Abstract
Insulin signaling and nutrient levels coordinate the metabolic response to feeding in the liver. Insulin signals in hepatocytes to activate Akt, which inhibits Foxo1 suppressing hepatic glucose production (HGP) and allowing the transition to the postprandial state. Here we provide genetic evidence that insulin regulates HGP by both direct and indirect hepatic mechanisms. Liver-specific ablation of the IR (L-Insulin Receptor KO) induces glucose intolerance, insulin resistance and prevents the appropriate transcriptional response to feeding. Liver-specific deletion of Foxo1 (L-IRFoxo1DKO) rescues glucose tolerance and allows for normal suppression of HGP and gluconeogenic gene expression in response to insulin despite lack of autonomous liver insulin signaling. These data indicate that, in the absence of Foxo1, insulin signals via an intermediary extra-hepatic tissue to regulate liver glucose production. Importantly, a hepatic mechanism distinct from the IR-Akt-Foxo1 axis exists to regulate glucose production.
Introduction
The dynamic regulation of liver glucose metabolism is essential for systemic carbohydrate homeostasis and organismal survival. During times of starvation, the liver produces the amount of glucose necessary to meet the metabolic demands of the body. This increased hepatic glucose production (HGP) during fasting results from an initial breakdown of glycogen stores before transitioning to gluconeogenesis from various precursors. In the postprandial state, this process is opposed by the rise of the hormone insulin, which suppresses HGP. However, in insulin-resistant disorders such as diabetes, insulin fails to regulate HGP, leading to elevated circulating glucose concentrations1, 2. Various models have been proposed to explain the inability of insulin to suppress HGP during diabetes, though there is still no consensus for the mechanism. Prevalent hypotheses include increased delivery of gluconeogenic precursors and fatty acids to the liver, accumulation of neutral lipids in liver, altered systemic cyto-adipokines, distorted glucagon-to-insulin ratios, and defective hepatic insulin signaling3.
Since excess HGP drives fasting hyperglycemia in diabetes, the elucidation of the mechanisms of how insulin regulates hepatic metabolism in normal and pathological livers has received substantial attention. In liver, insulin signals through its receptor (IR), insulin receptor substrates (Irs) and phosphoinositide 3’-kinase to activate the serine/threonine kinase Akt, which utilizes several distinct downstream pathways to modulate liver metabolism4. In hepatocytes, insulin promotes protein translation and cell growth by activating the mammalian target of rapamycin complex 1 (mTORC1) via Akt-mediated phosphorylation and inactivation of the tuberous sclerosis protein 1 and 2 complex5. Insulin also induces lipogenesis and glycogen synthesis via Akt-dependent mechanisms6, 7, 8. Finally, insulin stimulates an Akt-dependent inhibitory phosphorylation of Foxo1, which is thought to be the master regulator of key gluconeogenic genes leading to the subsequent regulation of glucose output2, 9, 10, 11, 12, 13. In support of this notion, ablating hepatic Foxo1 in insulin-resistant models improves glucose homeostasis14, 15. However, deletion of liver Foxo1 in lean mice only modestly reduces fasting glucose and hypoglycemia occurs only after prolonged fasting, leading to uncertainty concerning the role of Foxo1 in normal liver glucose metabolism10, 16.
A reduction of the IR-IRS-Akt pathway activity in mouse livers results in glucose intolerance, systemic insulin resistance, and a failure to suppress appropriately glucose production in response to insulin14, 17, 18. Concomitant deletion of Foxo1 normalizes the glucose intolerance and hyperinsulinemia triggered by hepatic deletion of Irs or Akt14, 17. Surprisingly, mice with liver specific deletion of Akt and Foxo1 (L-AktFoxo1TKO) still respond to insulin in vivo by suppressing gluconeogenic gene expression and HGP. Furthermore, L-AktFoxo1TKO mice adapt appropriately to the postprandial state despite lacking canonical liver insulin signaling17. These data are inconsistent with the established model of hepatic insulin action and suggest Akt is not an obligate intermediate for insulin action under all conditions. Importantly, these data suggest the existence in liver of a signaling pathway parallel to the Akt-Foxo1 axis that is capable of regulating HGP. At least two alternative mechanisms can be formulated to explain the insulin effects in L-AktFoxo1TKO mice: 1) insulin acts directly on the liver via the IR-Irs pathway; however, a bifurcation occurs prior to Akt thereby activating an unidentified parallel pathway independent of Akt to suppress HGP19 or 2) insulin acts non-autonomously via a peripheral tissue to regulate HGP.
In the study reported in this manuscript, we tested the second hypothesis by deleting insulin receptor specifically and exclusively in liver, with and without concomitant deletion of Foxo1. We reasoned that if insulin were suppressing HGP by acting via an intermediary, non-hepatic cell type, which then sends a signal to liver, the effect of insulin should not depend on the presence of its receptor on hepatocytes.
Results
Acute liver-specific deletion of IR
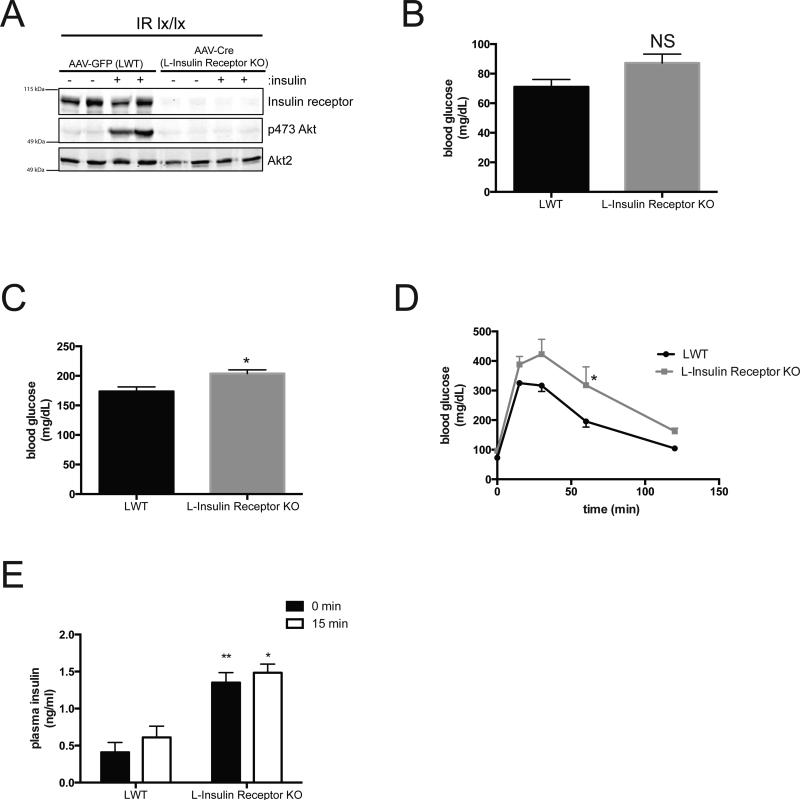
To minimize potential secondary and/or compensatory responses from congenital hepatic insulin receptor (IR) deficiency, we generated a model of acute, liver-specific knockout of the IR. Adult IRloxP/loxP mice were injected with an adeno-associated virus containing a liver-specific promoter (thyroxine-binding globulin) driving Cre recombinase to delete the IR (L-Insulin Receptor KO)8. IRloxP/loxP (LWT) mice injected with virus expressing GFP instead of Cre served as a control. A western blot confirmed efficient deletion of IR in primary hepatocytes isolated from mice following the virus injection (Figure 1A). Akt phosphorylation at Ser473 in response to insulin was undetectable in hepatocytes isolated from L-Insulin Receptor KO mice. In contrast to the major metabolic abnormalities reported in the congenital IR liver knockout mouse (LIRKO)18, L-Insulin Receptor KO mice displayed normal fasting glucose levels (Figure 1B) and modest postprandial hyperglycemia following 4 h of refeeding (Figure 1C). As expected, L-Insulin Receptor KO mice were glucose intolerant (Figure 1D) and hyperinsulinemic 15 min following an intraperitoneal glucose injection (Figure 1E).
Figure 1. Acute deletion of liver insulin receptor leads to glucose intolerance and enhanced Foxo1 activity.
(A) Western blot of primary hepatocytes from mice treated with 10 nM insulin for 15 minutes and probed for specific proteins as indicated. AAVGFP=LWT mice, AAV-Cre=L-Insulin Receptor KO mice. (B,C) Blood glucose concentrations in mice after overnight fast (B) and overnight fasted following by 4 h refeeding of normal chow (C). NS:not significant * P<0.05. n=5 mice. (E) Intraperitoneal glucose tolerance test (2 g per kg) on overnight fasted mice. n=5 mice per group. * p<0.05 vs LWT (F) Insulin levels before and after 15 minutes following glucose injection IP. n=5 mice per group. *** p<0.001, ** p<0.01 vs LWT. All data are presented as mean ± s.e.m. Statistical analysis was performed using Students's t followed by two-tailed analysis. A P <0.05 was considered statistically significant.
Hepatic Foxo1 deletion in L-Insulin Receptor KO mice
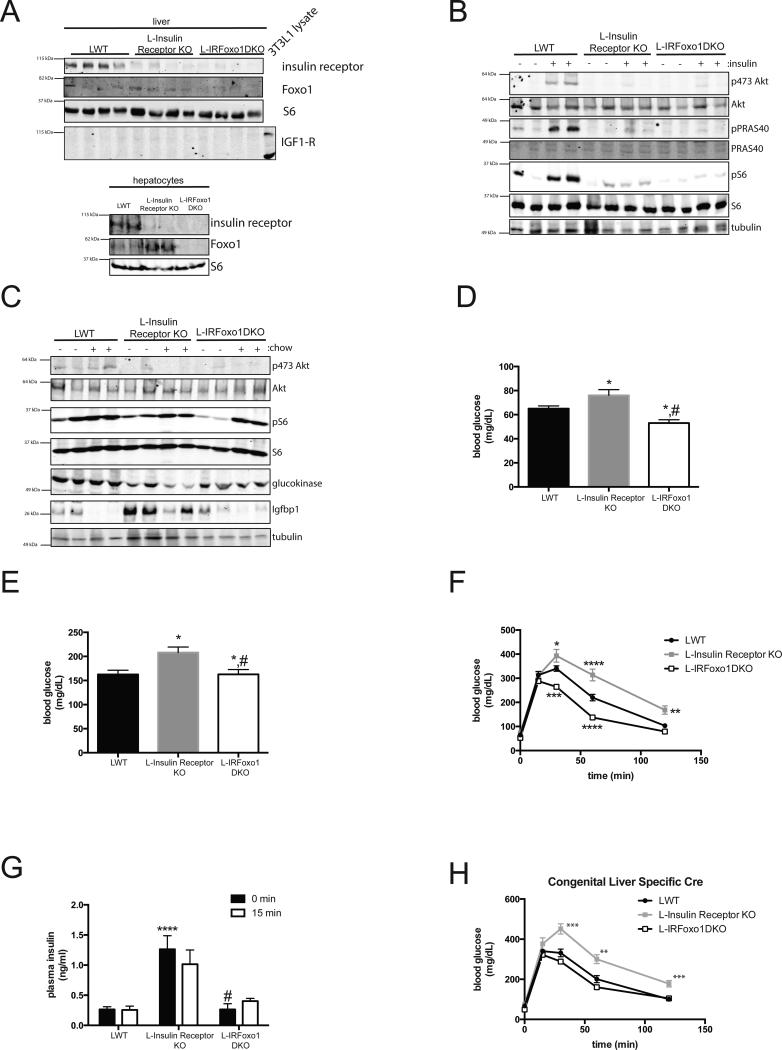
Insulin signals via its receptor to activate Akt and suppress Foxo1-dependent transcription. Hepatic Foxo1 deletion reverses the metabolic abnormalities resulting from liver-specific knockout of insulin receptor substrates (Irs) or Akt14, 17. In order to determine whether constitutive Foxo1 activation is responsible for the abnormal glucose metabolism following hepatic IR deletion, adult IRloxP/loxP;FoxO1loxP/loxP mice were injected with AAV Tbg-Cre to generate liver-specific deletion of IR and Foxo1 (L-IRFoxo1DKO). Mice injected with virus expressing GFP instead of Cre served as controls (LWT). A western blot confirmed excision of the IR and Foxo1 in primary hepatocytes and liver lysates from L-IRFoxo1DKO mice (Figure 2A). L-Insulin Receptor KO and L-IRFoxo1DKO mice failed to activate canonical insulin targets (phosphorylation of Akt, S6, and PRAS40) following a supraphysiological dose of insulin (Figure 2B). However, feeding elicited significant S6 phosphorylation in all genotypes, supporting the notion that hepatic insulin receptor is not required for the activation of the mTorc1 complex by nutrients (Figure 2C)20.
Figure 2. Liver Foxo1 deletion normalizes glucose levels and hyperinsulinemia in L-Insulin Receptor KO mice despite lack of hepatic insulin signaling.
(A) Western blot from primary hepatocyte and liver lysates of mice from indicated gentoypes probed for specific proteins as indicated. (B) Western blot of liver lysates in mice fasted overnight and I.P injected with either saline or insulin (2mU/g) and probed for specific proteins as indicated. (C) Western blot of liver lysates in mice fasted overnight and refed normal chow for 4 h and probed for specific proteins as indicated. (D,E) Blood glucose concentrations in mice after overnight fast (D) and overnight fasted following by 4 h refeeding of normal chow (E). * p<0.05 vs LWT, #p<0.05 vs L-Insulin Receptor KO. LWT group includes total of 18 mice in fasting group with n=10 IRloxP/loxP and n=8 IRloxP/loxP;Foxo1loxP/loxP and in refed group includes total of 9 mice in fasting group with n=4 IRloxP/loxP and n=5 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=12 for fasting group and n=6 for refed group. L-IRFoxo1DKO includes n=22 for fasting group and n=7 for refed group. (F) Intraperitoneal glucose tolerance test (2 g per kg) on overnight fasted mice. LWT group includes total of 8 mice with n=6 IRloxP/loxP and n=2 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=7 and L-IRFoxo1DKO includes n=8. *p<0.05 vs LWT, ****p<0.0001 vs LWT (G) Insulin levels before and after 15 minutes following glucose injection. LWT group includes total of 8 mice with n=6 IRloxP/loxP and n=2 IRloxP/loxP;Foxo1loxP/loxP, L-Insulin Receptor KO includes n=6 and L-IRFoxo1DKO includes n=13, for fasting group. LWT group includes total of 3 mice with n=2 IRloxP/loxP and n=1 IRloxP/loxP;Foxo1loxP/loxP, L-Insulin Receptor KO includes n=3 and L-IRFoxo1DKO includes n=8 for 15 min group. ****p<0.0001 vs LWT, #p<0.05 vs L-Insulin Receptor KO (H) Intraperitoneal glucose tolerance test (2 g per kg) on overnight fasted mice. n=7-10 mice. LWT group includes total of 6 mice with n=5 IRloxP/loxP and n=1 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=8 and L-IRFoxo1DKO includes n=5. **p<0.01 vs LWT, ****p<0.0001 vs LWT. All data are presented as mean ± s.e.m. Statistical analysis was performed using one-way analysis of variance followed by Tukey post-test when more than two groups were compared, two-way analysis of variance followed by Bonferroni when two conditions were involved. A P <0.05 was considered statistically significant.
Concomitant deletion of Foxo1 restored Igfbp1 protein to LWT levels while also partially increasing glucokinase protein concentration albeit not to the levels present in LWT mice (Figure 2C). Deletion of Foxo1 normalized the fasting (Figure 2D) and postprandial hyperglycemia in the L-Insulin Receptor KO (Figure 2E). In addition, L-IRFoxo1DKO mice demonstrated improved glucose tolerance (Figure 2F) and Foxo1 deletion rescued the hyperinsulinemia caused by hepatic deletion of the IR (Figure 2G). The relatively normal metabolic phenotype of the L-IRFoxo1DKO mice was assessed in an alternative model, i.e. the congenital liver specific knockout generated with a liver-specific Cre transgene. Consistent with data with acute recombination, deletion of Foxo1 normalized the glucose intolerance caused by liver-specific deletion of IR (Figure 2H). These data support the concept that a significant increase in Foxo1 activity in the L-Insulin Receptor KO mice as a result of defective Akt activation is responsible for the diabetic like phenotype of the L-Insulin Receptor KO mice.
Insulin suppresses HGP in vivo in L-IRFoxo1DKO mice
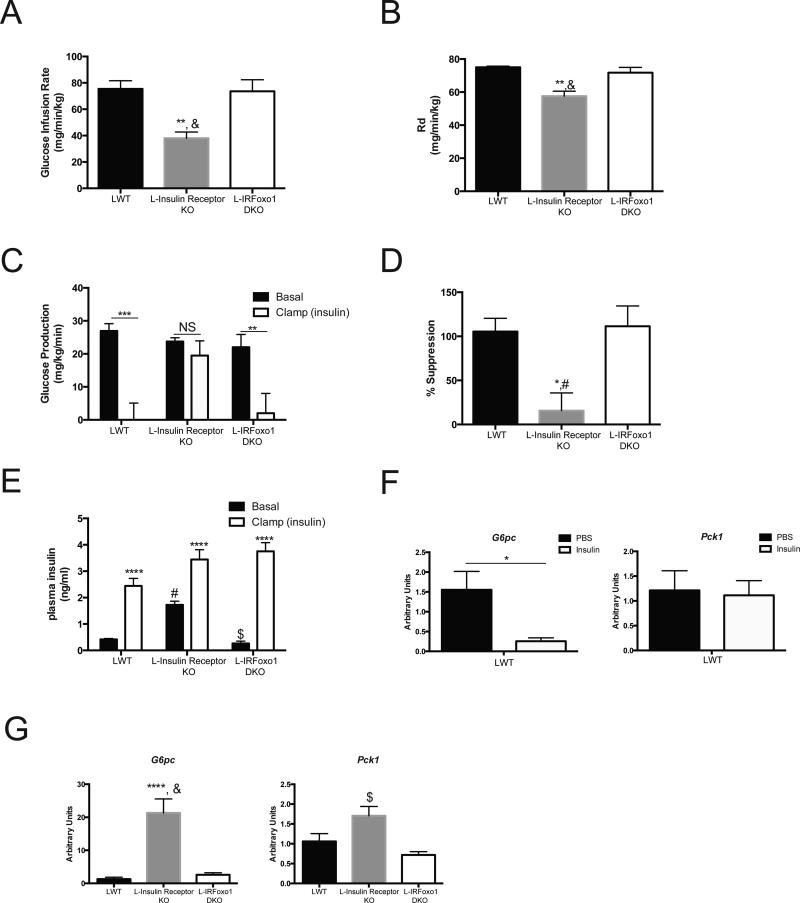
Despite the lack of hepatic insulin signaling in L-IRFoxo1DKO, the wildtype fasting glucose and insulin levels suggest these mice have normal HGP. However, these data do not provide information about the ability of insulin to regulate HGP. To ascertain whole-body insulin sensitivity directly and determine if insulin can suppress HGP independent of liver insulin signaling, hyperinsulinemic-euglycemic clamps in awake, unrestrained mice were performed. During insulin infusion under glucose clamp conditions, L-Insulin Receptor KO mice displayed a lower glucose infusion rate (Figure 3A), a 25% decrease in glucose disposal (Figure 3B), and a failure to suppress hepatic glucose production (Figure 3C,D) compared to LWT mice. Concomitant deletion of Foxo1 in L-IRFoxo1DKO mice normalized the glucose infusion rate (Figure 3A) confirming that Foxo1 deletion reversed the insulin resistance conferred by loss of the IR. L-IRFoxo1DKO mice had a normal rate of glucose disposal (Figure 3B) and, most importantly, insulin suppressed hepatic glucose production to the same extent as in LWT mice (Figure 3C,D). As noted previously, in this hyperinsulinemic-euglycemic clamp paradigm, insulin reduced glucose-6-phosphatase (G6pc) without affecting phosphoenolpyruvate carboxykinase (Pck1) expression in wildtype mice (Figure 3F)6. At the end of the clamp procedure, levels of G6pc and Pck1 in L-Insulin Receptor KO mice were significantly elevated compared to both LWT and L-IRFoxo1DKO mice (Figure 3G). The data support the hypothesis that insulin signals via an extra-hepatic tissue to regulate hepatic glucose production and the expression of G6pc.
Figure 3. L-IRFoxo1DKO are insulin responsive during euglycemic clamps.
Hyperinsulinemic-euglycemic clamps were perfromed on unrestrained 5 h fasted mice using a 2.5 mU/ming/kg infusion of insulin. (A) Steady state glucose infusion rate (B) rate of glucose disposal (C) hepatic glucose production during basal and insulin portions of the clamp (D) percent suppression of hepatic glucose production during the clamp portion compared to basal period (E) insulin levels during during basal and insulin portions of the clamp. *p<0.05 vs LWT, **p<0.01 vs LWT, ***p<0.001 vs LWT, ****p<0.0001 vs LWT #p<0.05 vs Insulin Receptor KO, &p<0.01 vs L-Insulin Receptor KO, $p<0.0001 vs L-Insulin Receptor KO. LWT group includes total of 4 mice with n=2 IRloxP/loxP and n=2 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=6 and L-IRFoxo1DKO includes n=4. ****p<0.0001 vs LWT $p<0.0001 vs L-Insulin Receptor KO. (F) Relative expression of G6pc and Pck1 under euglycemic conditions with/without infusion of 2.5 mU/min/kg of insulin for 120 mins. *p<0.05 vs PBS of indicated genotype. n=3 (PBS), n=6 (insulin). (G) Relative expression of G6pc and Pck1 under euglycemic conditions with infusion of 2.5 mU/min/kg of insulin for 120 mins. LWT group includes total of 4 mice with n=2 IRloxP/loxP and n=2 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=4 and L-IRFoxo1DKO includes n=4. ****p<0.0001 vs LWT $p<0.0001 vs L-Insulin Receptor KO. All data are presented as mean ± s.e.m. Statistical analysis was performed using one-way analysis of variance followed by Tukey post-test. A P <0.05 was considered statistically significant.
Feeding suppresses gluconeogenic genes in L-IRFoxo1DKO mice
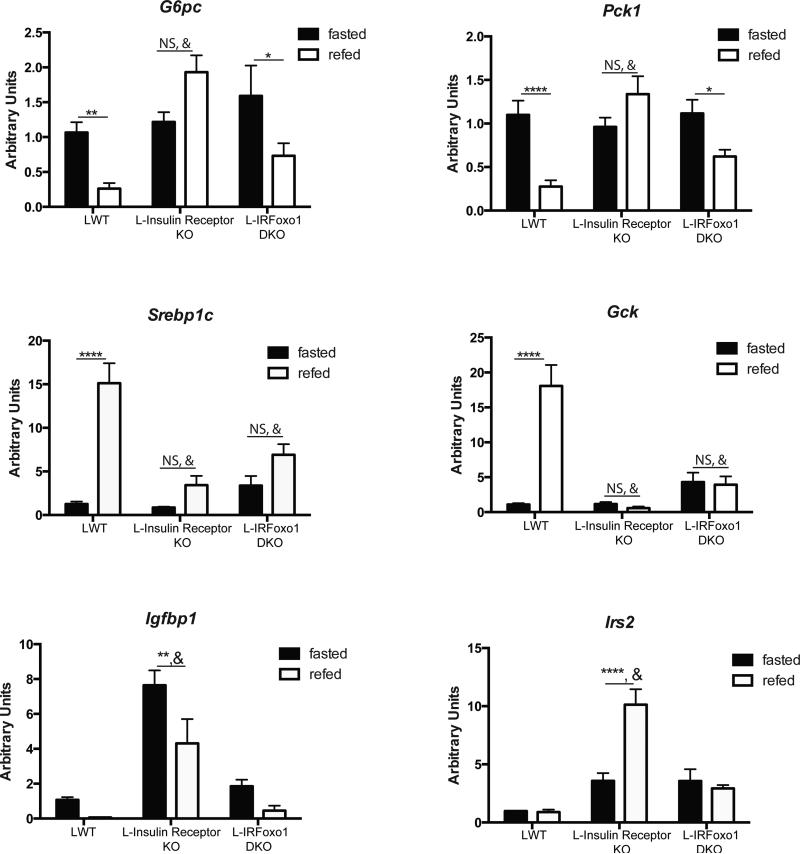
L-IRFoxo1DKO mice are glucose tolerant and insulin suppresses hepatic glucose production despite lacking hepatic insulin signaling. These data support previous observations that mice lacking Akt isoforms and Foxo1 in the liver can respond to insulin to reduce glucose production and transition to the fed state17. In addition to rescuing the defects in glucose metabolism, deletion of Foxo1 restored the inability of both the Irs and Akt liver double knockouts to regulate genes involved in gluconeogenesis14, 17. To test if hepatic insulin action is required for the changes in gene expression in response to feeding, mice were subjected to an overnight fast and refed. In LWT mice, 4h after refeeding there was the expected gene changes in expression, with suppression of G6pc, Pck1, Igfbp1 and induction of Srebp1c and Gck (Figure 4). Similar to previous models of defective hepatic insulin action, L-Insulin Receptor KO mice failed to reduce G6pc, Pck1 or increase Srebp1 and Gck mRNA in response to feeding; in addition, there was augmented expression of the Foxo1 target genes Igfbp1 and Irs2 (Figure 4). Despite the loss of hepatic insulin signaling in L-IRFoxo1DKO mice, refeeding suppressed both G6pc and Pck1 expression but failed to induce Srebp1 and Gck expression (Figure 4). Thus, hepatic insulin signaling is dispensable for the normal prandial response on gluconeogenic genes yet is required for the induction of Gck and the master lipogenic transcription factor, Srebp1c.
Figure 4. Metabolic transcriptional response to feeding in the absense of hepatic insulin signaling.
Hepatic gene expression in LWT, L-Insulin Receptor KO, and LIRFoxo1DKO following an overnight fast and 4 h of refeeding normal chow. LWT group includes total of 9 mice with n=5 IRloxP/loxP and n=4 IRloxP/loxP;Foxo1loxP/loxP. L-Insulin Receptor KO includes n=6 and L-IRFoxo1DKO includes n=4-5 *p<0.05 vs fasted condition of indicated genotype, **p<0.01 vs fasted condition of indicated genotype, ***p<0.001 vs fasted condition of indicated genotype, ****p<0.0001 vs fasted condition of indicated genotype, #p<0.0001 vs fasted LWT, &p<0.0001 vs fed LWT. All data are presented as mean ± s.e.m. Statistical analysis was performed using two-way analysis of variance followed by Bonferroni post-test. A P <0.05 was considered statistically significant.
Insulin fails to suppress genes in vitro in L-IRFoxo1DKO mice
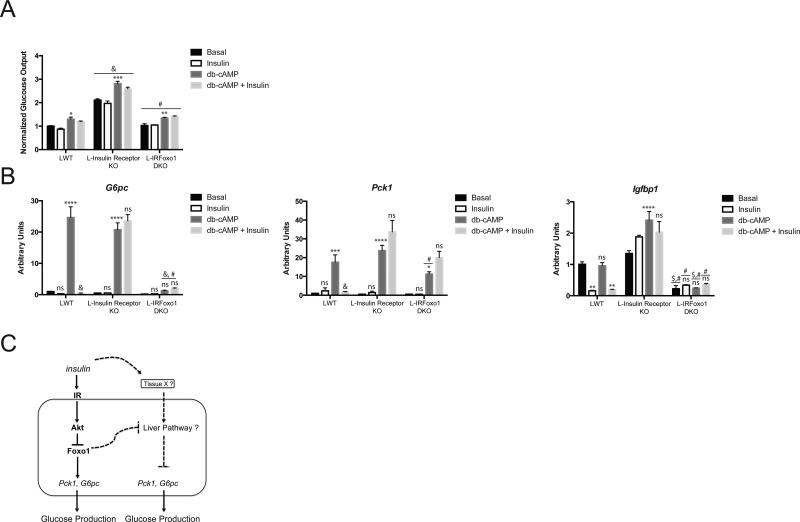
To confirm that insulin was acting cell-nonautonomously to regulate HGP and gluconeogenic genes in L-IRFoxo1DKO mice, primary hepatocytes were isolated from LWT, L-Insulin Receptor KO, and L-IRFoxo1DKO mice. Glucose production was measured over 6 h in response to db-cAMP, insulin, and db-cAMP and insulin. In all genotypes, db-cAMP stimulated glucose production approximately 30-40% (Figure 5A). L-Insulin Receptor KO mice exhibited increased glucose production that was normalized with concomitant Foxo1 deletion during all treatments (Figure 5A). G6pc and Pck1 expression was induced in response to db-cAMP in both LWT and L-Insulin Receptor KO. However, L-IRFoxo1DKO mice exhibited a defect in gluconeogenic gene expression in response to db-cAMP (Figure 5B). Insulin did not affect glucose production in isolated hepatocytes in any genotype or condition. However, insulin significantly reduced G6pc, Pck1, and Igfbp1 mRNA in hepatocytes isolated from LWT but not from L-Insulin Receptor KO or L-IRFoxo1DKO mice.
Figure 5. Glucose Production and gluconeogenic gene response to insulin and cAMP in isolated primary hepatocytes.
Primary hepatocytes were isolated from indicated genotype and treated with either 0.1 mM db-cAMP and/or 0.1 μM insulin for 6 h. (A) Glucose concentration in media was determined and expressed normalized to protein concentration. (B) Gene expression analysis was performed at the end of the treatments. Data is from a representative experiment and presented as mean ± SEM. *p<0.05 vs basal condition of indicated genotype, **p<0.01 vs basal condition of indicated genotype, ***p<0.001 vs basal condition of indicated genotype, ****p<0.001 vs basal condition of indicated genotype , #p<0.0001 vs L-Insulin Receptor KO, &p<0.0001 vs LWT for indicated treatment condition. All data are presented as mean ± s.e.m. Statistical analysis was performed using two-way analysis of variance followed by Bonferroni post-test. A P <0.05 was considered statistically significant. (C) In hepatocytes, insulin stimulates the kinase Akt thus inactivating the transcription factor Foxo1, which leads to the suppression of gluconeogenic genes and glucose production. In addition to the canonical IR-Akt liver pathway, there is an insulin responsive non-hepatic tissue (tissue X) capable of communicating with the liver to regulate hepatic glucose output and gluconeogenic genes. An unidentified liver pathway receives the insulin signal via tissue X to suppress gluconeogenic genes and glucose output in response to insulin. Deletion in liver of the IR, Irs, or Akt gene or high fat diet feeding leads to abberant Foxo1 activity, which represses this unidentified liver pathway. Ablation of hepatic Foxo1 alleviates this suppressive effect allowing insulin to regulate hepatic glucose metabolism.
Discussion
In this study, we used genetic loss of function experiments in mice to test the requirement for hepatic insulin action for the insulin-dependent regulation of glucose metabolism and transcription in mouse liver. Data presented herein confirm previous studies that signaling via the hepatic insulin receptor is essential for normal glucose metabolism in adult mice18. Substantial data support a model in which insulin regulates glucose metabolism by signaling through Akt-mediated inactivation of the transcription factor, Foxo1, thus contributing to the metabolic transition that accompanies the intake of food. Nonetheless, insulin suppressed hepatic glucose output during euglycemic clamps in mice with livers deficient for the IR and Foxo1. These data refute the idea that the canonical model of hepatic insulin action in liver represents the exclusive pathway by which the hormone suppresses hepatic glucose output. Moreover, the persistence of hepatic effects of insulin in L-IRFoxo1DKO mice strongly supports the hypothesis that, at least under some conditions, insulin can act cell non-autonomously to suppress hepatic glucose output and regulate gene expression17. The hypothesis that indirect mechanisms contribute to the regulation of insulin mediated suppression of HGP dates back almost 60 years 23. In support of this idea, restoring hepatic insulin signaling is not sufficient to fully suppress HGP in mice with systemic loss of the insulin receptor 24. Data presented herein provide genetic evidence that insulin uses both an intra and extra-hepatic mechanisms to regulate liver metabolism.
Previous studies using liver specific insulin receptor knockout (LIRKO) mice have reported robust defects in glucose homeostasis and peripheral insulin resistance18, 25. LIRKO mice develop profound insulin resistance as adults; however, the lack of the insulin receptor from birth also leads to liver failure in aged mice. To avoid the complications of congenital liver specific deletion, we employed a model that relies on acute deletion of the insulin receptor in adult mice8. L-Insulin Receptor KO mice displayed mild glucose intolerance and insulin resistance without the confounding peripheral insulin resistance and pronounced fasting hyperglycemia in the LIRKO mice. Moreover, the mild hyperglycemia of the acute IR deletion was less severe than in acute liver specific deletion of both Akt isoforms17. This difference in severity may be due to significant basal Akt activity independent of insulin action, leading to partial inhibition of Foxo1, even in fasting, non-obese mice17.
Considerable data refute the idea that insulin signals though a hepatocyte, cell autonomous pathway in L-IRFoxo1DKO mice. Insulin failed to promote phosphorylation of the canonical targets Akt and S6 in L-Insulin Receptor KO and L-IRFoxo1DKO mice. Though it has been suggested IGF1-R can be induced in liver by stresses such as regeneration26, we did not detect IGF1-R protein in livers from LWT, L-Insulin Receptor KO or L-IRFoxo1DKO mice (Figure 2A). High concentrations of insulin were completely without effect on isolated hepatocytes lacking insulin receptors with or without concomitant deletion of Foxo1 (Figure 5B).
One of the major unanswered questions that arise from this study is the identity of the extra-hepatic tissue that is binding insulin and transmitting the signal to suppress hepatic glucose output and gluconeogenic gene expression. Based on recent work, perhaps the most likely site of insulin action is the central nervous system (CNS), which has been proposed to serve as an organizing center for metabolic control21, 27, 28. In support of a direct communication between the CNS and liver, intra-cerebral ventricular infusion of insulin suppresses HGP without activation of hepatic insulin signaling through the hepatic vagus nerve29, 30. Moreover, mice with insulin receptor deficiency in AgRP neurons fail to suppress HGP in response to peripheral insulin infusion during euglycemic clamps31. However, infusion of insulin in the brain of dogs only increases liver glycogen synthesis without affecting glucose output, questioning the significance of a CNS mechanisms in the regulation of HGP32, 33. Future studies are needed to assess the contribution of insulin action in the CNS to the regulation of HGP in mice with livers lacking autonomous insulin action.
In addition to the CNS, a more classical target of insulin action is adipose tissue. It has been hypothesized that the insulin-dependent suppression of lipolysis limits gluconeogenesis by reduction in circulating glycerol and free fatty acids (FFAs)34. Preventing the fall of FFAs during hyperinsulinemic-euglycemic clamps by infusing a lipid/heparin mixture antagonizes the reduction of hepatic glucose output in dogs35. Moreover, pharmacological suppression of lipolysis alone is sufficient to reduce HGP independent of changes in insulin levels36. The other well-established pathway for regulating HGP is through the counter-regulatory hormone glucagon, whose secretion by the alpha cells of the pancreas is itself reduced by insulin3, 37. Indeed, the decline in portal glucagon concentrations during hyperinsulinemic-euglycemic clamps in dogs correlates well with reductions in HGP38. However, a reduction in peripheral glucagon during clamp experiments in mice has been difficult to observe25. Defining the relative contributions of these cell non-autonomous pathways for the regulation of HGP by insulin will be of significant interest.
Since these data establish an extra-hepatic pathway in the regulation of HGP, there must exist a liver autonomous mechanism to receive the signal initiated by insulin's interaction with a non-hepatic tissue. In L-Insulin Receptor KO mice, this unidentified liver autonomous pathway is suppressed by aberrant Foxo1 activity, preventing insulin's regulation of hepatic glucose output. Upon deletion of Foxo1, as in L-IRFoxo1DKO mice, the suppressive effect of Foxo1 on this pathway is lost allowing insulin to signal to the liver via an intermediary peripheral tissue to suppress hepatic glucose output and gluconeogenic gene expression (Figure 5C). The identity of this pathway remains unknown and presents a novel therapeutic target for the regulation of HGP independent of hepatic insulin action.
Though deletion of Foxo1 in IR null livers restored the response of gluconeogenic genes to nutritional regulation, lipogenic genes such as Srebp1c and Gck remained unresponsive to refeeding in the absence of hepatic insulin receptors (Figure 4). Following a meal, insulin and nutrients activate the mTORC1 signaling pathway which leads to increased lipogenesis and lipogenic gene expression, in part by inducing the transcription and posttranslational processing of SREBP1c8, 39. However, refeeding fails to increase Srebp1c and Gck gene expression in the liver of L-IRFoxo1DKO to the same extent as LWT mice. Indeed, the hepatic insulin receptor is required for SREBP1c induction and de novo lipogenesis in vivo20. The failure to induce lipogenic genes in LIRFoxo1DKO mice suggests strongly that insulin can stimulate lipid synthesis only by a cell autonomous pathway.
In summary, we have demonstrated that the abnormal hepatic glucose metabolism and insulin resistance resulting from lack of hepatic insulin receptor is dependent on a constitutively active Foxo1. Upon Foxo1 deletion, the insulin-dependent regulation of glucose metabolism in vivo was largely restored, supporting the idea that reducing Foxo1 activity in insulin resistant disorders may have beneficial effects15, 40. In addition, we provided genetic evidence that an extra-hepatic insulin-responsive tissue communicates with the liver to regulate glucose output. Elucidation of this pathway may provide a novel target for the treatment of insulin resistant disorders such as diabetes mellitus.
Materials and Methods
Mice
Male mice were used in all experiments. The IRloxP/loxP and Foxo1loxP/loxP mice were described previously 10, 41. IRloxP/loxP;Foxo1loxP/loxP mice were generated by crossing the IRloxP/loxP (C57Bl/6J) with the Foxo1loxP/loxP (FVB) mice. These mice were on a 129-C57BL/6J-FVB mixed background. Mice were injected at 6- to 8-weeks of age with 1011 genomic copies per mouse with adeno-associated-virus containing a liver specific promoter, thyroxine-binding globulin (TBG) promoter driving either GFP or Cre recombinase to generate LWT, L-Insulin Receptor KO, or L-IRFoxo1DKO8. The LWT group consisted of pooled GFP injected IRloxP/loxP and IRloxP/loxP;Foxo1loxP/loxP mice. GFP injected IRloxP/loxP and IRloxP/loxP;Foxo1loxP/loxP mice did not significantly differ in any observed measurement therefore data is shown as the pooled averages from both genotypes. Experiments were performed 2 to 3 weeks after virus injection. To generate liver-specific congenital knockouts, IRloxP/loxP and IRloxP/loxP;Foxo1loxP/loxP mice were crossed to an Alb-Afp-cre mouse42 (kind gift from Klaus Kaestner) and litter-mate floxed mice served as controls. For the fasting and refeeding experiments, mice were deprived of food for 16 h overnight, then sacrificed (for the fasted groups) or refed with normal chow (Laboratory Rodent Diet 5001) for an additional 4 h before sacrifice (for the refed group). For insulin injection, 16 h fasted mice were i.p. injected with 3% BSA in saline or insulin at 2 mU/g of body weight 20 min prior to sacrificing the mice and excising the liver for rapid freezing in liquid nitrogen. All mice experiments were reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health.
Liver protein extraction and western blotting
Livers were freeze-clamped and stored at −80 °C until further processing. Protein lysates were extracted from frozen livers with a modified RIPA buffer (150 mM NaCl, 50 mM Tris, pH 7.6, 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% SDS, supplemented with protease (Roche) and phosphatase (Sigma) inhibitors. Cleared supernatants were extracted from cellular debris following 15 min centrifugation at 17,000 g. The following antibodies were used for immunoblotting at a dilution of 1:1000: insulin receptor, IGF1-R, Foxo1, p-Ser 473 Akt, Akt, pPRAS 40, PRAS, p-S6 Ser 240 and Ser 244, S6 were from Cell Signaling Technology; antibody to Igfbp1 was purchased from Santa Cruz Biotechnology; antibodies to GFP and tubulin were from Sigma; antibody to Gck was provided by M.A. Magnuson (Vanderbilt University). Complete scans of key immunoblots are provided in Supplementary Fig. 1.
mRNA isolation and real-time PCR
Total RNA was isolated from frozen livers using the NucleoSpin RNA kit from Clontech. Complementary DNA was synthesized using Moloney murine leukemia virus reverse transcriptase and the relative expression of the genes of interest was quantified by real-time PCR using the SYBR Green Dye-based assay. Primer sequences are provided in Supplementary Table 1.
Primary hepatocytes isolation
Hepatocytes were isolated from random fed mice using a 2-step collagenase/DNAse digestion protocol43. For glucose output studies, primary hepatocytes from indicated genotypes were isolated and plated in M199 media containing 10% FBS, 100 nM T3, 500 nM dexamethasone, and 1 nM insulin. Following attachment, cells were changed to M199 media containing 100 nM dexamethasone, and 1 nM insulin were indicated and incubated overnight. Cells were washed switched to glucose output media (DMEM no glucose) and incubated with 10 mM lactate 1 mM pyruvate, 100 nM dexamethasone, and 100 nM insulin and/or 100 uM db-cAMP were indicated. Glucose production was determined by the measurement of glucose in the media over a 6 hour time course using hexokinase glucose assay. Cells were lysed in modified RIPA buffer described above and subjected to western blot analysis or protein normalization for glucose output studies. Alternatively, cells were harvested for RNA isolation and subsequent gene expression analysis as described above.
Metabolic Measurements
For glucose tolerance test, overnight fasted mice were given glucose at 2 g/kg body weight via intraperitoneal injection. Blood glucose concentrations in the mice were monitored by tail bleeding at 0, 15, 30, 60 and 120 min after glucose injection. Insulin concentrations were measured from plasma collected from the mice before and 15 min following glucose injection.
Hyperinsulinemic-euglycemic clamp
Euglycemic clamps were performed on unrestrained and awake 8-10 week old mice6. Indwelling catheters were placed into mice 5-7 days prior to clamp experiments. Mice were fasted for 4 hr and a priming 5 μCi [3-3H]glucose (Perkin Elmer HPLC purified) bolus followed by 0.05 μCi/min infusion was initiated for 120 minutes. Following the basal period, mice were given a bolus of 150 mU/kg insulin (or PBS) and a 0.1 μCi/min [3-3H]glucose infusion with insulin was initiated (6 h from start of fast). The rate of insulin infusion was 2.5 mU/kg/min, and the glucose infusion rate was adjusted to keep euglycemic levels between 100 and 130 mg/dl. PBS without glucose was infused in the control groups for gene expression analysis. Blood was taken by tail bleeding, and blood glucose levels were measured every 10 min during the clamp. Rd and HGP were calculated from the blood sampling every 10 min during the steady state portion of (80-120 min). At the end of the 2 hr clamp, mice were sacrificed by pentobarbital injection, and livers were quickly removed, freeze clamped in liquid nitrogen, and stored at −80°C for future uses.
Statistical analysis
All data are presented as mean ± s.e.m. Statistical analysis was performed using one-way analysis of variance followed by Tukey post-test when more than two groups were compared, two-way analysis of variance followed by Bonferroni post-test when two conditions were involved and a Students's t test with two-tailed analysis when only two groups of data were concerned. A P <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors would like to thank D. Accili (Columbia University) for sharing the Foxo1loxP/loxP mice and C. Ronald Kahn (Joslin) for sharing the IRloxP/loxP mice. This work was supported by the US National Institutes of Health grant R01 DK056886 (M.J.B.), NRSA individual postdoctoral fellowship F32 DK101175 (P.M.T.) and the Samuel Chiaffa Memorial Fund (P.M.T.)
Footnotes
Author Contributions
P.M.T. conceived of the hypothesis, designed and performed the experiments and analyzed the data. Q.C., B.R.M. provided technical assistance. M.J.B. conceived the hypothesis and directed the project. P.M.T. and M.J.B. prepared the manuscript.
Competing Financial Interests
P.M.T, Q.C., B.R.M. declare no competing financial interests. M.J.B is an employee of Pfizer, Inc.
References
- 1.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 3.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- 5.Menon S, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan M, et al. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Cell Metab. 2013;18:99–105. doi: 10.1016/j.cmet.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan M, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rena G, et al. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. Embo J. 2002;21:2263–2271. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 12.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 13.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC- 1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 14.Dong XC, et al. Inactivation of hepatic Foxo1 by insulin signaling is required foradaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel VT, et al. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55:2042–2050. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- 16.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 17.Lu M, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michael MD, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 19.Cheng Z, White MF. The AKTion in non-canonical insulin signaling. Nat Med. 2012;18:351–353. doi: 10.1038/nm.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas JT, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers MG, Jr., Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- 22.Ramnanan CJ, Edgerton DS, Cherrington AD. Evidence against a physiologic role for acute changes in CNS insulin action in the rapid regulation of hepatic glucose production. Cell Metab. 2012;15:656–664. doi: 10.1016/j.cmet.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine R, Fritz IB. The relation of insulin to liver metabolism. Diabetes. 1956;5:209–219. doi: 10.2337/diab.5.3.209. discussion, 219-222. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest. 2005;115:1314–1322. doi: 10.1172/JCI23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher SJ, Kahn CR. Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest. 2003;111:463–468. doi: 10.1172/JCI16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nature clinical practice Endocrinology & metabolism. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 27.Gelling RW, et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue H, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Konner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Ramnanan CJ, et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest. 2011;121:3713–3723. doi: 10.1172/JCI45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramnanan CJ, et al. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes. 2013;62:74–84. doi: 10.2337/db12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis GF, Vranic M, Harley P, Giacca A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes. 1997;46:1111–1119. doi: 10.2337/diab.46.7.1111. [DOI] [PubMed] [Google Scholar]
- 35.Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98:741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittelman SD, Bergman RN. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab. 2000;279:E630–637. doi: 10.1152/ajpendo.2000.279.3.E630. [DOI] [PubMed] [Google Scholar]
- 37.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers SR, Diamond MP, Adkins-Marshall BA, Williams PE, Stinsen R, Cherrington AD. Effects of small changes in glucagon on glucose production during a euglycemic, hyperinsulinemic clamp. Metabolism. 1991;40:66–71. doi: 10.1016/0026-0495(91)90194-2. [DOI] [PubMed] [Google Scholar]
- 39.Yecies JL, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 42.Parviz F, et al. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- 43.Miller RA, et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.