Abstract
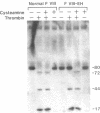
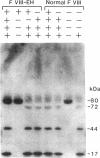
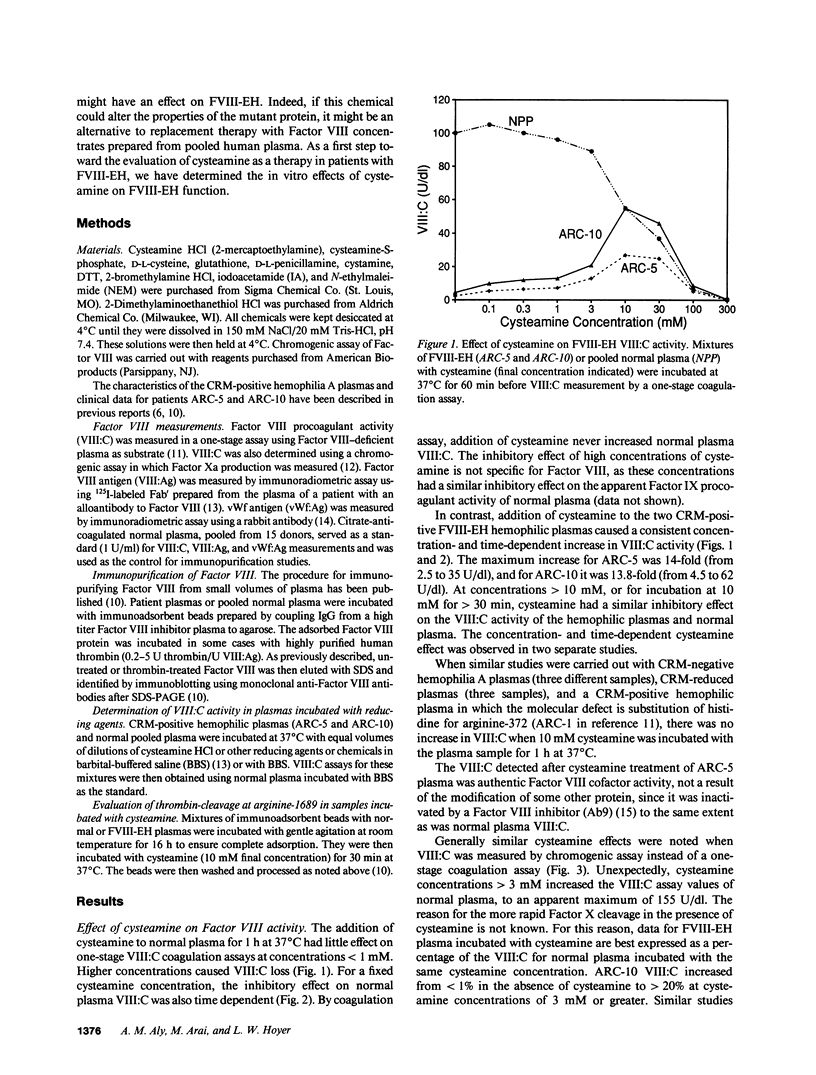
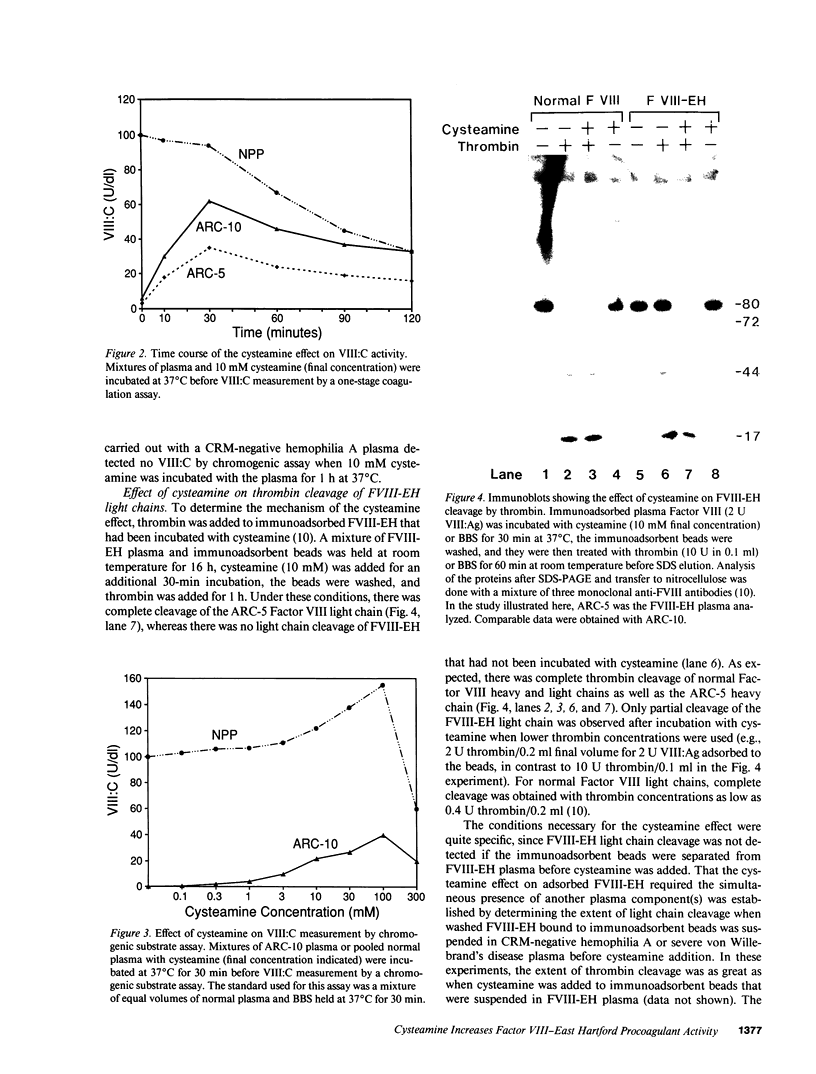
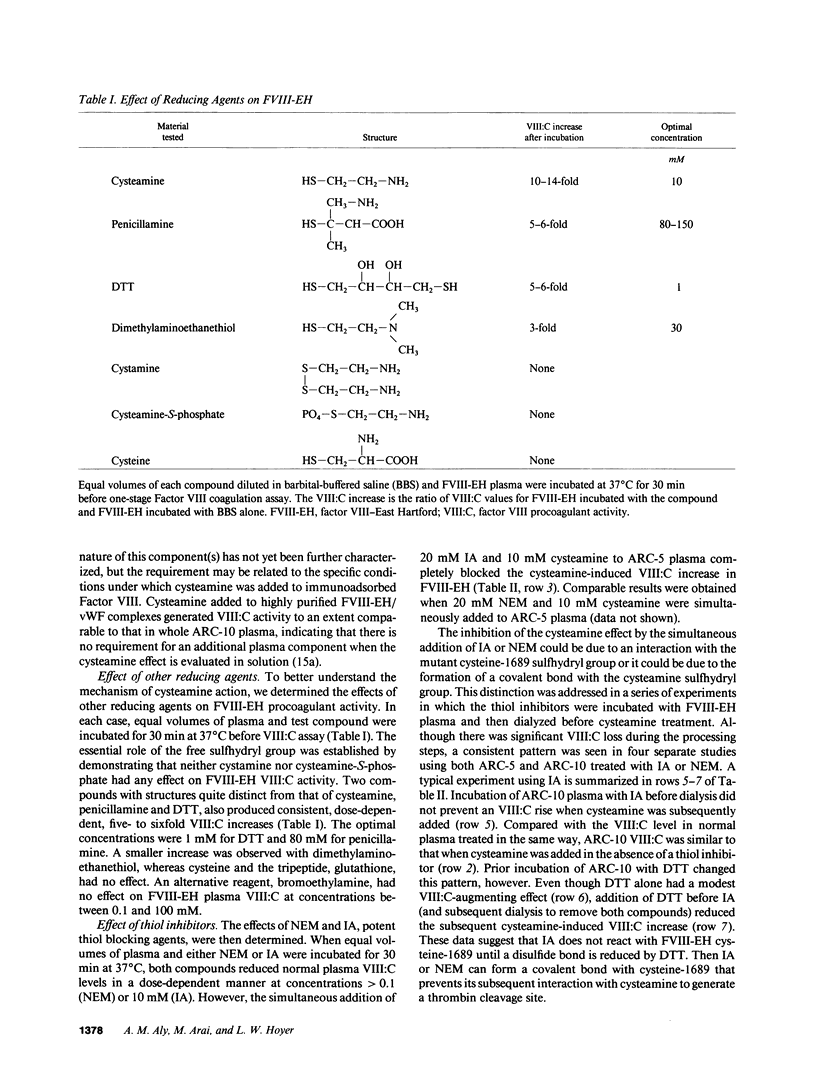
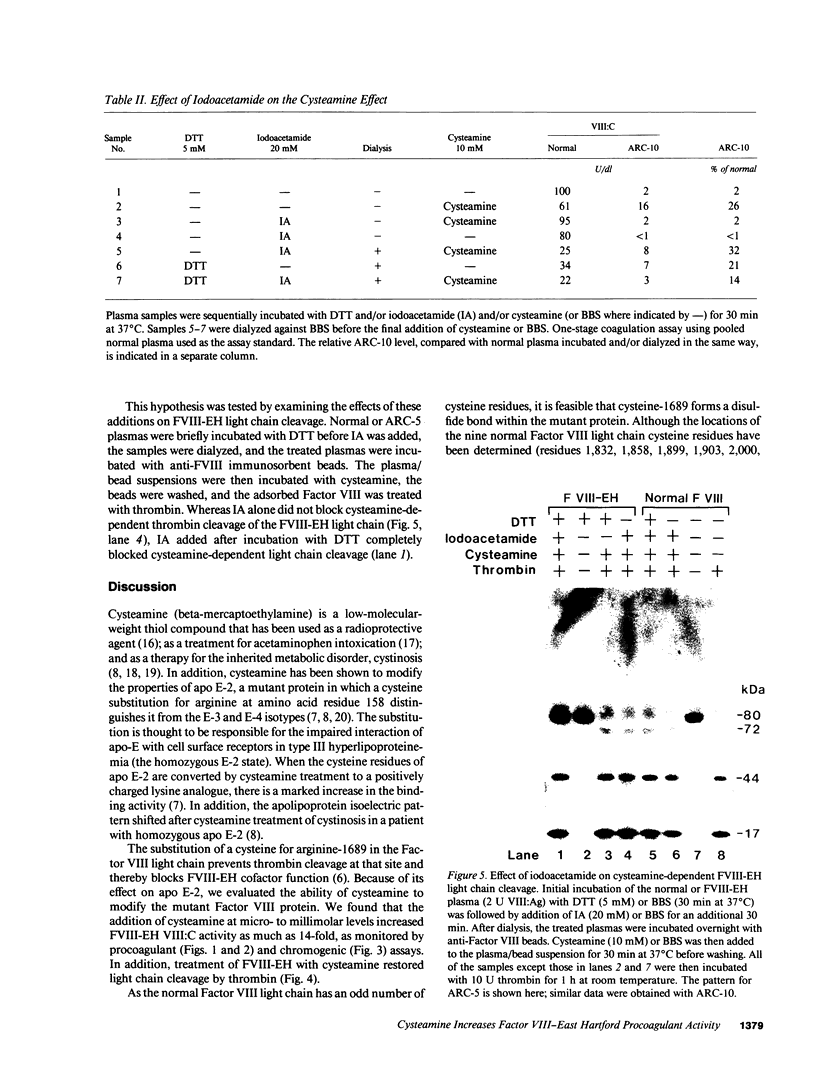
We have recently identified the molecular defect responsible for cross-reacting material-positive hemophilia A in two unrelated patients in which the substitution of cysteine for arginine-1689 (Factor VIII-East Hartford[FVIII-EH]) abolishes a critical Factor VIII light chain thrombin cleavage site. As other mutant proteins with a cysteine for arginine substitution have been modified in the presence of cysteamine, we have determined the effect of this and other reducing agents on FVIII-EH function. Cysteamine concentrations between 0.1 and 10 mM caused dose- and time-dependent increases in FVIII-EH VIII:C activity, as much as 14-fold (to 35 and 62 U/dl for the two patients tested). Comparable data were obtained in a standard one-stage VIII:C coagulation assay and in a chromogenic substrate assay measuring Factor Xa generation. Thrombin cleavage of the FVIII-EH light chain in the presence of cysteamine was documented by immunoadsorption and analysis. Cystamine and cysteamine-S-phosphate, similar compounds that do not possess a free thiol group, had no effect. Cysteamine augmentation of FVIII-EH VIII:C was abolished by the simultaneous addition of N-ethyl maleimide or iodoacetamide, but these sulfhydryl blocking agents did not prevent the VIII:C increase and light chain cleavage by thrombin if the plasma samples were dialyzed to remove the inhibitors before adding the cysteamine. However, incubation with DTT before iodoacetamide prevented the cysteamine effect after dialysis. These data suggest that when isolated from patient plasma, FVIII-EH cysteine-1689 is present in a disulfide bond. This bond is cleaved by cysteamine to form a new mixed disulfide, a pseudolysine that restores a thrombin cleavage site that is essential for procoagulant function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly A. M., Hoyer L. W. Factor VIII-East Hartford (arginine 1689 to cysteine) has procoagulant activity when separated from von Willebrand factor. J Clin Invest. 1992 May;89(5):1382–1387. doi: 10.1172/JCI115726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M., Higuchi M., Antonarakis S. E., Kazazian H. H., Jr, Phillips J. A., 3rd, Janco R. L., Hoyer L. W. Characterization of a thrombin cleavage site mutation (Arg 1689 to Cys) in the factor VIII gene of two unrelated patients with cross-reacting material-positive hemophilia A. Blood. 1990 Jan 15;75(2):384–389. [PubMed] [Google Scholar]
- Arai M., Inaba H., Higuchi M., Antonarakis S. E., Kazazian H. H., Jr, Fujimaki M., Hoyer L. W. Direct characterization of factor VIII in plasma: detection of a mutation altering a thrombin cleavage site (arginine-372----histidine). Proc Natl Acad Sci U S A. 1989 Jun;86(11):4277–4281. doi: 10.1073/pnas.86.11.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRECKENRIDGE R. T., RATNOFF C. D. Studies on the nature of the circulating anticoagulant directed against antihemophilic factor: with notes on an assay for anthemophilic factor. Blood. 1962 Aug;20:137–149. [PubMed] [Google Scholar]
- Corden B. J., Schulman J. D., Schneider J. A., Thoene J. G. Adverse reactions to oral cysteamine use in nephropathic cystinosis. Dev Pharmacol Ther. 1981;3(1):25–30. doi: 10.1159/000457418. [DOI] [PubMed] [Google Scholar]
- Diuguid D. L., Rabiet M. J., Furie B. C., Furie B. Molecular defects of factor IX Chicago-2 (Arg 145----His) and prothrombin Madrid (Arg 271----cys): arginine mutations that preclude zymogen activation. Blood. 1989 Jul;74(1):193–200. [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Ireland H., Panico M., Di Marzo V., Blench I., Morris H. R. Formation of a covalent disulfide-linked antithrombin-albumin complex by an antithrombin variant, antithrombin "Northwick Park". J Biol Chem. 1987 Oct 5;262(28):13381–13384. [PubMed] [Google Scholar]
- Fisher E. A., Gahl W. A. Cysteamine in treatment of type III hyperlipidaemia? Lancet. 1982 Nov 20;2(8308):1131–1132. doi: 10.1016/s0140-6736(82)92789-1. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Zimmerman T. S. Thrombin proteolysis of purified factor viii procoagulant protein: correlation of activation with generation of a specific polypeptide. Blood. 1983 Apr;61(4):807–811. [PubMed] [Google Scholar]
- Gahl W. A., Bashan N., Tietze F., Bernardini I., Schulman J. D. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science. 1982 Sep 24;217(4566):1263–1265. doi: 10.1126/science.7112129. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Gregg R. E., Hoeg J. M., Fisher E. In vivo alteration of a mutant human protein using the free thiol cysteamine. Am J Med Genet. 1985 Feb;20(2):409–417. doi: 10.1002/ajmg.1320200226. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Thoene J. G., Schneider J. A., O'Regan S., Kaiser-Kupfer M. I., Kuwabara T. NIH conference. Cystinosis: progress in a prototypic disease. Ann Intern Med. 1988 Oct 1;109(7):557–569. doi: 10.7326/0003-4819-109-7-557. [DOI] [PubMed] [Google Scholar]
- Galanakis D. K., Henschen A., Peerschke E. I., Kehl M. Fibrinogen Stony Brook, a heterozygous A alpha 16Arg----Cys dysfibrinogenemia. Evaluation of diminished platelet aggregation support and of enhanced inhibition of fibrin assembly. J Clin Invest. 1989 Jul;84(1):295–304. doi: 10.1172/JCI114154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryl M. S., Hoyer L. W. Immunologic studies of antihemophilic factor (AHF, VIII:C). VI. Characterization of antigenic determinants using human antibodies. Clin Immunol Immunopathol. 1982 May;23(2):517–526. doi: 10.1016/0090-1229(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Kogan S., Levinson B., Tuddenham E. G. Mutations of factor VIII cleavage sites in hemophilia A. Blood. 1988 Sep;72(3):1022–1028. [PubMed] [Google Scholar]
- Hoyer L. W., Breckenridge R. T. Immunologic studies of antihemophilic factor (AHF, factor VIII): cross-reacting material in a genetic variant of hemophilia A. Blood. 1968 Dec;32(6):962–971. [PubMed] [Google Scholar]
- Hoyer L. W. Immunologic studies of antihemophilic factor (AHF, factor VIII). IV. Radioimmunoassay of AHF antigen. J Lab Clin Med. 1972 Dec;80(6):822–833. [PubMed] [Google Scholar]
- Hughes R. D., Gazzard B. G., Hanid M. A., Trewby P. N., Murray-Lyon I. M., Davis M., Williams R., Bennet J. R. Controlled trial of cysteamine and dimercaprol after paracetamol overdose. Br Med J. 1977 Nov 26;2(6099):1395–1395. doi: 10.1136/bmj.2.6099.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. H., Davie E. W. Blood coagulation factors V and VIII: structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood. 1988 Mar;71(3):539–555. [PubMed] [Google Scholar]
- Koide T., Odani S., Takahashi K., Ono T., Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984 Jan;81(2):289–293. doi: 10.1073/pnas.81.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarchick J., Hoyer L. W. Immunoradiometric measurement of the factor VIII procoagulant antigen. J Clin Invest. 1978 Nov;62(5):1048–1052. doi: 10.1172/JCI109209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell M. B., Peake I. R., Taylor S. A., Lillicrap D. P., Giddings J. C., Bloom A. L. Factor IX Cardiff: a variant factor IX protein that shows abnormal activation is caused by an arginine to cysteine substitution at position 145. Br J Haematol. 1989 Aug;72(4):556–560. doi: 10.1111/j.1365-2141.1989.tb04323.x. [DOI] [PubMed] [Google Scholar]
- Miyata T., Terukina S., Matsuda M., Kasamatsu A., Takeda Y., Murakami T., Iwanaga S. Fibrinogens Kawaguchi and Osaka: an amino acid substitution of A alpha arginine-16 to cysteine which forms an extra interchain disulfide bridge between the two A alpha chains. J Biochem. 1987 Jul;102(1):93–101. doi: 10.1093/oxfordjournals.jbchem.a122046. [DOI] [PubMed] [Google Scholar]
- O'Brien D. P., Tuddenham E. G. Purification and characterization of factor VIII 1,689-Cys: a nonfunctional cofactor occurring in a patient with severe hemophilia A. Blood. 1989 Jun;73(8):2117–2122. [PubMed] [Google Scholar]
- Pittman D. D., Kaufman R. J. Proteolytic requirements for thrombin activation of anti-hemophilic factor (factor VIII). Proc Natl Acad Sci U S A. 1988 Apr;85(8):2429–2433. doi: 10.1073/pnas.85.8.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdie J. W. A comparative study of the radioprotective effects of cysteamine, WR-2721, and WR-1065 in cultured human cells. Radiat Res. 1979 Feb;77(2):303–311. [PubMed] [Google Scholar]
- Reber P., Furlan M., Beck E. A., Finazzi G., Buelli M., Barbui T. Fibrinogen Bergamo I (A alpha 16Arg----Cys): susceptibility towards thrombin following aminoethylation, methylation or carboxamidomethylation of cysteine residues. Thromb Haemost. 1985 Aug 30;54(2):390–393. [PubMed] [Google Scholar]
- Schwaab R., Ludwig M., Kochhan L., Oldenburg J., McVey J. H., Egli H., Brackmann H. H., Olek K. Detection and characterisation of two missense mutations at a cleavage site in the factor VIII light chain. Thromb Res. 1991 Feb 1;61(3):225–234. doi: 10.1016/0049-3848(91)90098-h. [DOI] [PubMed] [Google Scholar]
- Seghatchian M. J., Miller-Andersson M. A colorimetric evaluation of factor VIII: C potency. Med Lab Sci. 1978 Oct;35(4):347–354. [PubMed] [Google Scholar]
- Shima M., Ware J., Yoshioka A., Fukui H., Fulcher C. A. An arginine to cysteine amino acid substitution at a critical thrombin cleavage site in a dysfunctional factor VIII molecule. Blood. 1989 Oct;74(5):1612–1617. [PubMed] [Google Scholar]
- Smolin L. A., Clark K. F., Thoene J. G., Gahl W. A., Schneider J. A. A comparison of the effectiveness of cysteamine and phosphocysteamine in elevating plasma cysteamine concentration and decreasing leukocyte free cystine in nephropathic cystinosis. Pediatr Res. 1988 Jun;23(6):616–620. doi: 10.1203/00006450-198806000-00018. [DOI] [PubMed] [Google Scholar]
- Thoene J. G., Oshima R. G., Crawhall J. C., Olson D. L., Schneider J. A. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976 Jul;58(1):180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Ota K., Moroi M., Matsuda M. An apparently higher molecular weight gamma-chain variant in a new congenital abnormal fibrinogen Tochigi characterized by the replacement of gamma arginine-275 by cysteine. Blood. 1988 Feb;71(2):480–487. [PubMed] [Google Scholar]