26, 0.62]), indicating a medium effect for BT relative to control conditions overall (p < .001). This finding suggests that the average patient receiving BT treatment fared better than 66% of those in the control conditions.
Hypothesis 2: BT versus control—Primary outcomes (frequency and severity of use).
Consistent with prediction, BT also outperformed control conditions across pooled primary measures (frequency and severity of use). Using a random effects analysis, we obtained a mean overall effect size of Hedges’ g = 0.49 (SE = 0.11, 95% CI: [0.26, 0.71]), indicating an effect for BT relative to control conditions (p < .001). This suggests that the average patient receiving BT fared better than approximately 69% of those in the control conditions on frequency and severity of use measures.
Hypothesis 3: BT versus control—secondary outcomes (psychosocial).
Consistent with our prediction, BT also significantly outperformed control conditions on secondary measures (measures of psychosocial functioning). Using a Hedges’ g random effects analysis, we obtained a mean overall effect size of Hedges’ g = 0.42 (SE = 0.12, 95% CI: [0.19, 0.65], p < .001). This suggests that the average patient receiving BT fared better than 66% of those in the control conditions on measures of psychosocial functioning.
Analysis of Heterogeneity
The primary overall heterogeneity analysis (I2) suggested that 46% of the variance in effect sizes was due to true between-study differences (Q(9) = 16.44, p = .06), though this effect was not significant. Thus, a more conservative random effects analysis was used. Figure 1 is a forest plot of the bias corrected (Hedges’ g) between-group (controlled) effect sizes and 95% CIs for each study with BT and control conditions using all outcome variables combined (Hypothesis 1).
Hypothesis 4: Moderators—Effect size as a function of dose, sample size, and publication year.
Contrary to prediction, a meta-regression analysis showed there was not a significant relationship between dose (number of treatment sessions) and effect sizes (β = .02, p = .43). There was also not a significant relationship between publication year and effect size (β = .01, p = .73) or between sample size and effect size (β = .00, p = .70). In addition, there was not a significant difference in effect sizes between studies that did not assess for abuse or dependence as an inclusion criteria versus studies that only enrolled participants diagnosed as dependent (p = .29) or studies using in-person versus non-in-person treatments (p = .80). There were also no significant differences between group and individual treatment formats in the in-person treatments (p = .78). Finally, there was a significant difference between active control groups and WL control group, such that BTs evidenced larger effect sizes than active control groups when compared to WL control groups (Hedges’ g = 0.56, SE = 0.09, 95% CI: [0.73, 6.48], p < .001) (Hedges’ g = 0.14, SE = 0.12, 95% CI: [0.39, 1.16], p = .25). A table detailing the key variables utilized in the moderator analysis is available upon request.
Publication Bias “The File Drawer Problem”
Several authors suggest there may be a potential discrepancy between the number of published trials and the total number that are completed (Bakan, 1967; McNemar, 1960; Smart, 1964; Sterling, 1959). Therefore, any meta-analysis of published studies may be missing nonsignificant studies and therefore overestimate the overall effect size. Rosenthal (1991) and others have called this confound “The File Drawer Problem.” A conservative method of addressing this problem is to assume that the effect sizes of all current or future unpublished studies are equal to 0 and compute the number of such studies it would require to reduce the overall effect size to a non-significant level (Rosenthal & Rubin, 1988). This value may be referred to as the “fail-safe N.” Rosenthal (1991) suggested that findings may be considered robust if the required number of studies (X) to reduce the overall effect size to a nonsignificant level exceeded 5K + 10 which in this study would be 60. Analyses revealed that it would require more than 101 current or future unpublished studies with an effect size of 0 to bring the overall effect size of the primary analyses within the nonsignificant range, suggesting that the findings in this meta-analysis are robust.
Discussion
Major Findings
The meta-analysis of 10 randomized controlled BT trials (n = 2,027) largely supported the study hypotheses. First, consistent with prediction, BT outperformed control conditions when all outcome and time variables were combined (Hedges’ g = 0.44), indicating an effect for BT relative to control conditions overall. This suggests that the average patient receiving BT fared better than 66% of those in the control conditions. Second, consistent with prediction, BT outperformed control conditions on primary outcomes (Hedges’ g = .49), suggesting that the average patient receiving BT fared better than 69% of those in control conditions on frequency and severity of use measures. Third, consistent with prediction, BT significantly outperformed control conditions on secondary measures (Hedges’ g = 0.42), suggesting that the average patient receiving BT fared better than 66% of those in the control conditions on psychosocial measures. Fourth, there was not a significant relationship between effect sizes and publication year, sample (users vs. dependent), treatment format (either group vs. individual treatment or in person vs. Internet or telephone based), or sample size. The lack of differences seen among different treatment formats is compelling considering recent efforts to disseminate BTs in more cost-effective means (e.g., in-group format and through web or telephone-based delivery formats). Contrary to our prediction, there was not a dose–response relationship. Additionally, there was a significant difference in effect sizes between those studies with active control comparisons and those studies with WL controls, such that effect sizes of BT were larger for studies with WL control comparisons. This finding is unsurprising, as studies utilizing some form of treatment are likely to be more effective than WL due to treatment expectancies and therapist contact. However, it should also be noted that BT outperformed WL control treatments, but not active control treatments. Though the number of studies with active control treatments available for inclusion in the analysis was limited (i.e., three studies) and should thus be interpreted cautiously, this indicates that BT for cannabis use may not be robust against more active control treatments. Finally, publication bias analyses revealed that the findings in this meta-analysis are robust. It should be noted, however, that these moderator analyses may have been underpowered, given the relatively small number of studies with each moderator.
The results are promising, suggesting that current BTs are superior to control conditions (TAU, PsychPL, and WL). However, it is important to note that there is significant room for improvement. BT did not outperform active control treatments (TAU and PsychPL) and abstinence rates from use are still quite low. In our previous meta-analysis (Dutra et al., 2008), abstinence rates ranged only between 10% and 37%. Similarly, in a critical narrative review of the treatment outcome literature for cannabis dependence, McRae, Budney, and Brady (2003) concluded “studies suggest that many patients do not show a positive treatment response, indicating that cannabis dependence is not easily treated” (p. 369). Other studies have reported similar findings, and more recent clinical trials have extended such work by noting that in addition to full relapse, lapses are highly common and clinically significant. In fact, the most effective treatment to date for cannabis dependence (BT) yields only a 50% abstinence rate during the first 2-weeks of intervention (Budney, Moore, Rocha, & Higgins, 2006; Budney, Roffman, et al., 2007; Kadden et al., 2007). Moreover, of those who achieve abstinence during this time, about 1/2 relapse within 1 year (Budney, Roffman, et al., 2007; Dutra et al., 2008).
High relapse rates, however, are not unique to CUDs. Indeed, the vast majority of persons with any SUD do not fully benefit from existing intervention protocols. Thus, improving treatment effectiveness for CUDs may require developing augmentative treatments directly targeting the biobehavioral mechanisms underlying cannabis use maintenance and relapse. This strategy may be especially relevant to cannabis users, given that we were unable to find a dose–response relationship among existing behavioral treatments, suggesting that specialized, potent therapeutic ingredients may be required to reduce relapse in this population. While our findings suggest that specialized treatments may be necessary for relapse prevention, the lack of differences seen among different treatment formats suggest that more cost-effective treatment dissemination strategies (e.g., group treatment or web/telephone-based delivery) have the potential to be as efficacious for this population as individual, in-person treatment strategies.
A priori research points to the endocannabinoid system (Clapper, Mangieri, & Piomelli, 2009) as one augmentative potential treatment target. Cannabis users report that withdrawal symptoms are the primary reason for relapse (Bonn-Miller & Moos, 2009; Budney, Vandrey, Hughes, Thostenson, & Bursac, 2008; Cornelius, Chung, Martin, Clark, & Wood, 2007). Importantly, it appears that these withdrawal symptoms are strongly related to the downregulation of brain endocannabinoids resulting from frequent cannabis use (Bortolato et al., 2006, 2007; Clapper et al., 2009; Gobbi et al., 2005). Accordingly, increasing endocannabinoid levels during cannabis cessation attempts (i.e., during active BT) may improve outcomes by reducing withdrawal symptoms (Clapper et al., 2009). Therefore, reducing relapse rates, thereby increasing the effectiveness of CUD treatments by and large, may require pharmacological or psychological augmentative treatment strategies that can attenuate endocannabinoid withdrawal. Potentially effective strategies to accomplish this safely are currently being investigated, such as moderate-intensity aerobic exercise (Dietrich & McDaniel, 2004; Sparling, Giuffrida, Piomelli, Rosskopf, & Dietrich, 2003) and low-dose tetrahydrocannabinol (Budney, Vandrey, Hughes, Moore, & Bahrenburg, 2007; Haney et al., 2004). However, it is important to note that this area of research is still in the early stages, and there is currently a limited application of these strategies. Other pharmacological agents are under investigation, given that there are currently no FDA-approved medications available for CUDs.
Overall, as the most widely used recreational illicit drug, cannabis use requires public health attention and improved evidence-based clinical care. Meta-analyses contribute to this endeavor by examining the effects of RCTs that encompass a wide range of BT interventions, dose intensities, and sample characteristics. Our findings speak to both the specific weaknesses and strengths of current BT interventions, which may guide recommendations for treatment improvement. As mentioned previously, improving treatments outcomes by reducing relapse rates in problematic cannabis abusers may require augmentations to existing BTs in order to target specific core mechanisms accounting for cannabis use maintenance and relapse.
Limitations
Several limitations deserve comment. First, most RCTs in our review of the literature compared two BTs without including a control group (WL, PsychPL, or TAU); these studies were not included due to our interest in isolating the effects of BTs by comparing to control groups without BT components. Although there were twice as many studies as a previous meta-analysis, there were still only 10 trials currently that included such controls. Although it is costly to include such an additional condition, it is the only way to determine the relative efficacy of BT conditions. This limited number of studies may have also affected the power to detect moderators. Second, the current study did not compare different forms of behavioral treatments (e.g., relapse prevention, motivational enhancement, contingency management, and combinations of these treatments with CBT), as across studies, each version of behavioral treatment is not uniform. Further, two of the studies did not utilize in-person treatment conditions and instead used web-based (Rooke et al., 2013) or telephone-based interventions (Gates et al., 2012). However, our analysis showed no significant difference in effect sizes based on treatment format or based on individual versus group format within the in-person treatments. Finally, some of the RCTs included in the analysis did not assess for substance abuse/dependence and included cannabis “Users,” while others included only those participants diagnosed as dependent. However, all individuals in all included RCTs were seeking treatment for their cannabis use, suggesting problematic usage. Further, our analysis showed no significant difference in effect sizes between studies with “Users” versus dependent participants.
Summary and Conclusions
In sum, BT outperformed control conditions in across all outcome domains, including frequency and severity of use and psychosocial functioning. Across all treatment types, time points, and outcomes, the average patient receiving BT fared better than 66% of those in the control conditions. Although encouraging, there is still room for improvement, as abstinence rates are relatively low and BT, while outperforming WL control conditions, did not outperform active control conditions, which indicates a need for the development and evaluation of augmentative intervention strategies relevant to this population.
Acknowledgments
Portions of this research were funded by the National Institute on Drug Addiction K01 DA035930.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this article was in part supported by grants R01DA027533 and R34DA031038 from the National Institute of Health awarded to Jasper Smits, PhD, and Michael Zvolensky, PhD, and the National Institute on Drug Addiction K01 DA035930 awarded to Mark Powers, PhD.
Footnotes
Dr. Smits receives royalties from various book publishers unrelated to this study. All remaining authors report no competing interests.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anthony JC, Helzer JE. Syndromes of drug abuse and dependence. In: Robins LN, Reiger DA, editors. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, NY: Free Press; 1991. pp. 116–154. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the national comorbidity survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Bakan D. On method. San Francisco, CA: Jossey-Bass; 1967. [Google Scholar]
- Bender K, Tripodi S, Sarteschi C, Vaughn M. A meta-analysis of interventions to reduce adolescent cannabis use. Research on Social Work Practice. 2011;21:153–164. [Google Scholar]
- Bonn-Miller MO, Moos RH. Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addictive Behaviors. 2009;34:782–785. doi: 10.1016/J.Addbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ, Bernstein A. Marijuana use motives: Concurrent relations to frequency of past 30-day use and anxiety sensitivity among young adult marijuana smokers. Addictive Behaviors. 2007;32:49–62. doi: 10.1016/J.Addbeh.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Rothstein H. Comprehensive meta-analysis, a computer program for research synthesis. New Jersey, NJ: Biostat; 1999. [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/Sj.Npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biological Psychiatry. 2007;62:1103–1110. doi: 10.1016/J.Biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74:307–316. doi: 10.1037/0022-006x.74.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction Science and Clinical Practice. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and Alcohol Dependence. 2007;86:22–29. doi: 10.1016/J.Drugalc-dep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008;35:362–368. doi: 10.1016/J.Jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56:235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Journal of the American Medical Association. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. discussion 65–56. S0740-5472(01)00179-9 [pii] [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Gorelick DA. Quitting among non-treatment-seeking marijuana users: Reasons and changes in other substance use. American Journal of Addiction. 2006;15:297–302. doi: 10.1080/10550490600754341. P7580UX5Q815WK41 [pii] [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Clark DB, Wood DS. Cannabis withdrawal is common among comorbid adolescents seeking treatment. Alcoholism—Clinical and Experimental Research. 2007;31:118A. [Google Scholar]
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith G, Altman D, editors. Systematic reviews in health care: Meta-analysis in context. London, England: BMJ; 2001. pp. 285–312. [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: A comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001a;96:1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: Findings from the national survey of mental health and well-being. Social Psychiatry and Psychiatric Epidemiology. 2001b;36:219–227. doi: 10.1007/s001270170052. [DOI] [PubMed] [Google Scholar]
- Dietrich A, McDaniel WF. Endocannabinoids and exercise. British Journal of Sports Medicine. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Gates PJ, Norberg MM, Copeland J, Digiusto E. Randomized controlled trial of a novel cannabis use intervention delivered by telephone. Addiction. 2012;107:2149–2158. doi: 10.1111/j.1360-0443.2012.03953.x. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Piomelli D. Antidepressant-like activity and modulation of brain mono-aminergic transmission by blockade of anandamide hydrolysis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18620–18625. doi: 10.1073/Pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. The Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.13003101300310. [pii] [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hoch E, Noack R, Henker J, Pixa A, Hofler M, Behrendt S, Wittchen HU. Efficacy of a targeted cognitive-behavioral treatment program for cannabis use disorders (CANDIS) European Neuropsychopharmacology. 2012;22:267–280. doi: 10.1016/j.euroneuro.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Johnson K, Mullin JL, Marshall EC, Bonn-Miller MO, Zvolensky M. Exploring the mediational role of coping motives for marijuana use in terms of the relation between anxiety sensitivity and marijuana dependence. American Journal on Addictions. 2010;19:277–282. doi: 10.1111/J.1521-0391.2010.00041.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. National survey results on drug use from the monitoring the future study, 1975–1994. Bethesda, MD: National Institute on Drug Abuse; 1995. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the future: National survey results on drug use, 1975–2002. Bethesda, MD: National Institute on Drug Abuse; 2003. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National results on adolescent drug use. Bethesda, MD: National Institutes of Health; 2005. [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors. 2007;32:1220–1236. doi: 10.1016/J.Addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: A systematic meta-analysis. Archives of General Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Martin G, Copeland J. The adolescent cannabis check-up: Randomized trial of a brief intervention for young cannabis users. Journal of Substance Abuse Treatment. 2008;34:407–414. doi: 10.1016/j.jsat.2007.07.004. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Argeriou M. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McNemar Q. At random: Sense and nonsense. American Psychologist. 1960;15:295–300. [Google Scholar]
- McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: A review of the literature. Journal of Substance Abuse Treatment. 2003;24:369–376. doi: 10.1016/s0740-5472(03)00041-2. S0740547203000412 [pii] [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: A review of the literature. American Journal of Addiction. 2007;16:331–342. doi: 10.1080/10550490701525665. 782094591 [pii] [DOI] [PubMed] [Google Scholar]
- Peters EN, Nich C, Carroll KM. Primary outcomes in two randomized controlled trials of treatments for cannabis use disorders. Drug and Alcohol Dependence. 2011;118:408–416. doi: 10.1016/j.drugalcdep.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30:635–641. doi: 10.1016/J.Cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Powers MB, Sigmarsson SR, Emmelkamp PMG. A meta-analytic review of social phobia treatments. International Journal of Cognitive Therapy. 2008;1:94–113. [Google Scholar]
- Powers MB, Vedel E, Emmelkamp PMG. Behavioral couples therapy (BCT) for alcohol and drug use disorders: A meta-analysis. Clinical Psychology Review. 2008;28:952–962. doi: 10.1016/j.cpr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Powers MB, Zum Vörde Sive Vörding MB, Emmelkamp PMG. Acceptance and commitment therapy: A meta-analytic review. Psychotherapy and Psychosomatics. 2009;78:73–80. doi: 10.1159/000190790. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Roffman RA, Stephens RS, Simpson EE, Whitaker DL. Treatment of marijuana dependence: Preliminary results. Journal of Psychoactive Drugs. 1988;20:129–137. doi: 10.1080/02791072.1988.10524382. [DOI] [PubMed] [Google Scholar]
- Rooke S, Copeland J, Norberg M, Hine D, McCambridge J. Effectiveness of a self-guided web-based cannabis treatment program: Randomized controlled trial. Journal of Medical Internet Research. 2013;15(2):e26. doi: 10.2196/jmir.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. London, England: Sage; 1991. [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges L, editors. The handbook of research synthesis. New York, NY: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- Rosenthal R, Rubin DB. Comment: Assumptions and procedures in the file drawer problem. Statistical Science. 1988;3:120–125. [Google Scholar]
- Smart RG. The importance of negative results in psychological research. Canadian Psychologist. 1964;5A:225–232. [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. New York, NY: Pergamon; 1980. pp. 129–150. [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. NeuroReport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA. Predictors of marijuana treatment outcomes: The role of self-efficacy. Journal of Substance Abuse. 1993;5:341–354. doi: 10.1016/0899-3289(93)90003-t. [DOI] [PubMed] [Google Scholar]
- Sterling TD. Publication decisions and their possible effects on inferences drawn from tests of significance or vice versa. Journal of the American Statistical Association. 1959;54:30–40. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 national survey on drug use and health: National findings. Rockville, MD: Department of Health and Human Services; 2009. [Google Scholar]
- The Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- Weiner MD, Sussman S, McCuller WJ, Lichtman K. Factors in marijuana cessation among high-risk youth. Journal of Drug Education. 1999;29:337–357. doi: 10.2190/PN5U-N5XB-F0VB-R2V1. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Horowitz JD, Powers MB, Telch MJ. Psychological approaches in the treatment of specific phobias: A meta-analysis. Clinical Psychology Review. 2008;28:1021–1037. doi: 10.1016/j.cpr.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Mühlig S, Sonntag D, Bühringer G, Wittchen HU. Review on psychotherapeutic interventions for cannabis disorders. Journal of Addiction Research and Practice. 2004;50:334–342. [Google Scholar]


